Effects of Joint Lavage with Dimethylsulfoxide on LPS-Induced Synovitis in Horses—Clinical and Laboratorial Aspects
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sample Size Calculation
2.3. Randomization
2.4. Induction of Synovitis and Joint Lavage
2.5. Lameness Evaluation
2.6. Synovial Fluid Analysis
2.7. Statistical Analysis
3. Results
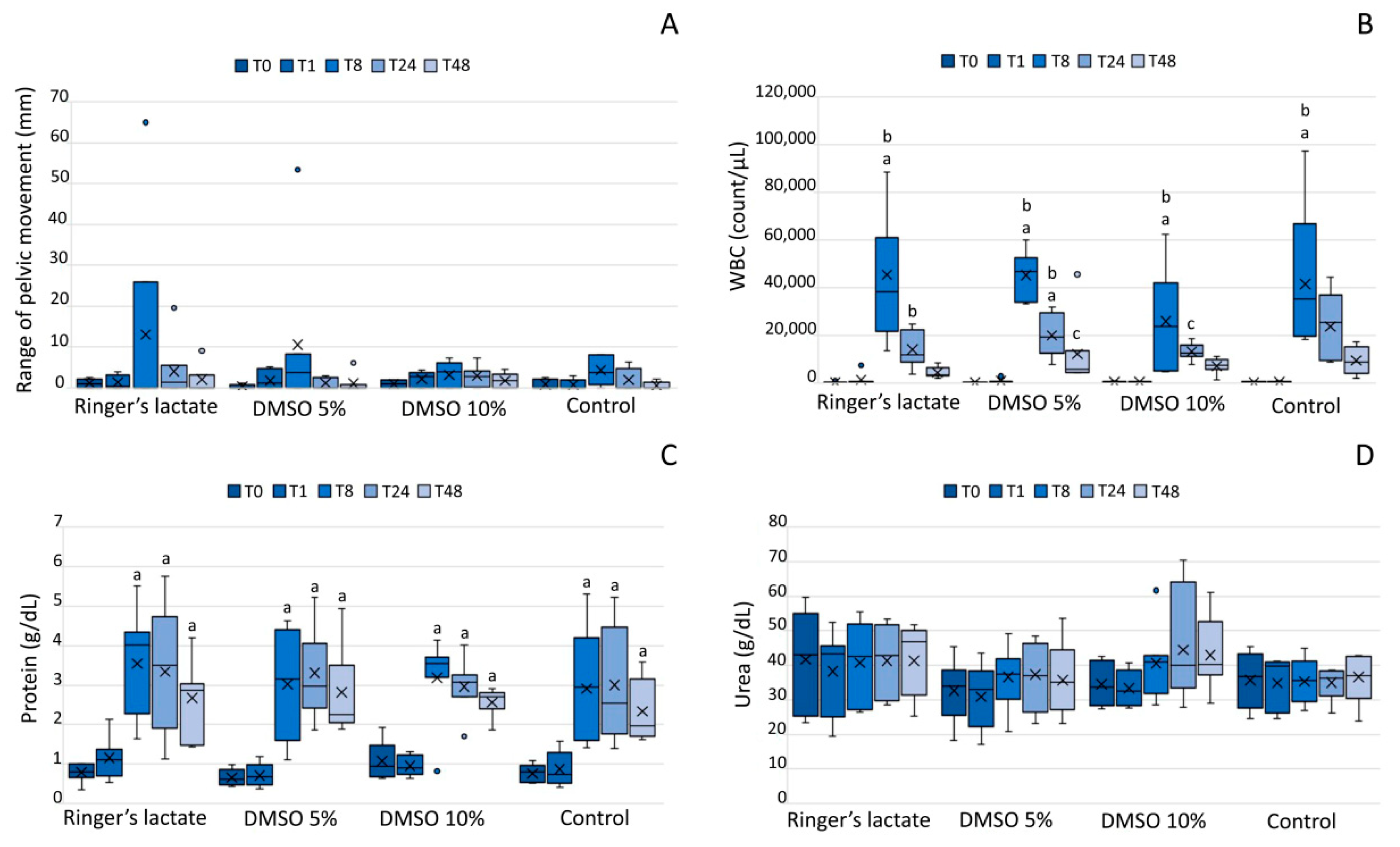
3.1. Lameness Evaluation
3.2. White Blood Cell Count of the Synovial Fluid
3.3. Total Protein and UreaConcentrations in the Synovial Fluid
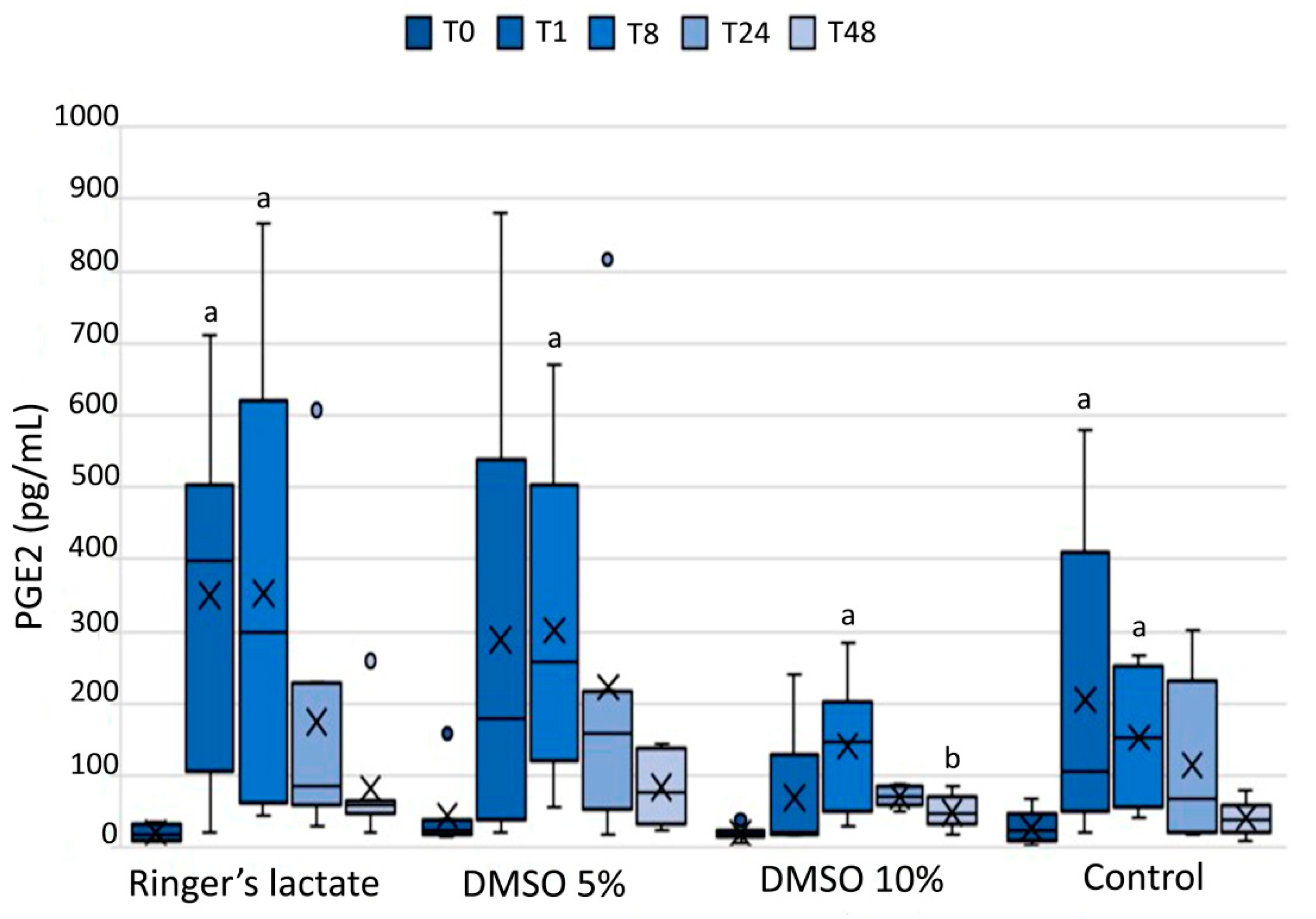
3.4. Prostaglandin E2 in the Synovial Fluid
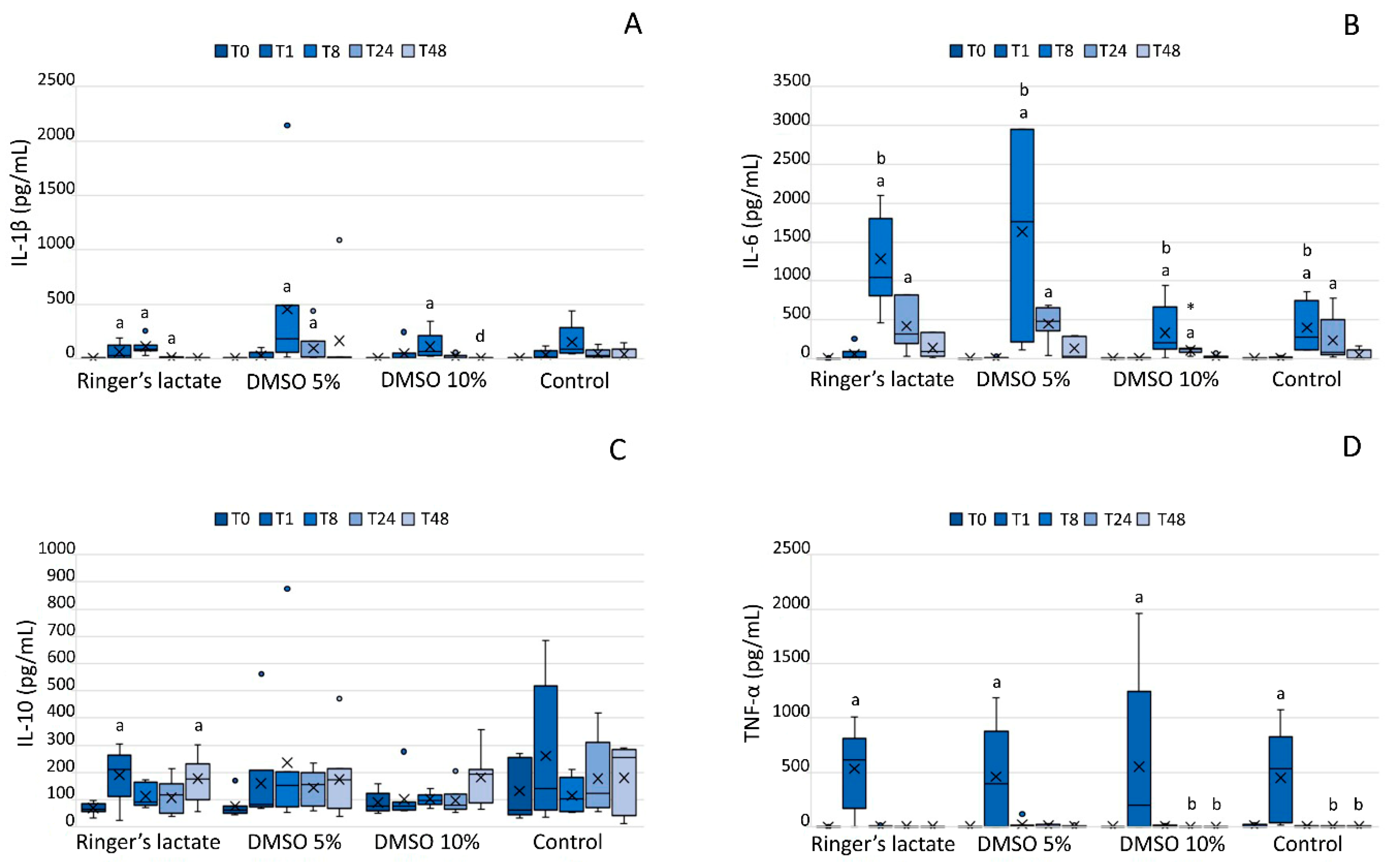
3.5. InterleukinLevels in the Synovial Fluid
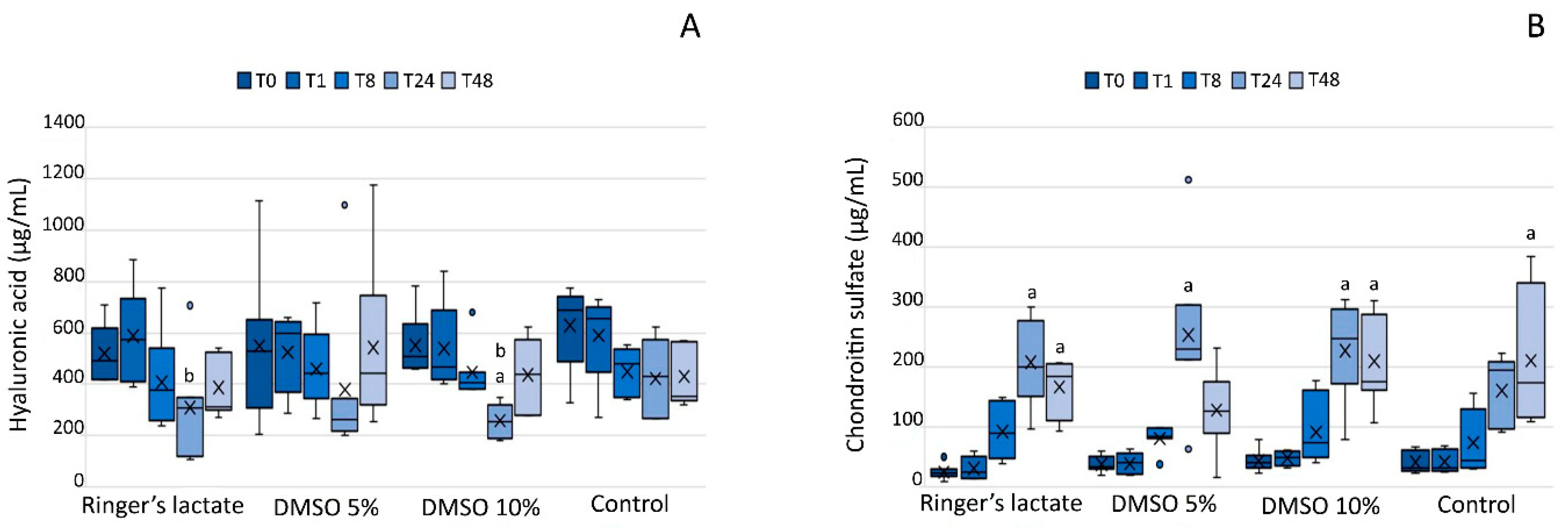
3.6. Glycosaminoglycans in the Synovial Fluid
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ashwood-Smith, M.J. Radioprotective and cryoprotective properties of dimethyl sulfoxide in cellular systems. Ann. N.Y. Acad. Sci. 1967, 141, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Brayton, C.F. Dimethyl sulfoxide (DMSO): A review. Cornell Vet. 1986, 76, 61–90. [Google Scholar] [PubMed]
- John, H.; Laudahn, G. Clinical experiences with the topical application of dmso in orthopedic diseases: Evaluation of 4180 cases. Ann. N.Y. Acad. Sci. 1967, 141, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.C.; Figueira-Coelho, J.; Martins-Silva, J.; Saldanha, C. Multidisciplinary utilization of dimethyl sulfoxide: Pharmacological, cellular, and molecular aspects. Biochem. Pharmacol. 2003, 65, 1035–1041. [Google Scholar] [CrossRef]
