Deciphering the Role of Bovine Viral Diarrhea Virus Non-Structural NS4B Protein in Viral Pathogenesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal and Ethical Statement
2.2. Samples
2.3. Cells and Viruses
2.4. NS4B Expression in Mammalian Cells
2.5. Virus Neutralization Assay (VNA)
2.6. SDS-PAGE and Western Blot
2.7. Indirect ELISA
2.8. Statistical Analysis
3. Results
3.1. BVDV Vaccination Elicits a Potent Post-Immunization Antibody Response
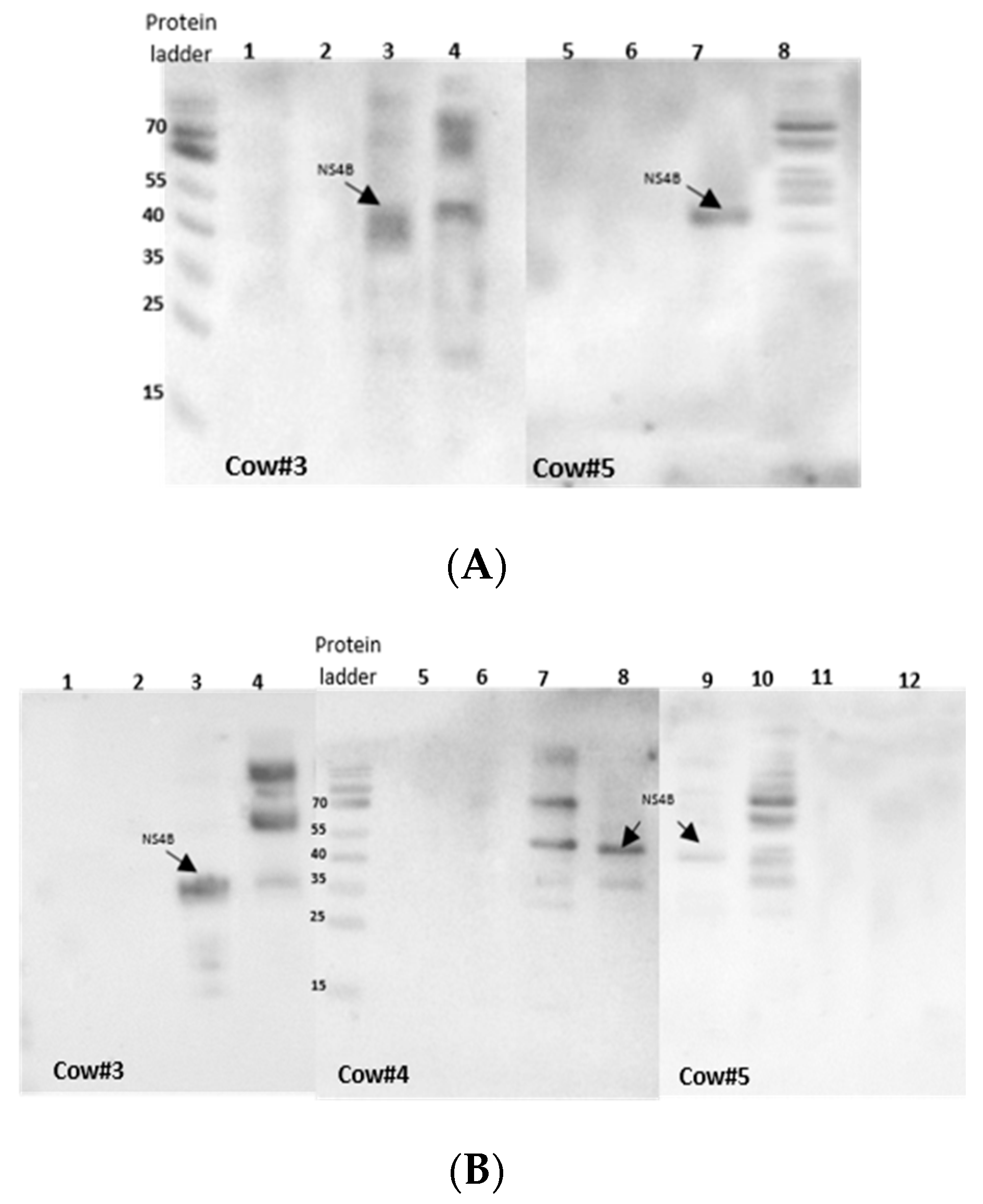
3.2. Antigenic Evaluation of NS4B Using Western Blot and ELISA
4. Discussion
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Houe, H. Economic impact of BVDV infection in dairies. Biologicals 2003, 31, 137–143. [Google Scholar] [CrossRef]
- Lanyon, S.R.; Hill, F.I.; Reichel, M.P.; Brownlie, J. Bovine viral diarrhoea: Pathogenesis and diagnosis. Vet. J. 2014, 199, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Neill, J.D. Molecular biology of bovine viral diarrhea virus. Biologicals 2013, 41, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Weiskircher, E.; Aligo, J.; Ning, G.; Konan, K.V. Bovine viral diarrhea virus NS4B protein is an integral membrane protein associated with Golgi markers and rearranged host membranes. Virol. J. 2009, 6, 185. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; McMullan, L.K.; Rice, C.M. Isolation and Characterization of Noncytopathic Pestivirus Mutants Reveals a Role for Nonstructural Protein NS4B in Viral Cytopathogenicity. J. Virol. 2001, 75, 10651–10662. [Google Scholar] [CrossRef] [PubMed]
- Suda, Y.; Murakami, S.; Horimoto, T. Bovine viral diarrhea virus non-structural protein NS4B induces autophagosomes in bovine kidney cells. Arch. Virol. 2018, 164, 255–260. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, X.; Wu, C.; Xu, H.; Liu, H. Current Drug Discovery for Anti-hepatitis C Virus Targeting NS4B. Curr. Top. Med. Chem. 2016, 16, 1362–1371. [Google Scholar] [CrossRef]
- Xie, X.; Zou, J.; Wang, Q.-Y.; Shi, P.-Y. Targeting dengue virus NS4B protein for drug discovery. Antivir. Res. 2015, 118, 39–45. [Google Scholar] [CrossRef]
- Sillanpää, M.; Melén, K.; Porkka, P.; Fagerlund, R.; Nevalainen, K.; Lappalainen, M.; Julkunen, I. Hepatitis C virus core, NS3, NS4B and NS5A are the major immunogenic proteins in humoral immunity in chronic HCV infection. Virol. J. 2009, 6, 84. [Google Scholar] [CrossRef]
- Lazaro-Olán, L.; Mellado-Sánchez, G.; García-Cordero, J.; Escobar-Gutiérrez, A.; Santos-Argumedo, L.; Gutiérrez-Castañeda, B.; Cedillo-Barrón, L. Analysis of Antibody Response in Human Dengue Patients from the Mexican Coast Using Recombinant Antigens. Vector Borne Zoonotic Dis. 2008, 8, 69–80. [Google Scholar] [CrossRef]
