Prevalence and Diversity of Avian Influenza Virus Hemagglutinin Sero-Subtypes in Poultry and Wild Birds in Bangladesh
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Bird Capture
2.3. Sample Collection, Competitive Enzyme-Linked Immunosorbent Assay and Haemagglutination Inhibition Test
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bouvier, N.M.; Palese, P. The biology of influenza viruses. Vaccine 2008, 26 (Suppl. 4), D49–D53. [Google Scholar] [CrossRef] [PubMed]
- MacLachlan, N.J.; Dubovi, E.J. (Eds.) Chapter 21—Orthomyxoviridae. In Fenner’s Veterinary Virology, 5th ed.; Academic Press: Boston, MA, USA, 2017; pp. 389–410. [Google Scholar] [CrossRef]
- Swayne, D.; Suarez, D. Highly pathogenic avian influenza. Rev. Sci. Tech. Off. Int. Epizoot. 2000, 19, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yuan, H.; Gao, R.; Zhang, J.; Wang, D.; Xiong, Y.; Fan, G.; Yang, F.; Li, X.; Zhou, J. Clinical and epidemiological characteristics of a fatal case of avian influenza A H10N8 virus infection: A descriptive study. Lancet 2014, 383, 714–721. [Google Scholar] [CrossRef]
- Yuen, K.-Y.; Chan, P.; Peiris, M.; Tsang, D.; Que, T.; Shortridge, K.; Cheung, P.; To, W.; Ho, E.; Sung, R. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet 1998, 351, 467–471. [Google Scholar] [CrossRef]
- Dhama, K. Avian/Bird Flu Virus: Poultry Pathogen Having. J. Med. Sci. 2013, 13, 301–315. [Google Scholar] [CrossRef]
- Li, K.; Guan, Y.; Wang, J.; Smith, G.; Xu, K.; Duan, L.; Rahardjo, A.; Puthavathana, P.; Buranathai, C.; Nguyen, T. Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature 2004, 430, 209–213. [Google Scholar] [CrossRef]
- Hamid, M.; Rahman, M.; Ahmed, S.; Hossain, K. Status of poultry industry in bangladesh and the role of private sector for its development. Asian J. Poultry Sci. 2017, 11, 1–13. [Google Scholar] [CrossRef]
- Alam, A.; Chowdhury, M.; Sobhan, I. Biodiversity of Tanguar Haor: A Ramsar Site of Bangladesh Volume I: Wildlife; IUCN Bangladesh: Dhaka, Bangladesh, 2012; 234p. [Google Scholar]
- Ahmed, S.S.; Ersbøll, A.K.; Biswas, P.K.; Christensen, J.P.; Hannan, A.S.; Toft, N. Ecological determinants of highly pathogenic avian influenza (H5N1) outbreaks in Bangladesh. PLoS ONE 2012, 7, e33938. [Google Scholar] [CrossRef]
- Hassan, M.M.; Hoque, M.A.; Debnath, N.C.; Yamage, M.; Klaassen, M. Are poultry or wild birds the main reservoirs for avian influenza in Bangladesh? Ecohealth 2017, 14, 490–500. [Google Scholar] [CrossRef]
- Webster, R.G.; Bean, W.J.; Gorman, O.T.; Chambers, T.M.; Kawaoka, Y. Evolution and ecology of influenza A viruses. Microbiol. Mol. Biol. Rev. 1992, 56, 152–179. [Google Scholar] [CrossRef]