- Elmali, N.; Baysal, O.; Harma, A.; Esenkaya, I.; Mizrak, B. Effects of resveratrol in inflammatory arthritis. Inflammation 2006, 30, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Picoli, T.; Barbosa, J.S.; D’vila Vargas, G.; de Oliveira Hübner, S.; Fischer, G. Toxicidade e eficiência do dimetilsulfóxido (dmso) no congelamento de células madin-darby bovine kidney (mdbk). Sci. Anim. Health 2015, 3, 159–168. [Google Scholar] [CrossRef]
- Kay, A.G.; Rooney, P.; Kearney, J.; Pegg, D.E. Evaluation of dmso transport in human articular cartilage: Vehicle solutions and effects on cell function. Cryo Lett. 2015, 36, 187–194. [Google Scholar]
- Elisia, I.; Nakamura, H.; Lam, V.; Hofs, E.; Cederberg, R.; Cait, J.; Hughes, M.R.; Lee, L.; Jia, W.; Adomat, H.H.; et al. DMSO represses inflammatory cytokine production from human blood cells and reduces autoimmune arthritis. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- McIlwraith, C.W.; Frisbie, D.D.; Kawcak, C.E. The horse as a model of naturally occurring osteoarthritis. Bone Jt. Res. 2013, 1, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, A.T.; Palitti, F.; Hill, M.A.; Stevens, D.L.; Ahnström, G. Influence of DMSO on Carbon K ultrasoft X-rays induced chromosome aberrations in V79 Chinese hamster cells. Mutat. Res. Fundam. Mol. Mech. Mutagenes. 2010, 691, 23–26. [Google Scholar] [CrossRef]
- Repine, J.E.; Eaton, J.W.; Anders, M.W.; Hoidal, J.R.; Fox, R.B. Generation of Hydroxyl Radical by Enzymes, Chemicals, and Human Phagocytes In Vitro. J. Clin. Investig. 2008, 64, 1642–1651. [Google Scholar] [CrossRef] [PubMed]
