Differences between the Filling Velocities of the Left and Right Heart Ventricle in Racing Pigeons (Columba livia F. Domestica) and the Influence of Anesthesia with Isoflurane
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
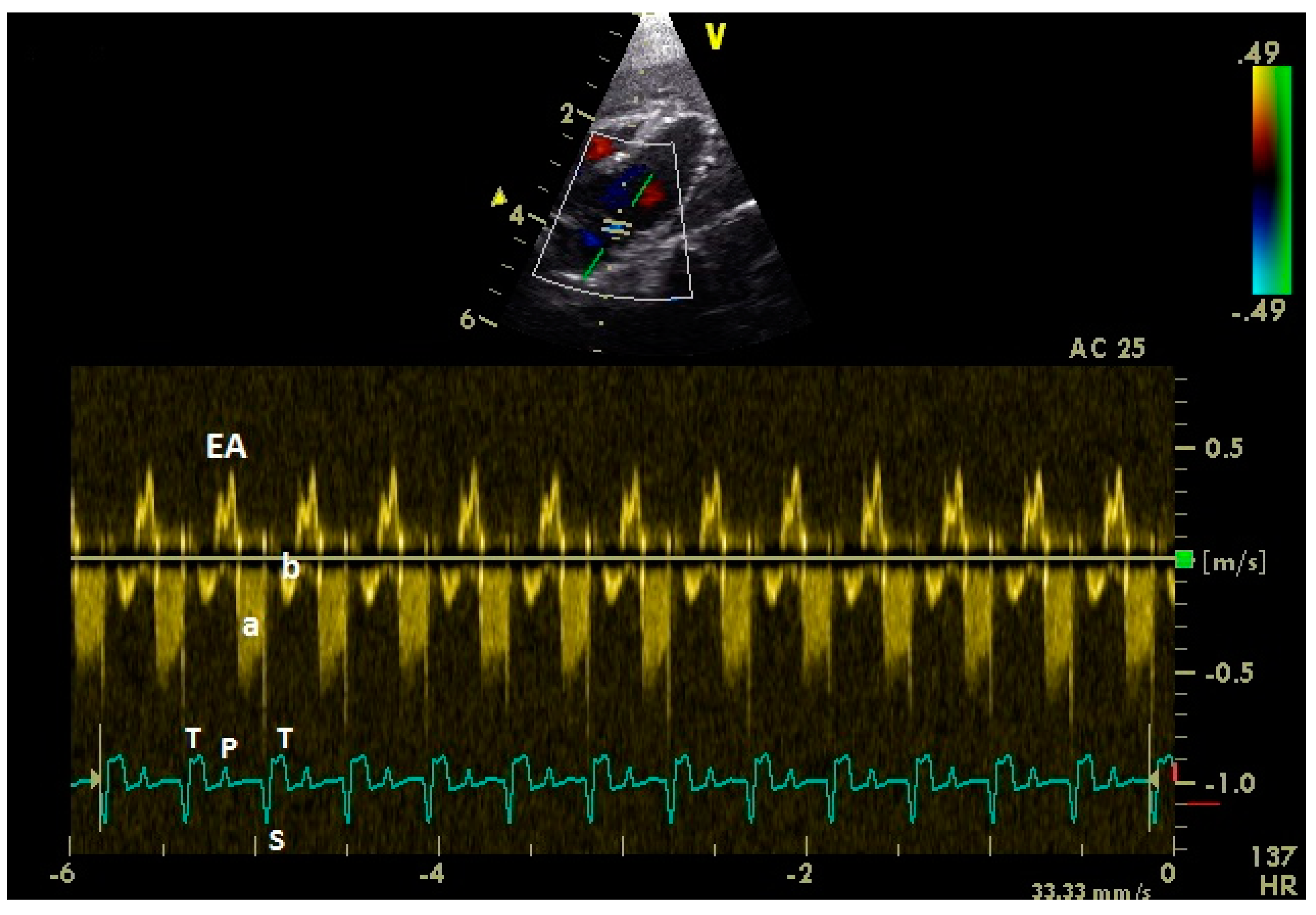
2.2. Doppler-Sonographic Examination
2.3. Anesthesia
2.4. Statistical Analysis
3. Results
3.1. Diastolic Ventricular Filling Blood Flow Velocities in Conscious Racing Pigeons
3.2. Influence of Isoflurane Anesthesia on Diastolic Blood Flow Velocities
3.3. Influence of Heart Rate (HR), Body Indices, Age, and Sex on the Diastolic Blood Flow Velocities
3.4. Examination of the Diastolic Blood Flow with Color Doppler Flow Imaging
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Waibl, H.; Sinowatz, F. Kreislaufapparat und Lymphatisches System. In Lehrbuch der Anatomie der Haustiere, Band V, Anatomie der Vögel, 2nd ed.; Schummer, A., Vollmerhaus, B., Eds.; Verlag Paul Parey: Berlin/Hamburg, Germany, 1992; pp. 282–330. [Google Scholar]
- Hummel, G. Anatomie und Physiologie der Vögel; Verlag Eugen Ulmer GmbH & Co.: Stuttgart, Germany, 2000; pp. 83–103. [Google Scholar]
- Prosheva, V.I.; Kaseva, N.N.; Dernovoj, B. Morpho-functional characterization of the heart of Gallus gallus domesticus with special reference to the right muscular atrioventricular valve. J. Anat. 2019, 235, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Davies, F. The conducting system of birds heart. J. Anat. 1930, 64, 9. [Google Scholar]
- Szabo, E.; Viragh, S.; Challice, C.E. The structure of the atrioventricular conducting system in the avian heart. Anat. Rec. 1986, 215, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Prosheva, V.I.; Kaseva, N.N. Location and functional characterization of the right atrioventricular pacemaker ring in the adult avian heart. J. Morphol. 