- Vandegrift, K.J.; Sokolow, S.H.; Daszak, P.; Kilpatrick, A.M. Ecology of avian influenza viruses in a changing world. Ann. N. Y. Acad. Sci. 2010, 1195, 113. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.; Chaitaweesub, P.; Parakamawongsa, T.; Premashthira, S.; Tiensin, T.; Kalpravidh, W.; Wagner, H.; Slingenbergh, J. Free-grazing ducks and highly pathogenic avian influenza, Thailand. Emerg. Infect. Dis. 2006, 12, 227. [Google Scholar] [CrossRef]
- Parvin, R.; Kamal, A.H.; Haque, M.E.; Chowdhury, E.H.; Giasuddin, M.; Islam, M.R.; Vahlenkamp, T.W. Genetic characterization of highly pathogenic H5N1 avian influenza virus from live migratory birds in Bangladesh. Virus Genes 2014, 49, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; Hoque, M.A.; Ujvari, B.; Klaassen, M. Live bird markets in Bangladesh as a potentially important source for Avian Influenza Virus transmission. Prev. Vet. Med. 2018, 156, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.T.-Y.; Wang, J.; Shen, Y.; Zhou, B.; Duan, L.; Cheung, C.-L.; Ma, C.; Lycett, S.J.; Leung, C.Y.-H.; Chen, X. The genesis and source of the H7N9 influenza viruses causing human infections in China. Nature 2013, 502, 241–244. [Google Scholar] [CrossRef]
- Ma, C.; Lam, T.T.-Y.; Chai, Y.; Wang, J.; Fan, X.; Hong, W.; Zhang, Y.; Li, L.; Liu, Y.; Smith, D.K. Emergence and evolution of H10 subtype influenza viruses in poultry in China. J. Virol. 2015, 89, 3534–3541. [Google Scholar] [CrossRef]
- Hénaux, V.; Samuel, M.D. Avian influenza shedding patterns in waterfowl: Implications for surveillance, environmental transmission, and disease spread. J. Wildl. Dis. 2011, 47, 566–578. [Google Scholar] [CrossRef]
- Costa, T.P.; Brown, J.D.; Howerth, E.W.; Stallknecht, D.E. Variation in viral shedding patterns between different wild bird species infected experimentally with low-pathogenicity avian influenza viruses that originated from wild birds. Avian Pathol. 2011, 40, 119–124. [Google Scholar] [CrossRef]
- Germeraad, E.A.; Sanders, P.; Hagenaars, T.J.; Jong, M.C.M.d.; Beerens, N.; Gonzales, J.L. Virus Shedding of Avian Influenza in Poultry: A Systematic Review and Meta-Analysis. Viruses 2019, 11, 812. [Google Scholar] [CrossRef]
- Grear, D.A.; Dusek, R.J.; Walsh, D.P.; Hall, J.S. No evidence of infection or exposure to highly pathogenic avian influenzas in peridomestic wildlife on an affected poultry facility. J. Wildl. Dis. 2017, 53, 37–45. [Google Scholar] [CrossRef][Green Version]