- Colucci, M.; Maione, F.; Bonito, M.C.; Piscopo, A.; Di Giannuario, A.; Pieretti, S. New insights of dimethyl sulphoxide effects (DMSO) on experimental in vivo models of nociception and inflammation. Pharmacol Res. 2008, 57, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Welch, R.D.; DeBowes, R.M.; Liepold, H.W. Evaluation of the effects of intra-articular injection of dimethylsulfoxide on normal equine articular tissues. Am. J. Vet. Res. 1989, 50, 1180–1182. [Google Scholar] [PubMed]
- Welch, R.D.; Watkins, J.P.; DeBowes, R.M.; Leipold, H.W. Effects of intra-articular administration of dimethylsulfoxide on chemically induced synovitis in immature horses. Am. J. Vet. Res. 1991, 52, 934–939. [Google Scholar]
- Adair, H.S.; Goble, D.O.; Vanhooser, S.; Blackford, J.T.; Rohrbach, B.W. Evaluation of use of dimethyl sulfoxide for intra-articular lavage in clinically normal horses. Am. J. Vet. Res. 1991, 52, 333–336. [Google Scholar]
- Smith, G.; Bertone, A.L.; Kaeding, C.; Simmons, E.J.; Apostoles, S. Anti-inflammatory effects of topically applied dimethyl sulfoxide gel on endotoxin-induced synovitis in horses. Am. J. Vet. Res. 1998, 59, 1149–1152. [Google Scholar] [PubMed]
- Auer, D.E.; Ng, J.C.; Reilly, J.S.; Seawright, A.A. Anti-inflammatory drugs inhibit degradation of equine synovial fluid induced by free radicals. Aust. Vet. J. 1991, 68, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.L.; Macdonald, M.H.; Tesch, A.M.; Willits, N.H. In vitro evaluation of the effect of dimethyl sulfoxide