2016, 277, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Prosheva, V.; Dernovoj, B.; Kharin, S.; Kaseva, N.; Shklyar, T.; Blyakhman, F. Does the right muscular atrioventricular valve in the avian heart perform two functions? Comp. Biochem. Physiol. 2015, 184, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Pees, M.; Krautwald-Junghanns, M.-E. Cardiovascular physiology and diseases of pet birds. Vet. Clin. N. Am. Exot. Anim. Pract. 2009, 12, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Pees, M.; Krautwald-Junghanns, M.-E.; Straub, J. Evaluation and treating the cardio-vasculare system. In Clinical Avian Medicine; Harrison, G.J., Lightfoot, T., Eds.; Spix Publishing: Palm Beach, FL, USA, 2006; pp. 379–394. [Google Scholar]
- Carrani, F.; Gelli, D.; Salvadori, M.; Aloisi, M. A preliminary echocardiographic initial approach to diastolic and systolic function in medium and large parrots. In Proceedings of the 7th European Association of Avian Veterinarians, Puerto de la Cruz, Teneriffa, Spain, 22–26 April 2003; pp. 145–149. [Google Scholar]
- Boskovic, M.; Krautwald-Junghanns, M.-E.; Failing, K.; Schneider, M. Möglichkeiten und Grenzen echokardiographischer Untersuchungen bei Tag- und Nachtgreifvögeln (Accipitriformes, Falconiformes, Strigiformes). Tierarztl. Prax. 1999, 27, 334–341. [Google Scholar]
- Pees, M. Echokardiographische Untersuchungen bei Psittaciformes unter Besonderer Berücksichtigung des Kongo-Graupapageis (Psittacus erithacus erithacus). Ph.D. Thesis, University of Leipzig, Leipzig, Germany, 2001. [Google Scholar]
- Pees, M.; Straub, J.; Krautwald-Junghanns, M.-E. Echocardiographic examinations of 60 African grey parrots and 30 other psittacine birds. Vet. Rec. 2004, 155, 73–76. [Google Scholar] [CrossRef]