- Hoye, B.J.; Fouchier, R.A.; Klaassen, M. Host behaviour and physiology underpin individual variation in avian influenza virus infection in migratory Bewick’s swans. Proc. Biol. Sci. 2012, 279, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Hoque, A. Risk of Spill-Over of Diseases (in Particular Avian Influenza) from Wild Aquatic Birds in North Queensland. Ph.D. Thesis, James Cook University, Townsville, Australia, 2011. [Google Scholar]
- Selleck, P. Influenza A Virus: A Competitive ELISA for the Detection of Antibodies to Influenza A Viruses in Equine Sera; CSIRO Livestock Industries, CSIRO Publishing: Canberra, Australia, 2008. [Google Scholar]
- Sarker, R.D.; Giasuddin, M.; Chowdhury, E.H.; Islam, M.R. Serological and virological surveillance of avian influenza virus in domestic ducks of the north-east region of Bangladesh. BMC Vet. Res. 2017, 13, 180. [Google Scholar] [CrossRef] [PubMed]
- Verhagen, J.H.; Lexmond, P.; Vuong, O.; Schutten, M.; Guldemeester, J.; Osterhaus, A.D.; Elbers, A.R.; Slaterus, R.; Hornman, M.; Koch, G. Discordant detection of avian influenza virus subtypes in time and space between poultry and wild birds; Towards improvement of surveillance programs. PLoS ONE 2017, 12, e173470. [Google Scholar] [CrossRef] [PubMed]
- Krauss, S.; Obert, C.A.; Franks, J.; Walker, D.; Jones, K.; Seiler, P.; Niles, L.; Pryor, S.P.; Obenauer, J.C.; Naeve, C.W. Influenza in migratory birds and evidence of limited intercontinental virus exchange. PLoS Pathog. 2007, 3, e167. [Google Scholar] [CrossRef] [PubMed]
- Pepin, K.M.; Wang, J.; Webb, C.T.; Hoeting, J.A.; Poss, M.; Hudson, P.J.; Hong, W.; Zhu, H.; Guan, Y.; Riley, S. Anticipating the prevalence of avian influenza subtypes H9 and H5 in live-bird markets. PLoS ONE 2013, 8, e56157. [Google Scholar] [CrossRef] [PubMed]
- Tolba, H.M.; Elez, R.M.A.; Elsohaby, I.; Ahmed, H.A. Molecular identification of avian influenza virus subtypes H5N1 and H9N2 in birds from farms and live bird markets and in respiratory patients. PeerJ 2018, 6, e5473. [Google Scholar] [CrossRef]
- Morales, A.C., Jr.; Hilt, D.A.; Williams, S.M.; Pantin-Jackwood, M.J.; Suarez, D.L.; Spackman, E.; Stallknecht, D.E.; Jackwood, M.W. Biologic characterization of H4, H6, and H9 type low pathogenicity avian influenza viruses from wild birds in chickens and turkeys. Avian Dis. 2009, 53, 552–562. [Google Scholar] [CrossRef]
- Martin, V.; Sims, L.; Lubroth, J.; Pfeiffer, D.; Slingenbergh, J.; Domenech, J. Epidemiology and ecology of highly pathogenic avian influenza with particular emphasis on South East Asia. Dev. Biol. 2006, 124, 23–36. [Google Scholar]