on equine articular cartilage matrix metabolism. Vet. Surg. 2000, 29, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Moses, V.S.; Hardy, J.; Bertone, A.L.; Weisbrode, S.E. Effects of anti-inflammatory drugs on lipopolysaccharide-challenged and -unchallenged equine synovial explants. Am. J. Vet. Res. 2001, 62, 54–60. [Google Scholar] [CrossRef]
- Schleining, J.A.; Reinertson, E.L. Evidence for dimethyl sulphoxide (DMSO) use in horses. Part 1: DMSO as a topical and intra-articular anti-inflammatory agent. Equine Vet. Educ. 2007, 19, 545–546. [Google Scholar] [CrossRef]
- Schleining, J.A.; Reinertson, E.L. Evidence for dimethyl sulphoxide (DMSO) use in horses. Part 2: DMSO as a parenteral anti-inflammatory agent and as a pharmacological carrier. Equine Vet. Educ. 2007, 19, 598–599. [Google Scholar] [CrossRef]
- Silva, M.M.; Hagen, S.C.F.; Vendruscolo, C.D.P.; Baccarin, R.Y.A.; Spagnolo, J.D.; Yamada, A.L.M.; Stievani, F.; Correia da Silva, L.C.L. The correlation between score-based protocol for equine joint assessment and subsequent arthroscopic intervention outcomes. Braz. J. Vet. Res. Anim. Sci. 2019. [Google Scholar] [CrossRef]
- Baccarin, R.Y.A.; Rasera, L.; Machado, T.S.L.; Michelacci, Y.M. Relevance of synovial fluid chondroitin sulphate as a biomarker to monitor polo pony joints. Can. J. Vet. Res. 2014, 78, 50–60. [Google Scholar] [PubMed]
- Machado, T.S.L.; Correia da Silva, L.C.L.; Baccarin, R.Y.A.; Michelacci, Y.M. Synovial fluid chondroitin sulphate indicates abnormal joint metabolism in asymptomatic osteochondritic horses. Equine Vet. J. 2012, 44, 404–411. [Google Scholar] [CrossRef]