- Straub, J.; Pees, M.; Schumacher, J.; Gompf, R.E.; Basade, H.; Kautwald-Junghanns, M.-E. Dopplerechocardiography in birds. In Proceedings of the 6th Main Conference of the European Association of Avian Veterinarians, Munich, Germany, 8–10 March 2001; pp. 92–94. [Google Scholar]
- Korbel, R.; Milovanovic, A.; Erhardt, W. Aerosacculare perfusion with isoflurane—An anaesthetic procedure for head surgery in birds. In Proceedings of the 1993 European Conference of the Association of Avian Veterinarians, Utrecht, The Netherlands, 3–6 March 1993; pp. 9–42. [Google Scholar]
- Korbel, R. Vergleichende Untersuchungen zur Inhalationsanäthesie mit Isofluran (Forene) und Sevofluran (SEVOrane) bei Haustauben (Columba livia Gmel., 1789, var. domestica) und Vorstellung eines Referenz-Narkoseprotokolls für Vögel. Tierärztl. Prax. 1998, 26, 211–223. [Google Scholar]
- Touzot-Jourde, G.; Hernandez-Divers, S.J.; Trim, C.M. Cardiopulmonary effects of controlled versus spontaneous ventilation in pigeons anesthetized for coelioscopy. J. Am. Vet. Med. Assoc. 2005, 227, 1424–1428. [Google Scholar] [CrossRef]
- Botman, J.; Dugdale, A.; Gabriel, F.; Vandeweerd, J.M. Cardiorespiratory parameters in the awake pigeon and during anaesthesia with isoflurane. Vet. Anaesth. Analg. 2016, 43, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Fox, P.R.; Bond, B.R.; Peterson, M.E. Echocardiographic reference values in healthy cats sedated with ketamine hydrochlorid. Am. J. Vet. Res. 1985, 46, 1479–1484. [Google Scholar] [PubMed]
- Yang, X.; Liu, Y.; Rhaleb, N.; Kurihara, N.; Kim, H.E.; Carretero, O.A. Echocardiographic assessment of cardiac function in conscious and anesthetized mice. Am. J. Physiol. Heart. Circ. Physiol. 1999, 277, H1967–H1974. [Google Scholar] [CrossRef] [PubMed]
