The Outcome and Economic Viability of Embryo Production Using IVF and SOV Techniques in the Wagyu Breed of Cattle
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
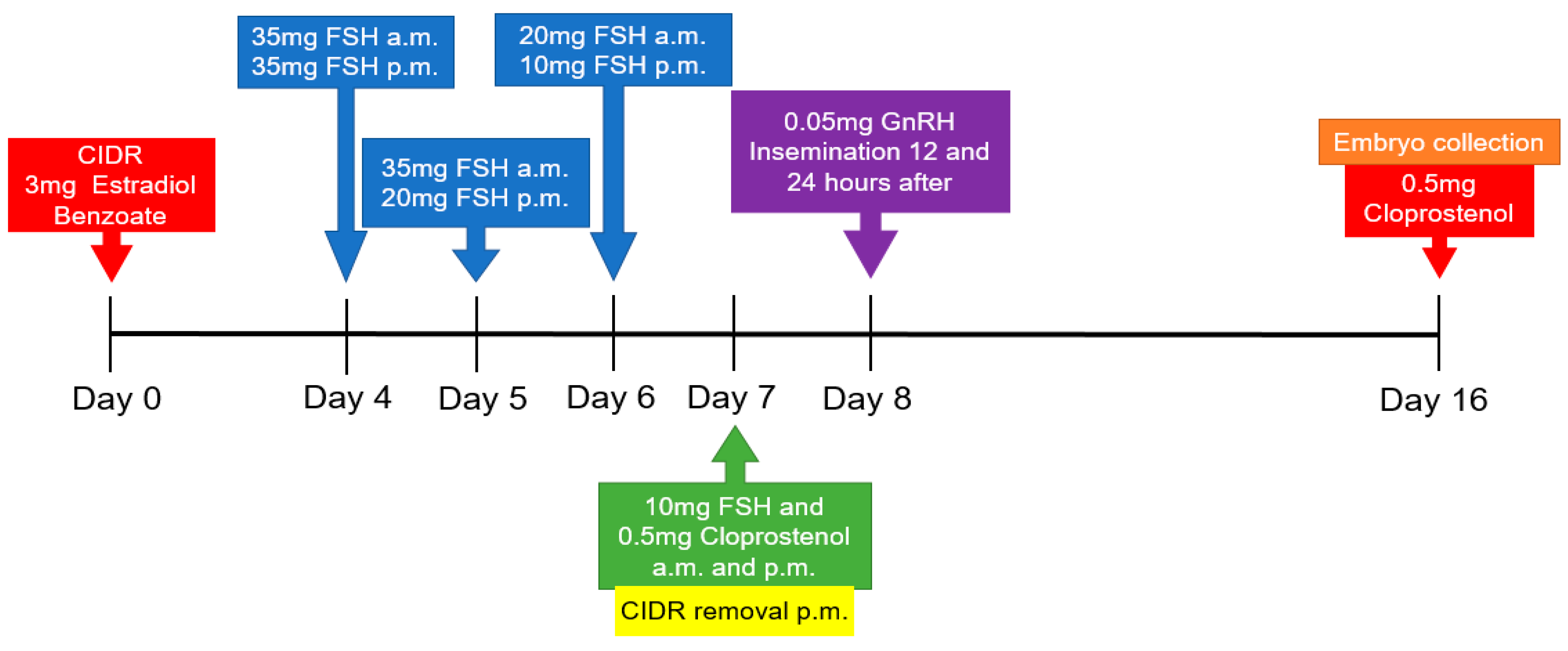
2.2. Superovulation Protocol and Follicular Aspiration
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- AWA. WHAT is Wagyu? American Wagyu Association—AWA. 2019. Available online: https://wagyu.org/breed-info/what-is-wagyu (accessed on 10 July 2019).
