The Outcome and CT Findings of Low-Dose Intensity Modulated Radiation Therapy with SQAP in a Cat with Thymoma
Abstract
1. Introduction
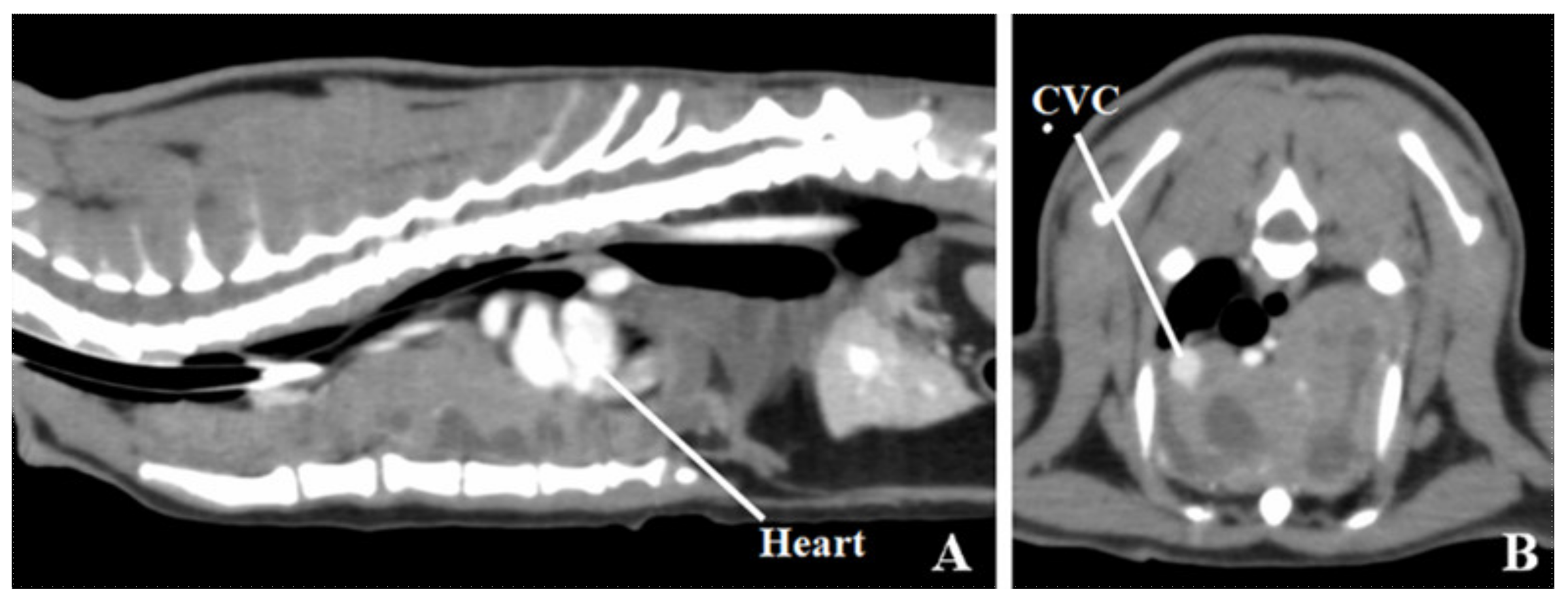
2. Case Presentation
3. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Sahara, H.; Ishikawa, M.; Takahashi, N.; Ohtani, S.; Sato, N.; Gasa, S.; Akino, T.; Kikuchi, K. In vivo anti-tumour effect of 3’-sulphonoquinovosyl 1’-monoacylglyceride isolated from sea urchin (Strongylocentrotus intermedius) intestine. Br. J. Cancer 1997, 75, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Ohta, K.; Mizushima, Y.; Hirata, N.; Takemura, M.; Sugawara, F.; Matsukage, A.; Yoshida, S.; Sakaguchi, K. Sulfoquinovosyldiacylglycerol, KM043, a New Potent Inhibitor of Eukaryotic DNA Polymerases and HIV-Reverse Transcriptase Type 1 from a Marine Red Alga, Gigartina tenella. Chem. Pharm. Bull. 1998, 46, 684–686. [Google Scholar] [CrossRef] [PubMed]
- Takakusagi, Y.; Naz, S.; Takakusagi, K.; Ishima, M.; Murata, H.; Ohta, K.; Miura, M.; Sugawara, F.; Sakaguchi, K.; Kishimoto, S.; et al. A Multimodal Molecular Imaging Study Evaluates Pharmacological Alteration of the Tumor Microenvironment to Improve Radiation Response. Cancer Res. 2018, 78, 6828–6837. [Google Scholar] [CrossRef] [PubMed]
- Sakimoto, I.; Ohta, K.; Yamazaki, T.; Ohtani, S.; Sahara, H.; Sugawara, F.; Sakaguchi, K.; Miura, M. α-Sulfoquinovosylmonoacylglycerol Is a Novel Potent Radiosensitizer Targeting Tumor Angiogenesis. Cancer Res. 2006, 66, 2287–2295. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, C.; Maruo, T.; Nishiyama, Y.; Hosaka, S.; Fukuyama, Y.; Sahara, H. Sulfoquinovosyl acyl panediol (SQAP) as a radiation sensitizer for dogs with tumors: A pilot study. Azabu Univ. Mag. 2020, 31, 53–59. [Google Scholar]
- Mori, Y.; Sahara, H.; Matsumoto, K.; Takahashi, N.; Yamazaki, T.; Ohta, K.; Aoki, S.; Miura, M.; Sugawara, F.; Sakaguchi, K.; et al. Downregulation of Tie2 gene by a novel antitumor sulfolipid, 3’-sulfoquinovosyl-1’-monoacylglycerol, targeting angiogenesis. Cancer Sci. 2008, 99, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Ohta, K.; Murata, H.; Mori, Y.; Ishima, M.; Sugawara, F.; Sakaguchi, K.; Miura, M. Remodeling of the tumor microenvironment by combined treatment with a novel radiosensitizer, {alpha}-sulfoquinovosylmonoacylglycerol ({alpha}-SQMG) and X-irradiation. Anticancer. Res. 2010, 30, 4397–4404. [Google Scholar] [PubMed]