- Palmer, J.L.; Bertone, A.L. Experimentally-induced synovitis as a model for acute synovitis in the horse. Equine Vet. J. 1994, 26, 492–495. [Google Scholar] [CrossRef]
- Neuenschwander, H.M.; Moreira, J.J.; Vendruscolo, C.P.; Fülber, J.; Seidel, S.R.T.; Michelacci, Y.M.; Baccarin, R.Y.A. Hyaluronic acid has chondroprotective and joint-preserving effects on LPS-induced synovitis in horses. J. Vet. Sci. 2019. [Google Scholar] [CrossRef]
- Cokelaere, S.M.; Plomp, S.G.M.; de Boef, E.; de Leeuw, M.; Bool, S.; van de Lest, C.H.A.; van Weeren, P.R.; Korthagen, N.M. Sustained intra-articular release of celecoxib in an equine repeated LPS synovitis model. Eur. J. Pharm. Biopharm. 2018, 128, 327–336. [Google Scholar] [CrossRef]
- Mccracken, M.J.; Kramer, J.; Keegan, K.G.; Lopes, M.; Wilson, D.A.; Reed, S.K.; La Carrubba, A.; Rasch, M. Comparison of an inertial sensor system of lameness quantification with subjective lameness evaluation. Equine Vet. J. 2012, 44, 652–656. [Google Scholar] [CrossRef]
- MacWilliams, P.S.; Friedrichs, K.R. Laboratory evaluation and interpretation of synovial fluid. Vet. Clin. North Am. Small Anim.Pract. 2003, 33, 153–178. [Google Scholar] [CrossRef]
- Hawkins, D.L.; MacKay, R.J.; Gum, G.G.; Colahan, P.T.; Meyer, J.C. Effects of intra-articularly administered endotoxin on clinical signs of disease and synovial fluid tumor necrosis factor, interleukin 6, and prostaglandin E2 values in horses. Am. J. Vet. Res. 1993, 54, 379–386. [Google Scholar]