- Amin, N.; Pupo, M.; Aguilar, A.; Vázquez, S.; Caballero, Y.; Ochoa, R.; Guzman, M.G.; Acosta, A. Recognition of a multiple antigen peptide containing sequence from mimotope of the dengue type 3 virus NS4B protein by human antibodies. Asian Pac. J. Trop. Med. 2016, 9, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Savardashtaki, A.; Sharifi, Z.; Hamzehlou, S.; Farajollahi, M.M. Analysis of Immumoreactivity of Heterologously Expressed Non-structural Protein 4B (NS4B) from Hepatitis C Virus (HCV) Genotype 1a. Iran. J. Biotechnol. 2015, 13, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Donis, R.O.; Corapi, W.; Dubovi, E.J. Neutralizing Monoclonal Antibodies to Bovine Viral Diarrhoea Virus Bind to the 56K to 58K Glycoprotein. J. Gen. Virol. 1988, 69, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Bolin, S.R. Immunogens of bovine viral diarrhea virus. Vet. Microbiol. 1993, 37, 263–271. [Google Scholar] [CrossRef]
- Mishra, N.; Rajukumar, K.; Pitale, S.S.; Prakash, A.; Nema, R.K.; Behera, S.P.; Dubey, S.C. Evidence of a humoral immune response against the prokaryotic expressed N-terminal autoprotease (Npro) protein of bovine viral diarrhoea virus. J. Biosci. 2010, 35, 79–86. [Google Scholar] [CrossRef]
- Agapov, E.V.; Murray, C.L.; Frolov, I.; Qu, L.; Myers, T.M.; Rice, C.M. Uncleaved NS2-3 Is Required for Production of Infectious Bovine Viral Diarrhea Virus. J. Virol. 2004, 78, 2414–2425. [Google Scholar] [CrossRef]
- Platt, R.; Kesl, L.; Guidarini, C.; Wang, C.; Roth, J.A. Comparison of humoral and T-cell-mediated immune responses to a single dose of Bovela ® live double deleted BVDV vaccine or to a field BVDV strain. Veter- Immunol. Immunopathol. 2017, 187, 20–27. [Google Scholar] [CrossRef]
- Gauger, P.C.; Vincent, A.L. Serum Virus Neutralization Assay for Detection and Quantitation of Serum-Neutralizing Antibodies to Influenza A Virus in Swine. Recent Results Cancer Res. 2014, 1161, 313–324. [Google Scholar] [CrossRef]
- Zoth, S.C.; Taboga, O. Multiple recombinant ELISA for the detection of bovine viral diarrhoea virus antibodies in cattle sera. J. Virol. Methods 2006, 138, 99–108. [Google Scholar] [CrossRef]
- Newman, F.K.; Frey, S.E.; Blevins, T.P.; Mandava, M.; Bonifacio, J.A.; Yan, L.; Belshe, R.B. Improved Assay to Detect Neutralizing Antibody following Vaccination with Diluted or Undiluted Vaccinia (Dryvax) Vaccine. J. Clin. Microbiol. 2003, 41, 3154–3157. [Google Scholar] [CrossRef]