- Chaves, A.A.; Weinstein, D.M.; Bauer, J.A. Non-invasive echocardiographic studies in mice influence of anesthetic regimen. Life Sci. 2001, 69, 213–222. [Google Scholar] [CrossRef]
- Kiatchoosakun, S.; Kikpatrick, D.; Hoit, B.D. Effects of tribromethanol anesthesia on echocardiographic assessment of left ventricular function in mice. Comp. Med. 2001, 51, 26–29. [Google Scholar]
- Roth, D.M.; Swaney, J.S.; Dalton, N.D.; Gilpin, E.A.; Ross, J., Jr. Impact of anesthesia on cardiac function during echocardiography in mice. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H2134–H2140. [Google Scholar] [CrossRef] [PubMed]
- Plante, E.; Lachanche, D.; Roussel, E.; Drolet, M.-C.; Arsenault, M.; Couet, J. Impact of Anesthesia on echocardiographic evaluation of systolic and diastolic function in rats. J. Am. Soc. Echocardiogr. 2006, 19, 1520–1525. [Google Scholar] [CrossRef]
- Stein, A.B.; Tiwari, S.; Thomas, P.; Hunt, G.; Levent, C.; Stoddard, M.F.; Tang, X.-L.; Bolli, R.; Wawn, B. Effects of anesthesia on ecocardographic essessment of left ventricular structure and function in rats. Basic Res. Cardiol. 2007, 102, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Stypmann, J.; Engelen, M.A.; Breithardt, A.-K.; Milberg, P.; Rothenburger, M.; Breithardt, O.A.; Breithardt, G.; Eckardt, L.; Poulsen Nautrup, C. Doppler echocardiography and tissue Doppler imaging in the healthy rabbit: Differences of cardiac function during awake and anaesthetized examination. Int. J. Cardiol. 2007, 115, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Straub, J.; Forbes, N.A.; Thielebein, J.; Pees, M.; Krautwald-Junghanns, M.-E. The effects of isoflurane anaesthesia on some Doppler-derived cardiac parameters in the Common Buzzard (Buteo buteo). Vet. J. 2003, 166, 273–276. [Google Scholar] [CrossRef]