- Owens, J.G.; Kamerling, S.G.; Stanton, S.R.; Keowen, M.L.; Prescott-Mathews, J.S. Effects of pretreatment with ketoprofen and phenylbutazone on experimentally induced synovitis in horses. Am. J. Vet. Res. 1996, 57, 866–874. [Google Scholar] [PubMed]
- Xing, L.; Remick, D.G. Mechanisms of Dimethyl Sulfoxide Augmentation of IL-1β Production. J. Immunol. 2005, 174, 6195–6202. [Google Scholar] [CrossRef] [PubMed]
- De Abreu Costa, L.; Ottoni, M.H.F.; Dos Santos, M.G.; Meireles, A.B.; De Almeida, V.G.; De Fátima Pereira, W.; Alves de Avelar-Freitas, B.; Eustaquio Alvim Brito-Melo, G. Dimethyl sulfoxide (DMSO) decreases cell proliferation and TNF-α, IFN-, and IL-2 cytokines production in cultures of peripheral blood lymphocytes. Molecules 2017, 22. [Google Scholar] [CrossRef]
- Bertuglia, A.; Pagliara, E.; Grego, E.; Ricci, A.; Brkljaca-Bottegaro, N. Pro-inflammatory cytokines and structural biomarkers are effective to categorize osteoarthritis phenotype and progression in Standardbred racehorses over five years of racing career. BMC Vet. Res. 2016, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.B.A.; Katsikis, P.D.; Chu, C.Q.; Thomssen, H.; Webb, L.M.C.; Maini, R.N.; Londei, M.; Feldmann, M. High level of interleukin-10 production by the activated t cell population within the rheumatoid synovial membrane. Arthritis Rheum. 1995. [Google Scholar] [CrossRef] [PubMed]
| Card | Right Tibiotarsal Joint | Left Tibiotarsal Joint | Card | Right Tibiotarsal Joint | Left Tibiotarsal Joint |
|---|---|---|---|---|---|
| 1 | A | B | 8 | A | C |
| 2 | C | D | 9 | C | A |