- Withrow, S.J. Miscellaneous tumors: Thymoma. In Small Animal Clinical Oncology, 2nd ed.; Withrow, S.J., MacEwen, E.G., Eds.; WB Saunders: Philadelphia, PA, USA, 1996; pp. 530–533. [Google Scholar]
- Patnaik, A.K.; Lieberman, P.H.; A Erlandson, R.; Antonescu, C. Feline Cystic Thymoma: A Clinicopathologic, Immunohistologic, and Electron Microscopic Study of 14 Cases. J. Feline Med. Surg. 2003, 5, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Aronsohn, M.G.; Schunk, K.L.; Carpenter, J.L.; King, N.W. Clinical and pathologic features of thymoma in 15 dogs. J. Am. Vet. Med Assoc. 1984, 184, 1355–1362. [Google Scholar]
- Gores, B.R.; Berg, J.; Carpenter, J.L.; Aronsohn, M.G. Surgical treatment of thymoma in cats: 12 cases (1987–1992). J. Am. Vet. Med Assoc. 1994, 204, 1782–1785. [Google Scholar]
- Smith, A.N.; Wright, J.C.; Wrjr, B.; LaRue, S.M.; Fineman, L.; Hogge, G.S.; E Kitchell, B.; E Hohenhaus, A.; Burk, R.L.; Dhaliwal, R.S.; et al. Radiation therapy in the treatment of canine and feline thymomas: A retrospective study (1985–1999). J. Am. Anim. Hosp. Assoc. 2001, 37, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Fuller, C.D.; Ramahi, E.H.; Aherne, N.; Eng, T.Y.; Thomas, J.C.R. Radiotherapy for Thymic Neoplasms. J. Thorac. Oncol. 2010, 5, S327–S335. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Murakami, M.; Kawabe, M.; Iwasaki, R.; Heishima, K.; Sakai, H.; Mori, T. Hypofractionated radiation therapy in the treatment of canine thymoma: Retrospective study of eight cases. Vet. Radiol. Ultrasound 2017, 58, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Dolera, M.; Malfassi, L.; Mazza, G.; Urso, G.; Sala, M.; Marcarini, S.; Carrara, N.; Pavesi, S.; Finesso, S.; Kent, M.S. Feasibility for using hypofractionated stereotactic volumetric modulated arc radiotherapy (vmat) with adaptive planning for treatment of thymoma in rabbits: 15 cases. Vet. Radiol. Ultrasound 2016, 57, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Andres, K.M.; Kent, M.; Siedlecki, C.T.; Mayer, J.; Brandão, J.; Hawkins, M.G.; Morrisey, J.K.; Quesenberry, K.; Valli, V.E.; Bennett, R.A. The use of megavoltage radiation therapy in the treatment of thymomas in rabbits: 19 cases. Vet. Comp. Oncol. 