- Parvin, R.; Begum, J.A.; Nooruzzaman, M.; Chowdhury, E.H.; Islam, M.R.; Vahlenkamp, T.W. Review analysis and impact of co-circulating H5N1 and H9N2 avian influenza viruses in Bangladesh. Epidemiol. Infect. 2018, 146, 1259–1266. [Google Scholar] [CrossRef]
- Peiris, J.M. Avian influenza viruses in humans. Rev. Sci. Tech. (Int. Off. Epizoot.) 2009, 28, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Barman, S.; Marinova-Petkova, A.; Hasan, M.K.; Akhtar, S.; El-Shesheny, R.; Turner, J.C.; Franks, J.; Walker, D.; Seiler, J.; Friedman, K. Role of domestic ducks in the emergence of a new genotype of highly pathogenic H5N1 avian influenza A viruses in Bangladesh. Emerg. Microbes Infect. 2017, 6, 1–13. [Google Scholar] [CrossRef]
- Halvorson, D.; Karunakaran, D.; Senne, D.; Kelleher, C.; Bailey, C.; Abraham, A.; Hinshaw, V.; Newman, J. Epizootiology of avian influenza: Simultaneous monitoring of sentinel ducks and turkeys in Minnesota. Avian Dis. 1983, 22, 77–85. [Google Scholar] [CrossRef]
- Jackwood, M.W.; Stallknecht, D.E. Molecular epidemiologic studies on North American H9 avian influenza virus isolates from waterfowl and shorebirds. Avian Dis. 2007, 51, 448–450. [Google Scholar] [CrossRef] [PubMed]
- Osborne, K.; Weinberg, J.; Miller, E. The European Sero-Epidemiology Network. Euro Surveill. Bull. Eur. Mal. Transm. Eur. Commun. Dis. Bull. 1997, 2, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Bi, Y.; Wong, G.; Gray, G.C.; Gao, G.F.; Li, S. Epidemiology, evolution, and recent outbreaks of avian influenza virus in China. J. Virol. 2015, 89, 8671–8676. [Google Scholar] [CrossRef] [PubMed]
| HA Subtype | H1 | H2 | H3 | H4 | H5 | H6 | H7 | H8 | H9 | H10 | H11 | H12 | H13 | H14 | H15 | H16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Migratory bird (n = 41, N = 170) | ●●●●●●●●●●●●●●●●● | ●●●●●●●●●●●●●● | ●● | ●●●●●●●●●●●●● | ●●●●●●●●●●●●●●●●●●●●●●●●●●● | ●●●●●●●●●● | ●●●●●●●●●●●●●●●●●●●●●●● | ●●●●●●●●●●●●●●●●● | ●●●● | ● | ||||||
| Resident wild bird (n = 36, N = 170) | ● | ●●●●●●●●● | ●●●●●●●●●●●●●●●●●●●●●●● | ●●●●●●●●●●● | ●● | |||||||||||
| Nomadic duck (n = 38, N = 170) | ● | ●●●●●●●●●●●●●●●●●●●●● | ●● | ●●●●●●●●●●●●●●●●●●●●●●●●●●●●● | ●●●●●●●●●●●●●● | ●● | ●●●●●● | |||||||||
| Household duck (n = 47, N = 170) | ● | ●●●●●●●●● | ●●●● | ●●●●●●●●●●●●●●●●●●●●●●●●●●●●● | ● | ●●●●●●●●●●●●●●●●●● | ●●●● | ● | ||||||||
| Household chicken (n = 7, N = 170) | ●●●●● | ●● | ● |
| AIV Subtype | 2012 (January–March) | 2013 (January–March) | 2014 (January–March) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MB | RW | ND | HD | HC | MB | RW | ND | HD | HC | MB | RW | ND | HD | HC | |