- Li, G.; Adam, A.; Luo, H.; Shan, C.; Cao, Z.; Fontes-Garfias, C.R.; Sarathy, V.V.; Teleki, C.; Winkelmann, E.R.; Liang, Y.; et al. An attenuated Zika virus NS4B protein mutant is a potent inducer of antiviral immune responses. NPJ Vaccines 2019, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Van Cleef, K.W.R.; Overheul, G.J.; Thomassen, M.C.; Marjakangas, J.M.; Van Rij, R.P. Escape Mutations in NS4B Render Dengue Virus Insensitive to the Antiviral Activity of the Paracetamol Metabolite AM404. Antimicrob. Agents Chemother. 2016, 60, 2554–2557. [Google Scholar] [CrossRef] [PubMed]
- Pouliot, J.J.; Thomson, M.; Xie, M.; Horton, J.; Johnson, J.; Krull, D.; Mathis, A.; Morikawa, Y.; Parks, D.; Peterson, R.; et al. Preclinical Characterization andIn VivoEfficacy of GSK8853, a Small-Molecule Inhibitor of the Hepatitis C Virus NS4B Protein. Antimicrob. Agents Chemother. 2015, 59, 6539–6550. [Google Scholar] [CrossRef]
- Xu, J.; Xie, X.; Ye, N.; Zou, J.; Chen, H.; White, M.A.; Shi, P.-Y.; Zhou, Z. Design, Synthesis, and Biological Evaluation of Substituted 4,6-Dihydrospiro[[1,2,3]triazolo[4,5- b]pyridine-7,3′-indoline]-2′,5(3 H)-dione Analogues as Potent NS4B Inhibitors for the Treatment of Dengue Virus Infection. J. Med. Chem. 2019, 62, 7941–7960. [Google Scholar] [CrossRef] [PubMed]
- Roth, C.; Cantaert, T.; Colas, C.; Prot, M.; Casadémont, I.; Levillayer, L.; Thalmensi, J.; Langlade-Demoyen, P.; Gerke, C.; Bahl, K.; et al. A Modified mRNA Vaccine Targeting Immunodominant NS Epitopes Protects Against Dengue Virus Infection in HLA Class I Transgenic Mice. Front. Immunol. 2019, 10, 1424. [Google Scholar] [CrossRef]
- Kuhs, K.A.L.; Toporovski, R.; Ginsberg, A.A.; Shedlock, D.J.; Weiner, D.B. Induction of Intrahepatic HCV NS4B, NS5A and NS5B-Specific Cellular Immune Responses following Peripheral Immunization. PLoS ONE 2012, 7, e52165. [Google Scholar] [CrossRef]
- Latimer, B.; Toporovski, R.; Yan, J.; Pankhong, P.; Morrow, M.P.; Khan, A.S.; Sardesai, N.Y.; Welles, S.L.; Jacobson, J.M.; Weiner, D.B.; et al. Strong HCV NS3/4a, NS4b, NS5a, NS5b-specific cellular immune responses induced in Rhesus macaques by a novel HCV genotype 1a/1b consensus DNA vaccine. Hum. Vaccines Immunother. 2014, 10, 2357–2365. [Google Scholar] [CrossRef] [PubMed]
- Berasain, C.; García-Granero, M.; Riezu-Boj, J.I.; Civeira, M.P.; Banales, J.M.; Borrás-Cuesta, F. Detection of anti-hepatitis C virus antibodies by ELISA using synthetic peptides. J. Hepatol. 1993, 18, 80–84. [Google Scholar] [CrossRef][Green Version]
- Valdés, K.; Alvarez, M.; Pupo, M.; Vázquez, S.; Rodríguez, R.; Guzmán, M.G. Human Dengue Antibodies against Structural and Nonstructural Proteins. Clin. Diagn. Lab. Immunol. 2000, 7, 856–857. [Google Scholar] [CrossRef]
- Kuno, G.; Vorndam, A.V.; Gubler, D.J.; Gómez, I. Study of anti-dengue NS1 antibody by Western blot. J. Med. Virol. 1990, 32, 102–108. [Google Scholar] [CrossRef]