2012, 10, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Kaser-Hotz, B.; Rohrer, C.R.; Fidel, J.L.; Nett, C.S.; Horauf, A.; Hauser, B. Radiotherapy in three suspect cases of feline thymoma. J. Am. Anim. Hosp. Assoc. 2001, 37, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Sarıhan, S.; Bayram, A.S.; Gebitekin, C.; Yerci, O.; Siğirli, D.; Sıgırlı, D.; Yercı, Ö. Thymic tumors and results of radiotherapy. Rep. Pract. Oncol. Radiother. 2018, 23, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Marks, L.B.; Bentzen, S.M.; Deasy, J.O.; Kong, F.M.; Bradley, J.D.; Vogelius, I.S.; El Naqa, I.; Hubbs, J.L.; Lebesque, J.V.; Timmerman, R.D.; et al. Radiation dose-volume effects in the lung. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S70–S76. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kutara, K.; Mochizuki, Y.; Ohnishi, A.; Mitsui, I.; Kanda, T.; Sugiyama, A.; Maeta, N.; Kobayashi, K.; Shimizu, Y.; Okamura, Y.; et al. The Outcome and CT Findings of Low-Dose Intensity Modulated Radiation Therapy with SQAP in a Cat with Thymoma. Vet. Sci. 2020, 7, 203. https://doi.org/10.3390/vetsci7040203
Kutara K, Mochizuki Y, Ohnishi A, Mitsui I, Kanda T, Sugiyama A, Maeta N, Kobayashi K, Shimizu Y, Okamura Y, et al. The Outcome and CT Findings of Low-Dose Intensity Modulated Radiation Therapy with SQAP in a Cat with Thymoma. Veterinary Sciences. 2020; 7(4):203. https://doi.org/10.3390/vetsci7040203
Chicago/Turabian StyleKutara, Kenji, Yohei Mochizuki, Akihiro Ohnishi, Ikki Mitsui, Teppei Kanda, Akihiko Sugiyama, Noritaka Maeta, Kosuke Kobayashi, Yuki Shimizu, Yasuhiko Okamura, and et al. 2020. "The Outcome and CT Findings of Low-Dose Intensity Modulated Radiation Therapy with SQAP in a Cat with Thymoma" Veterinary Sciences 7, no. 4: 203. https://doi.org/10.3390/vetsci7040203
APA StyleKutara, K., Mochizuki, Y., Ohnishi, A., Mitsui, I., Kanda, T., Sugiyama, A., Maeta, N., Kobayashi, K., Shimizu, Y., Okamura, Y., & Asanuma, T. (2020). The Outcome and CT Findings of Low-Dose Intensity Modulated Radiation Therapy with SQAP in a Cat with Thymoma. Veterinary Sciences, 7(4), 203. https://doi.org/10.3390/vetsci7040203