- Gotoh, T.; Nishimura, T.; Kuchida, K.; Mannen, H. The Japanese Wagyu beef industry: Current situation and future prospects—A review. Asian Australas. J. Anim. Sci. 2018, 31, 933–950. [Google Scholar] [CrossRef] [PubMed]
- Skarżyński, D.J.; Siemieniuch, M.J.; Majewska, M. Wagyu—Japanese black beef cattle. Med. Weter 2009, 65, 532–535. [Google Scholar]
- Hirooka, H. Marbled Japanese Black cattle. J. Anim. Breed. Genet. 2014, 131, 1–2. [Google Scholar] [CrossRef]
- Gotoh, T.; Joo, S.T. Characteristics and Health Benefit of Highly Marbled Wagyu and Hanwoo Beef. Korean J. Food Sci. Anim. Resour. 2016, 36, 709–718. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gotoh, T.; Albrecht, E.; Teuscher, F.; Kawabata, K.; Sakashita, K.; Iwamoto, H.; Wegner, J. Differences in muscle and fat accretion in Japanese Black and European cattle. Meat Sci. 2009, 82, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.S.; Paulino, P.V.R.; Das, A.K.; Wei, S.; Serao, N.V.L.; Fu, X.; Harris, S.M.; Dodson, M.V.; Du, M. Enhancement of adipogenesis and fibrogenesis in skeletal muscle of Wagyu compared with Angus cattle. J. Anim. Sci. 2013, 91, 2938–2946. [Google Scholar] [CrossRef]
- Wei, S.; Fu, X.; Liang, X.; Zhu, M.J.; Jiang, Z.; Parish, S.M.; Dodson, M.V.; Zan, L.; Du, M. Enhanced mitogenesis in stromal vascular cells derived from subcutaneous adipose tissue of Wagyu compared with those of Angus cattle. J. Anim. Sci. 2015, 93, 1015–1024. [Google Scholar] [CrossRef]
- Adams, T.H.; Walzem, R.L.; Smith, D.R.; Tseng, S.; Smith, S.B. Hamburger high in total, saturated and trans-fatty acids decreases HDL cholesterol and LDL particle diameter, and increases plasma TAG in mildly hypercholesterolaemic men. Br. J. Nutr. 2010, 103, 91–98. [Google Scholar] [CrossRef]
- Troy, D.J.; Tiwari, B.K.; Joo, S.T. Health implications of beef intramuscular fat consumption. Korean J. Food Sci. 2016, 36, 650–655. [Google Scholar] [CrossRef]
- Gilmore, L.A.; Walzem, R.L.; Crouse, S.F.; Smith, D.R.; Adams, T.H.; Vaidyanathan, V.; Cao, X.; Smith, S.B. Consumption of high-oleic acid ground beef increases HDL cholesterol concentration but both high- and low-oleic acid ground beef decrease HDL particle diameter in normocholesterolemic men. J. Nutr. 2011, 141, 1188–1194. [Google Scholar] [CrossRef]
- Lahey, R.; Wang, X.; Carley, A.; Lewandowski, E.D. Dietary fat supply to failing hearts determineds dynamic lipid signaling for nuclear receptor activation and oxidation of stored triglyceride. Circulation 2014, 130, 1790–1799. [Google Scholar] [CrossRef] [PubMed]
- Scraggs, E.; Zanella, R.; Wojtowicz, A.; Taylor, J.F.; Gaskins, C.T.; Reeves, J.J.; de Avila, J.M.; Neibergs, H.L. Estimation of inbreeding and effective population size of full-blood wagyu cattle registered with the American Wagyu Cattle Association. J. Anim. Breed. Genet. 2013, 131, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Sasaki, S.; Watanabe, T.; Nishimura, S.; Ideta, A.; Yamazaki, M.; Matsuda, K.; Yuzaki, M.; Sakimura, K.; Aoyagi, Y.; et al. Ionotropic glutamate receptor AMPA 1 is associated with ovulation rate. PLoS ONE 2010, 5, e13817. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, H.; Naito, A.; Fujii, T.; Sugimoto, M.; Takedomi, T.; Moriyasu, S.; Sakai, H.; Kageyama, S. Effects of Genetic Background on Responses to Superovulation in Japanese Black Cattle. J. Vet. Med. Sci. 2019, 81, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Zanella, R.; Lago, L.V.; Silva, A.N.; Pertille, F.; Silva, N.C.; Panetto, J.C.C.; Zanella, G.C.; Facioli, F.L.; Silva, M.V.G.B. Genetic Characterization of Indubrasil Cattle Breed Population. Vet. Sci. 2018, 5, 98. [Google Scholar] [CrossRef]
- Bó, G.A.; Mapletoft, R.J. Historical perspectives and recent research on superovulation in cattle. Theriogenology 2014, 81, 38–48. [Google Scholar] [CrossRef]
- Baruselli, P.S.; Souza, A.H.; Martins, C.M. Sexed Semen: Artificial Insemination and Embryo Transfer. Rev. Bras. Reprod. Anim. 2007, 31, 374–381. [Google Scholar]
- Kahi, A.K.; Hirooka, H. Genetic and economic evaluation of Japanese Black (Wagyu) cattle breeding schemes. J. Anim. Sci. 2005, 83, 2021–2032. [Google Scholar] [CrossRef]
- An, L.; Ling, P.; Zhu, X.; Liu, Y.; Zhang, F.; Ma, X.; Xu, B.; Wang, Y.; Du, Z.; Yang, L.; et al. Successful Vitrification of In Vivo Embryos Collected from Superovulated Japanese Black Cattle (Wagyu). Reprod. Domest. Anim. 2016, 51, 255–261. [Google Scholar] [CrossRef]
- MUB Beef Perform. Available online: https://www.mubbrasil.com.br/produtos-3/mub-beef/mub-beef-perform (accessed on 10 November 2019).