- Fudge, A.M. Laboratory Medicine Avian and Exotic Pets; W.B. Saunders Company: Philadelphia, PA, USA, 2000; p. 377. [Google Scholar]
- Lumeij, J.T.; Ritchie, B.W. Cardiology. In Avian Medicine, Principles and Application; Ritchie, B.W., Harrison, G.J., Harrison, L.R., Eds.; HBD Int’l Inc.: BS. Brentwood, TN, USA, 1994; pp. 694–722. [Google Scholar]
- Lumeij, J.T.; Stokhof, A.A. Electrocardiogram of the Racing Pigeon (Columba livia forma domestica). Res. Vet. Sci. 1985, 38, 275–278. [Google Scholar] [CrossRef]
- Murcia, L.M.M.; Bernal, L.J.; Montes, A.M.; Ayala, I. The normal electrocardiogram of the unanaesthetized competition ‘Spanish Pouler’ pigeon (Columba livia gutturosa). J. Vet. Med. A Physiol. Pathol. Clin. Med. 2005, 52, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Riedel, U. Die Ultrachalluntersuchung des Vogels am Beispiel der Brieftaube (Columba livia Forma Domestica) mit Hilfe der Schnittbildechokardiographie. Ph.D. Thesis, Justus-Liebig-Universität Gießen, Gießen, Germany, 1995. [Google Scholar]
- Schulz, M. Morphologische und funktionelle Messungen am Herzen von Brieftauben (Columba livia Forma Domestica) mit Hilfe der Schnittbildechokardiographie. Ph.D. Thesis, Justus-Liebig-Universität Gießen, Gießen, Germany, 1995. [Google Scholar]
- Boon, J.A. Manual of Veterinary Echocardiography, 1st ed.; Blackwell Publishing: Oxford, UK, 2006. [Google Scholar]
- Kirberger, R.M.; Bland-van den Berg, P.; Darazs, B. Doppler echocardiography in the normal dog: Part I Velocity findings and flow patterns. Vet. Radiol. Ultrasound 1992, 33, 370–379. [Google Scholar] [CrossRef]
- Kirberger, R.M.; Bland-van den Berg, P.; Grimbeek, R.J. Doppler echocardiography in the normal dog: Part II Factors influencing blood flow velocities and a comparison between left and right heart blood flow. Vet. Radiol. Ultrasound 1992, 33, 380–386. [Google Scholar] [CrossRef]