| 3 | B | A | 10 | D | B |
| 4 | D | C | 11 | C | B |
| 5 | B | C | 12 | A | C |
| 6 | A | D | 13 | B | A |
| 7 | B | D |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sotelo, E.D.P.; Vendruscolo, C.P.; Fülber, J.; Seidel, S.R.T.; Jaramillo, F.M.; Agreste, F.R.; Silva, L.C.L.C.d.; Baccarin, R.Y.A. Effects of Joint Lavage with Dimethylsulfoxide on LPS-Induced Synovitis in Horses—Clinical and Laboratorial Aspects. Vet. Sci. 2020, 7, 57. https://doi.org/10.3390/vetsci7020057
Sotelo EDP, Vendruscolo CP, Fülber J, Seidel SRT, Jaramillo FM, Agreste FR, Silva LCLCd, Baccarin RYA. Effects of Joint Lavage with Dimethylsulfoxide on LPS-Induced Synovitis in Horses—Clinical and Laboratorial Aspects. Veterinary Sciences. 2020; 7(2):57. https://doi.org/10.3390/vetsci7020057
Chicago/Turabian StyleSotelo, Eric Danilo Pauls, Cynthia Prado Vendruscolo, Joice Fülber, Sarah Raphaela Torquato Seidel, Fernando Mosquera Jaramillo, Fernanda Rodrigues Agreste, Luís Cláudio Lopes Correia da Silva, and Raquel Yvonne Arantes Baccarin. 2020. "Effects of Joint Lavage with Dimethylsulfoxide on LPS-Induced Synovitis in Horses—Clinical and Laboratorial Aspects" Veterinary Sciences 7, no. 2: 57. https://doi.org/10.3390/vetsci7020057
APA StyleSotelo, E. D. P., Vendruscolo, C. P., Fülber, J., Seidel, S. R. T., Jaramillo, F. M., Agreste, F. R., Silva, L. C. L. C. d., & Baccarin, R. Y. A. (2020). Effects of Joint Lavage with Dimethylsulfoxide on LPS-Induced Synovitis in Horses—Clinical and Laboratorial Aspects. Veterinary Sciences, 7(2), 57. https://doi.org/10.3390/vetsci7020057