- Wright, J.M. Manual of the International Embryo Transfer Society, 4th ed.; International Embryo Transfer Society: Champaign, IL, USA, 2010. [Google Scholar]
- Mapletoft, R.J.; García Guerra, A.; Dias, F.C.F.; Singh, J.; Adams, G.P. In Vitro and In Vivo embryo production in cattle superstimulated with FSH for 7 days. Anim. Reprod. 2015, 12, 383–388. [Google Scholar]
- Du, F.; Ma, X.; Xu, B.; Wang, Y.; Li, Y.; An, L.Y.; Yang, L.; Presicce, G.A. Japanese Black (Wagyu) cattle multiple-ovulation embryo transfer: Follicle-stimulating hormone source in a case study in south China. Reprod. Fertil. Dev. 2015, 27, 163–164. [Google Scholar] [CrossRef]

| Product | Total Dose | Cost (R$) | Cost ($) |
|---|---|---|---|
| CIDR | 1.90 mg | 20.00 | 4.00 |
| FSH | 165 mg | 218.00 | 43.60 |
| Cloprostenol | 1.50 mg | 12.00 | 2.40 |
| Estradiol Benzoate | 3.00 mg | 1.00 | 0.20 |
| GnRh | 0.05 mg | 19.00 | 3.80 |
| Semen | 2 | 200.00 | 40.00 |
| AI Service | 2 | 120.00 | 24.00 |
| Material SOV Collection | 60.00 | 12.00 | |
| Labor | 980.00 | 196.00 | |
| TOTAL | R$1630.00 | $326.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Facioli, F.L.; De Marchi, F.; Marques, M.G.; Michelon, P.R.P.; Zanella, E.L.; Caires, K.C.; Reeves, J.J.; Zanella, R. The Outcome and Economic Viability of Embryo Production Using IVF and SOV Techniques in the Wagyu Breed of Cattle. Vet. Sci. 2020, 7, 58. https://doi.org/10.3390/vetsci7020058
Facioli FL, De Marchi F, Marques MG, Michelon PRP, Zanella EL, Caires KC, Reeves JJ, Zanella R. The Outcome and Economic Viability of Embryo Production Using IVF and SOV Techniques in the Wagyu Breed of Cattle. Veterinary Sciences. 2020; 7(2):58. https://doi.org/10.3390/vetsci7020058
Chicago/Turabian StyleFacioli, Fernanda L., Flávia De Marchi, Mariana G. Marques, Paulo R. P. Michelon, Eraldo L. Zanella, Kyle C. Caires, Jerry J. Reeves, and Ricardo Zanella. 2020. "The Outcome and Economic Viability of Embryo Production Using IVF and SOV Techniques in the Wagyu Breed of Cattle" Veterinary Sciences 7, no. 2: 58. https://doi.org/10.3390/vetsci7020058
APA StyleFacioli, F. L., De Marchi, F., Marques, M. G., Michelon, P. R. P., Zanella, E. L., Caires, K. C., Reeves, J. J., & Zanella, R. (2020). The Outcome and Economic Viability of Embryo Production Using IVF and SOV Techniques in the Wagyu Breed of Cattle. Veterinary Sciences, 7(2), 58. https://doi.org/10.3390/vetsci7020058