- Oxorn, D.; Edelist, G.; Harrington, E.; Tsang, S. Echocardiographic assessment of left ventricular filling during isoflurane anaesthesia. Can. J. Anaesth. 1996, 43, 569–574. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sousa, M.G.; Carareto, R.; De-Nardi, A.B.; Brito, F.L.C.; Nunes, N.; Camacho, A. Effects of isoflurane on echocardiographic parameters in healthy dogs. Vet. Anaesth. Analg. 2008, 35, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Bonagura, J.D.; Miller, M.W.; Darke, P.G.G.D. Doppler echocardiography I pulsed-wave and continuous-wave examinations. Vet. Clin. N. Am. Small Anim. Pract. 1998, 28, 1325–1359. [Google Scholar] [CrossRef]
- Vastenberg, M.H.A.C.; Boroffka, S.A.E.B.; Schoemaker, N.J. Echocardiographic measurements in clinically healthy ferrets anesthetized with isoflurane. Vet. Radiol. Ultrasound 2004, 45, 228–232. [Google Scholar] [CrossRef]
- Fitzgerald, B.C.; Beaufrère, H. Cardiology. In Current Therapy in Avian Medicine and Surgery, 1st ed.; Speer, B.L., Ed.; ELSVIER: St. Louis, MO, USA, 2016; pp. 252–328. [Google Scholar]
- Masoudifard, M.; Bidgoli, V.R.; Madani, S.A.; Vajhi, A.; Davoodipoor, S.; Vali, Y. Normal echocardiographic findings in healthy pigeons. Iran. J. Vet. Med. 2016, 11, 7–13. [Google Scholar]
- Kawasaki, T.; Hoka, S.; Okamoto, H.; Okuyama, T.; Takahashi, S. The difference of isoflurane and Halothane in ventriculoarterial coupling in dogs. Anesth. Analg. 1994, 79, 681–686. [Google Scholar] [CrossRef]
- Klide, A.M. Cardiopulmonary effects of enflurane and isoflurane in the dog. Am. J. Vet. Res. 1976, 37, 127–131. [Google Scholar]