| (N = 10) | (N = 28) | (N = 0) | (N = 6) | (N = 0) | (N = 16) | (N = 6) | (N = 23) | (N = 5) | (N = 3) | (N = 15) | (N = 2) | (N = 15) | (N = 37) | (N = 4) | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| H1 | 8 (80) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 7 (43.8) | 1 (16.7) | 0 (0) | 0 (0) | 0 (0) | 2 (13.3) | 0 (0) | 1 (6.7) | 1 (2.7) | 0 (0) |
| H2 | 3 (30) | 8 (28.6) | 0 (0) | 2 (33.3) | 0 (0) | 6 (37.5) | 1 (16.7) | 17 (73.9) | 2 (40) | 0 (0) | 5 (33.3) | 0 (0) | 4 (26.7) | 5 (13.5) | 0 (0) |
| H3 | 2 (20) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| H4 | 7 (70) | 0 (0) | 0 (0) | 4 (66.6) | 0 (0) | 3 (18.8) | 0 (0) | 2 (8.7) | 0 (0) | 0 (0) | 3 (20) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| H5 | 2 (20) | 18 (64.3) | 0 (0) | 3 (50) | 0 (0) | 10 | 4 (66.7) | 16 (69.6) | 2 (40) | 3 (100) | 15 (100) | 2 (100) | 13 (86.7) | 24 (64.9) | 2 (50) |
| H6 | 7 (70) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (18.8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| H7 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| H8 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (20) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| H9 | 6 (60) | 6 (21.4) | 0 (0) | 2 (33.3) | 0 (0) | 7 (43.8) | 5 (83.3) | 9 (39.1) | 1 (20) | 2 (66.7) | 10 (66.7) | 0 (0) | 5 (33.3) | 15 (40.5) | 0 (0) |
| H10 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (8.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| H11 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| H12 | 5 (50) | 2 (7.1) | 0 (0) | 2 (33.3) | 0 (0) | 6 (37.5) | 0 (0) | 5 (21.7) | 0 (0) | 1 (33.3) | 6 (40) | 0 (0) | 1 (6.7) | 2 (5.4) | 0 (0) |
| H13 | 3 (30) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (6.3) | 0 (0) | 0 (0) | 1 (20) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| H14 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| H15 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| H16 | 1 (10) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
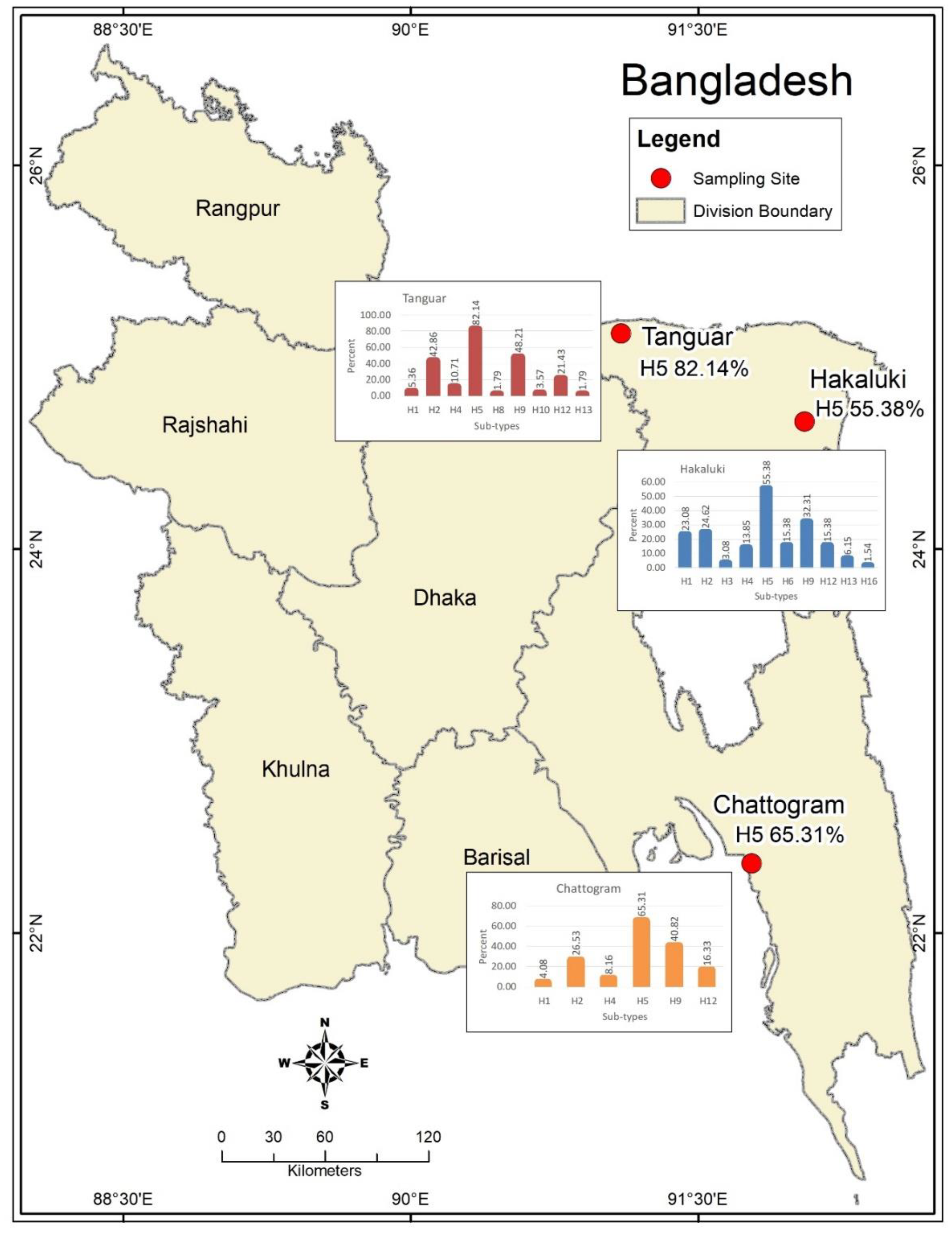
| AIV Subtype | Hakaluki Hoar | Tanguar Hoar | Chittagong | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MB | RW | ND | HD | HC | MB | RW | ND | HD | HC | MB | RW | ND | HD | HC | |
| (N = 23) | (N = 1) | (N = 15) | (N = 22) | (N = 4) | (N = 17) | (N = 3) | (N = 23) | (N = 13) | (N = 0) | (N = 1) | (N = 32) | (N = 0) | (N = 13) | (N = 3) | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| H1 | 14 (60.9) | 0 (0) | 1 (6.7) | 0 (0) | 0 (0) | 3 (17.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (3.1) | 0 (0) | 1 (7.7) | 0 (0) |
| H2 | 9 (39.1) | 0 (0) | 4 (26.7) | 3 (13.7) | 0 (0) | 5 (29.4) | 0 (0) | 17 (73.9) | 2 (15.4) | 0 (0) | 0 (0) | 9 (28.1) | 0 (0) | 4 (30.8) | 0 (0) |