- Wong, S.J.; Boyle, R.H.; Demarest, V.L.; Woodmansee, A.N.; Kramer, L.D.; Li, H.; Drebot, M.; Koski, R.A.; Fikrig, E.; Martin, D.A.; et al. Immunoassay Targeting Nonstructural Protein 5 To Differentiate West Nile Virus Infection from Dengue and St. Louis Encephalitis Virus Infections and from Flavivirus Vaccination. J. Clin. Microbiol. 2003, 41, 4217–4223. [Google Scholar] [CrossRef] [PubMed]
- Conrycantilena, C. Hepatitis C virus diagnostics: Technology, clinical applications and impacts. Trends Biotechnol. 1997, 15, 71–76. [Google Scholar] [CrossRef]
- Masalova, O.V.; Lakina, E.; Abdulmedzhidova, A.; Atanadze, S.; Semiletov, Y.; Shkurko, T.; Burkov, A.; Ulanova, T.; Pimenov, V.; Novikov, V.; et al. Characterization of monoclonal antibodies and epitope mapping of the NS4 protein of hepatitis C virus. Immunol. Lett. 2002, 83, 187–196. [Google Scholar] [CrossRef]
- Chang, J.; Seidel, C.; Ofenloch, B.; Jue, D.; Fields, H.; Khudyakov, Y. Antigenic Heterogeneity of the Hepatitis C Virus NS4 Protein as Modeled with Synthetic Peptides. Virology 1999, 257, 177–190. [Google Scholar] [CrossRef][Green Version]

| Cow Number | Pre-Immune Neutralization Titre Dilutions * | Post-Immune Neutralization Titre Dilutions * |
|---|---|---|
| 1 | - | 1:10,240 |
| 2 | - | 1:10,240 |
| 3 | 1:10,240 | 1:10,240 |
| 4 | - | 1:2560 |
| 5 | 1:5120 | 1:5120 |
| Animal | Status | VNA | W.B | ELISA |
|---|---|---|---|---|
| Cow 1 | Pre-Immunization | − | − | − |
| Post-immunization | + | − | − | |
| Cow 2 | Pre-Immunization | − | − | − |
| Post-immunization | + | − | − | |
| Cow3 | Pre-Immunization | + | + | + |
| Post-immunization | + | + | + | |
| Cow4 | Pre-Immunization | − | − | − |
| Post-immunization | + | + | − | |
| Cow5 | Pre-Immunization | + | + | + |
| Post-immunization | + | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bashir, S.; Kossarev, A.; Martin, V.C.; Paeshuyse, J. Deciphering the Role of Bovine Viral Diarrhea Virus Non-Structural NS4B Protein in Viral Pathogenesis. Vet. Sci. 2020, 7, 169. https://doi.org/10.3390/vetsci7040169
Bashir S, Kossarev A, Martin VC, Paeshuyse J. Deciphering the Role of Bovine Viral Diarrhea Virus Non-Structural NS4B Protein in Viral Pathogenesis. Veterinary Sciences. 2020; 7(4):169. https://doi.org/10.3390/vetsci7040169
Chicago/Turabian StyleBashir, Shahbaz, Andrey Kossarev, Violeta Cascon Martin, and Jan Paeshuyse. 2020. "Deciphering the Role of Bovine Viral Diarrhea Virus Non-Structural NS4B Protein in Viral Pathogenesis" Veterinary Sciences 7, no. 4: 169. https://doi.org/10.3390/vetsci7040169
APA StyleBashir, S., Kossarev, A., Martin, V. C., & Paeshuyse, J. (2020). Deciphering the Role of Bovine Viral Diarrhea Virus Non-Structural NS4B Protein in Viral Pathogenesis. Veterinary Sciences, 7(4), 169. https://doi.org/10.3390/vetsci7040169