- Deryck, Y.L.J.; Brimioulle, S.; Maggiorini, M.; de Canniere, D.; Naeije, R. Systemic vascular effects of isoflurane versus propofol anesthesia in dogs. Anesth. Analg. 1996, 83, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Eger, E.I., II. The pharmacology of isoflurane. Br. J. Anaesth. 1984, 56, 71–99. [Google Scholar]
- Steffey, E.P.; Howland, D., Jr. Isoflurane potency in dog and cat. Am. J. Vet. Res. 1978, 38, 1833–1836. [Google Scholar]
- Pagel, P.S.; Kampine, J.P.; Schmeling, W.T.; Warltier, D.C. Influence of volatile anesthetics on myocardial contractility in viva: Desflurane versus isoflurane. Anesthesiology 1991, 74, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Rüther, P. Untersuchungen zur Tageszeitabhängigen Rhythmik der Ruheherzfrequenz und Ausgewählter Arrhythmien bei der Brieftaube (Columba livia f. Domestica). Ph.D. Thesis, Free University of Berlin, Berlin, Germany, 1998. [Google Scholar]
- Prosheva, V.I.; Shmakov, D.N. Activation of the right atrioventricular valve in the avian heart. J. Evol. Biochem. Physiol. 1987, 23, 269–272. [Google Scholar]
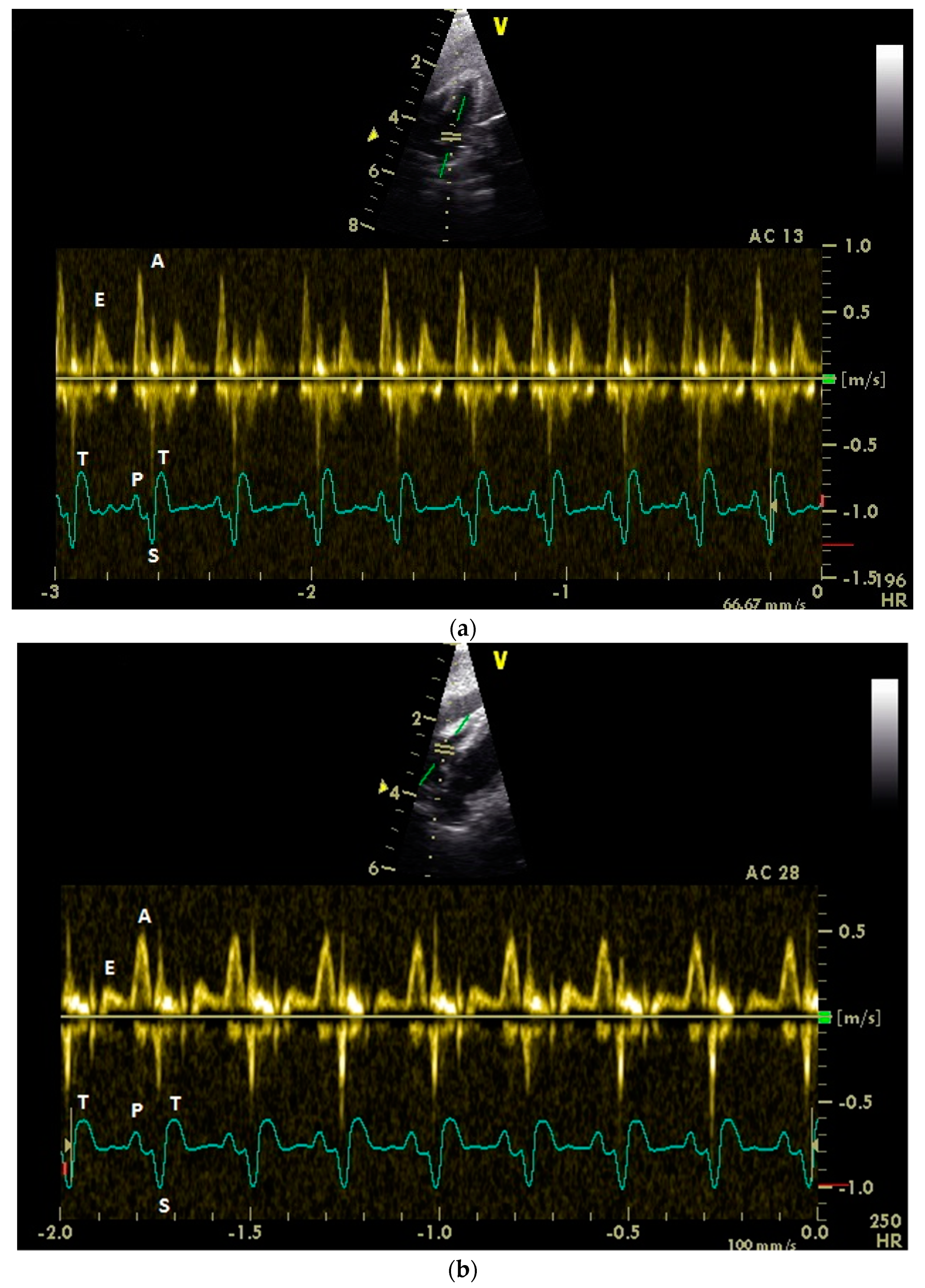
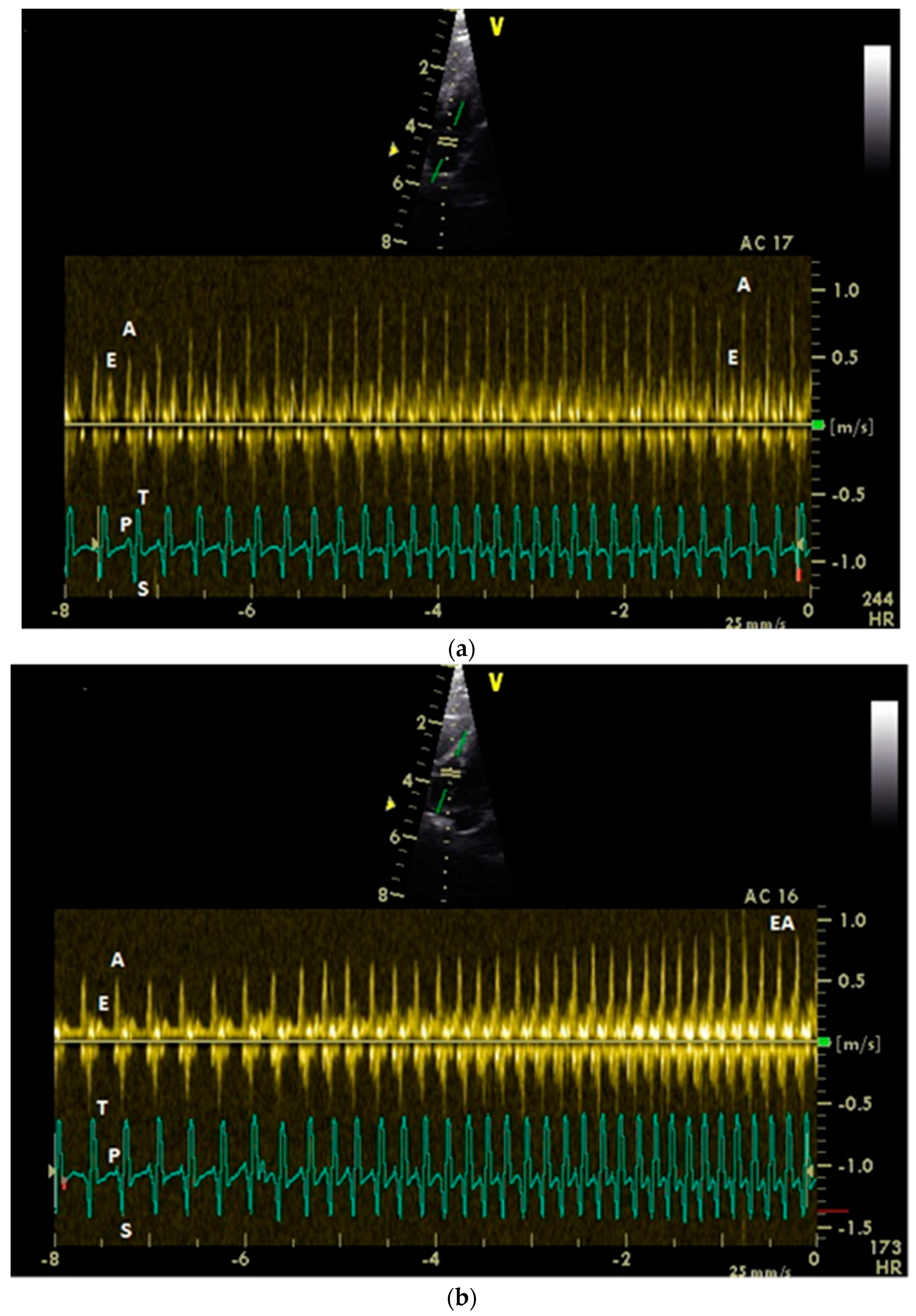
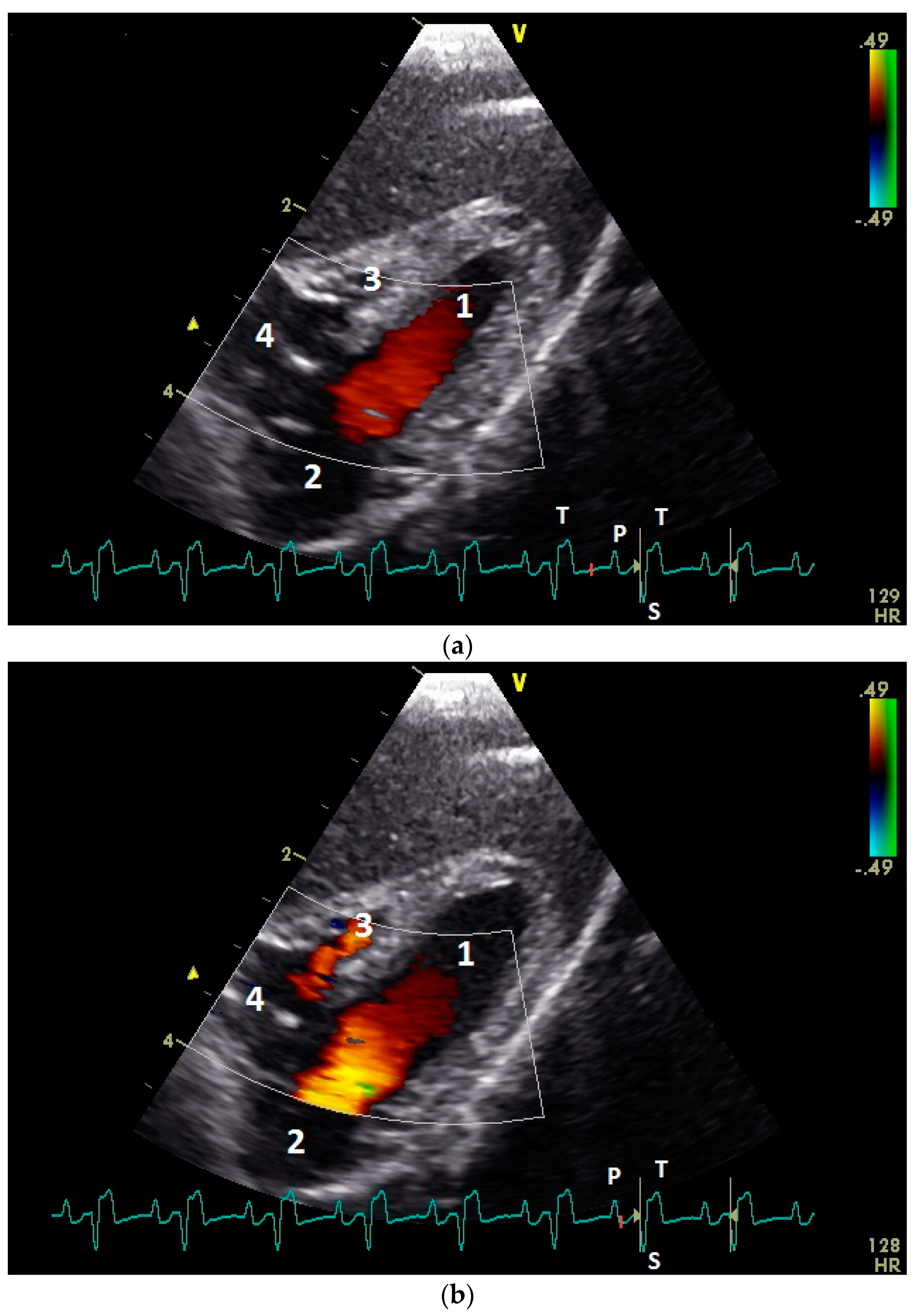
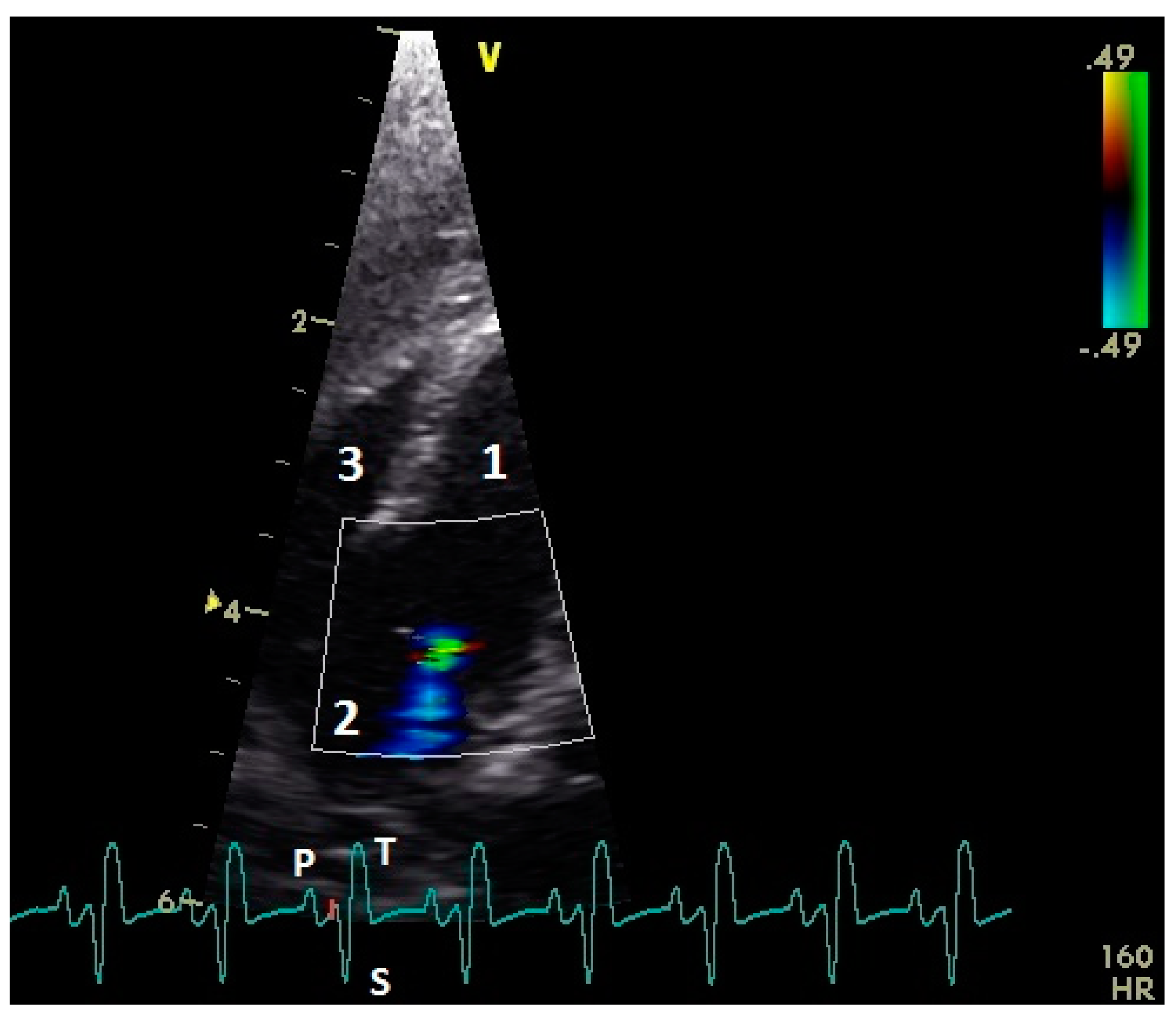
| Measuring Point | Peak E Velocity | Peak A Velocity | E : A Ratio | Peak EA Velocity | Angle Correction |
|---|---|---|---|---|---|
| Left AV valve | 0.37 ± 0.59 | 0.58 ± 0.20 | 0.72 ± 0.28 | 0.84 ± 0.01 | 28.4 ± 7.8 |
| (0.37; 0.25–0.53) | (0.57; 0.24–0.96) | (0.64; 0.31–1.63) | (0.84; 0.83–0.85) | (29.0; 12.0–45.0) | |
| Heart rate | 220.5 ± 41.3 | (270.0–360.0) | |||
| (225.0; 146.0–300.0) | |||||
| Left AV valve in anesthesia | 0.34 ± 0.07 (0.33; 0.26–0.52) | 0.42 ± 0.12 (0.41; 0.22–0.67) | 0.87 ± 0.32 (0.78; 0.44–1.79) | 0.55 ± 0.17 (0.55; 0.43–0.67) | 27.4 ± 7.9 (28.0; 0.0–45.0) |
| Heart rate | 148.4 ± 29.5 | (210.0–257.0) | |||
| (146.0; 99.0–244.0) | |||||