| H3 | 2 (8.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| H4 | 9 (39.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (23.5) | 0 (0) | 2 (8.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (30.8) | 0 (0) |
| H5 | 9 (39.1) | 1 (100) | 13 (86.7) | 11 (50) | 2 (50) | 17 (100) | 3 (100) | 16 (69.6) | 10 (76.9) | 0 (0) | 1 (100) | 20 (62.5) | 0 (0) | 8 (61.5) | 3 (100) |
| H6 | 10 (43.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| H7 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| H8 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (7.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| H9 | 12 (52.2) | 0 (0) | 5 (33.3) | 4 (18.2) | 0 (0) | 10 (58.8) | 1 (33.3) | 9 (39.1) | 7 (53.8) | 0 (0) | 1 (100) | 10 (31.3) | 0 (0) | 7 (53.8) | 2 (66.7) |
| H10 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (8.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| H11 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| H12 | 9 (39.1) | 0 (0) | 1 (6.7) | 0 (0) | 0 (0) | 7 (41.2) | 0 (0) | 5 (21.7) | 0 (0) | 0 (0) | 1 (100) | 2 (6.3) | 0 (0) | 4 (30.8) | 1 (33.3) |
| H13 | 4 (17.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (7.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| H14 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| H15 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| H16 | 1 (4.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, M.M.; El Zowalaty, M.E.; Islam, A.; Khan, S.A.; Rahman, M.K.; Järhult, J.D.; Hoque, M.A. Prevalence and Diversity of Avian Influenza Virus Hemagglutinin Sero-Subtypes in Poultry and Wild Birds in Bangladesh. Vet. Sci. 2020, 7, 73. https://doi.org/10.3390/vetsci7020073
Hassan MM, El Zowalaty ME, Islam A, Khan SA, Rahman MK, Järhult JD, Hoque MA. Prevalence and Diversity of Avian Influenza Virus Hemagglutinin Sero-Subtypes in Poultry and Wild Birds in Bangladesh. Veterinary Sciences. 2020; 7(2):73. https://doi.org/10.3390/vetsci7020073
Chicago/Turabian StyleHassan, Mohammad M., Mohamed E. El Zowalaty, Ariful Islam, Shahneaz A. Khan, Md. K. Rahman, Josef D. Järhult, and Md. A. Hoque. 2020. "Prevalence and Diversity of Avian Influenza Virus Hemagglutinin Sero-Subtypes in Poultry and Wild Birds in Bangladesh" Veterinary Sciences 7, no. 2: 73. https://doi.org/10.3390/vetsci7020073
APA StyleHassan, M. M., El Zowalaty, M. E., Islam, A., Khan, S. A., Rahman, M. K., Järhult, J. D., & Hoque, M. A. (2020). Prevalence and Diversity of Avian Influenza Virus Hemagglutinin Sero-Subtypes in Poultry and Wild Birds in Bangladesh. Veterinary Sciences, 7(2), 73. https://doi.org/10.3390/vetsci7020073