| Right AV valve | 0.22 ± 0.06 | 0.52 ± 0.10 | 0.43 ± 0.11 | 0.67 ± 0.01 | 22.2 ± 7.82 |
| (0.2; 0.12–0.42) | (0.51; 0.31–0.70) | (0.4; 0.25–0.71) | (0.67; 0.66–0.68) | (24.0; 8.0–40.0) | |
| Heart rate | 219.1 ± 34.7 | (300–360) | |||
| (215.5; 148–300) | |||||
| Right AV valve in anesthesia | 0.18 ± 0.06 (0.18; 0.03–0.38) | 0.47 ± 0.11 (0.45; 0.24–0.80) | 0.42 ± 0.24 (0.37; 0.09–1.58) | 0.37 ± 0.05 (0.43; 0.36–0.50) | 21.8 ± 7.6 (22.0; 0.0–35.0 |
| Heart rate | 148.7 ± 20.3 | 235.8 ± 31.6 | |||
| (147.5; 100.0–197.0) | (240.0; 189.0–270.0) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Legler, M.; Koy, L.; Kummerfeld, N.; Fehr, M. Differences between the Filling Velocities of the Left and Right Heart Ventricle in Racing Pigeons (Columba livia F. Domestica) and the Influence of Anesthesia with Isoflurane. Vet. Sci. 2019, 6, 79. https://doi.org/10.3390/vetsci6040079
Legler M, Koy L, Kummerfeld N, Fehr M. Differences between the Filling Velocities of the Left and Right Heart Ventricle in Racing Pigeons (Columba livia F. Domestica) and the Influence of Anesthesia with Isoflurane. Veterinary Sciences. 2019; 6(4):79. https://doi.org/10.3390/vetsci6040079
Chicago/Turabian StyleLegler, Marko, Lajos Koy, Norbert Kummerfeld, and Michael Fehr. 2019. "Differences between the Filling Velocities of the Left and Right Heart Ventricle in Racing Pigeons (Columba livia F. Domestica) and the Influence of Anesthesia with Isoflurane" Veterinary Sciences 6, no. 4: 79. https://doi.org/10.3390/vetsci6040079
APA StyleLegler, M., Koy, L., Kummerfeld, N., & Fehr, M. (2019). Differences between the Filling Velocities of the Left and Right Heart Ventricle in Racing Pigeons (Columba livia F. Domestica) and the Influence of Anesthesia with Isoflurane. Veterinary Sciences, 6(4), 79. https://doi.org/10.3390/vetsci6040079




