Case Reports for Topical Treatment of Corneal Ulcers with a New Matrix Therapy Agent or RGTA® in Dogs
Abstract
1. Introduction
2. Materials and Methods
2.1. Dogs
2.2. Clinical Evaluation
2.3. Clinical Tests
2.4. Schirmer Tear Test
2.5. Fluorescein Stain
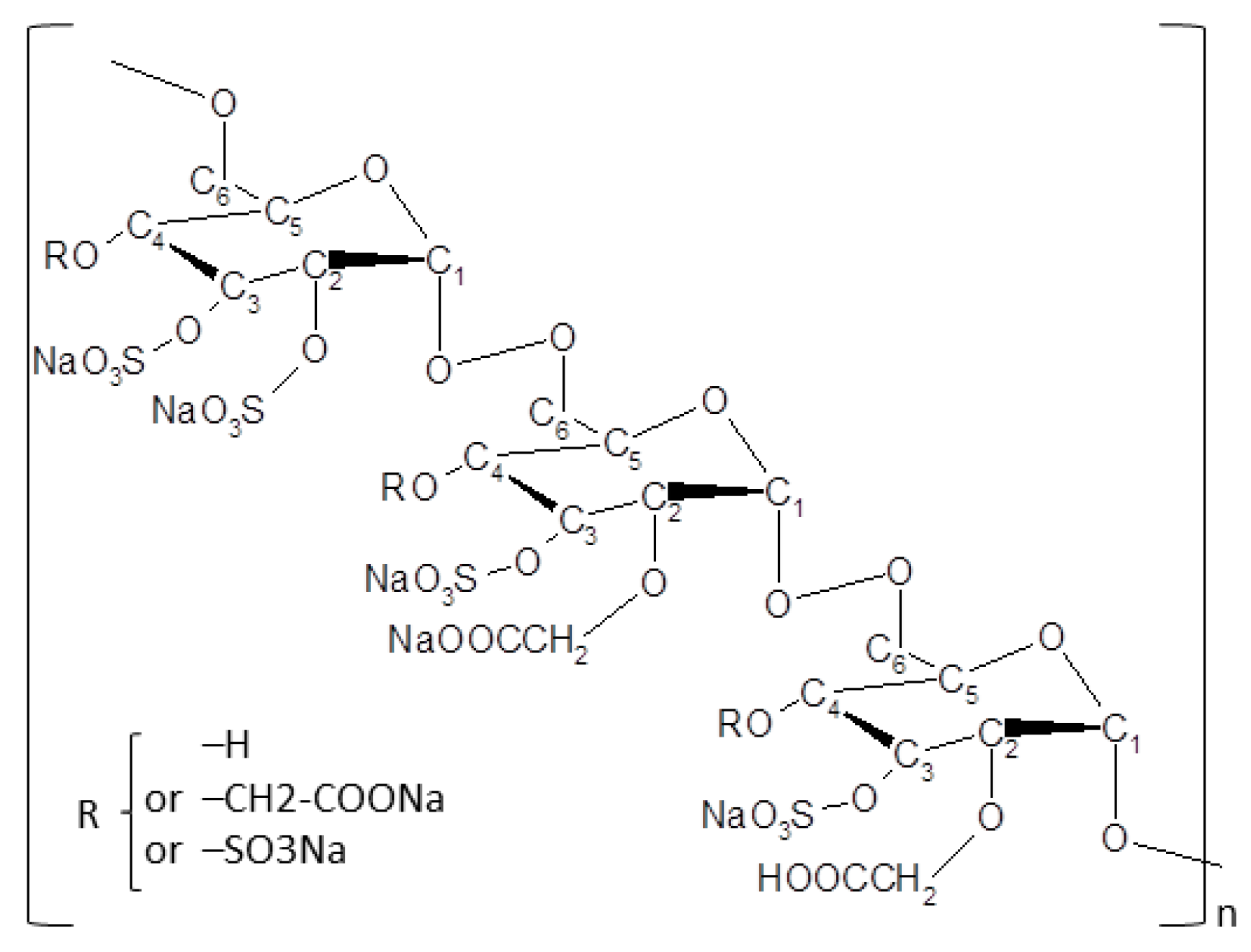
2.6. New Regenerative Matrix Treatment
2.7. Statistical Analysis
3. Results
3.1. New Regenerative Matrix Treatment
3.2. Treatment and Follow-up
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| KCS | Keratoconjunctivitis sicca or dry eye syndrome |
| OD (oculus dexter) | Right eye |
| OS (oculus sinister) | Left eye |
| OU (oculus uterque) | Both eyes |
| PO | per os |
| q3d | Quaque 3 die = every 3 days |
Appendix A
- 13 years old, Shih-Tzu, female/spayed.
- History: KCS (i.e., dry eye syndrome), Glaucoma.
- Prior Treatments: OU: Dorzolamide, Neopolydex, cyclosporine 2%, and artificial tears.
- Diagnostics: 8/31/15 OS superficial ulcer.
- Treatments: OU: Dorzolamide, cyclosporine 2%, and artificial tears.
- OS: Clerapliq® 1 drop q3days, Ofloxacin.
- Recheck: 9/17/15 OS ulcer healed.
- No more rechecks.
- 10 years old, Boston Terrier, male/intact.
- History: OD squinting and erosion treated by referring veterinarian two months prior to presentation.
- 6/29/2015 Grid Keratotomy.
- Prior Treatments: Atropine/terramycin/serum/remand/flurbiprofen.
- Findings: OD supero-temporal paracentral 3 mm erosion with poorly adherent margins, temporal paracentral 2–3+ edema.
- Diagnostics: 7/24/2015 OD endothelial dystrophy and persistent erosion.
- Treatments: 7/24/2015 OD: Diamond burr debridement; Ofloxacin 1; NaCl 5% solution.
- Systemic: Tramadol 50 mg PO as needed for pain.
- Recheck: 8/6/2015 persistent ulcer.
- Treatments: Diamond burr debridement repeated and continued above topical meds.
- Recheck: 8/17/2015 OD complete epithelialization but nonadherent central to paracentral; 3+ edema; peripheral superficial vascularization.
- Treatments: OD: Clerapliq® 1 drop q3d; ofloxacin; NaCl 5% ointment; strip.
- Systemic: Tramadol 50 mg as needed for pain.
- Recheck: 8/31/2015 OD complete epithelialization with fluorescein stipple over vascular ridge with bullae in temporal paracentral region, adherent epithelium challenged with cotton tip applicator, 3+ edema.
- Treatments: OD: Clerapliq® 1 drop q3d; Neopolydex; ofloxacin; NaCl 5% ointment; strip.
- Systemic: Tramadol 50 mg PO as needed for pain.
- Recheck: 9/21/2015 complete epithelialization with no fluorescein stipple and no bullae, adherent epithelium in all quadrants, 2+ edema, attenuated vascularization.
- Treatments: None.
- 7 years old, French Bulldog, male/neutered.
- History: 8/20/2015 diagnosed with OD erosion by referring veterinarian.
- Treatments: Tobramycin q4h and serum q4h.
- Findings: OD temporal paracentral corneal erosion with nonadherent margins and subepithelial fluorescein uptake, 1+ edema, 9:00 peripheral superficial pigmentation.
- Diagnostics: 8/24/2015 OD persistent epithelial erosion.
- Treatments: OD: Geographic diamond burr debridement; tobramycin; serum; Remand, corneal repair gel.
- Systemic: Clavamox 125 mg until gone; Rimadyl 25 mg until gone; tramadol 50 mg as needed for pain.
- Recheck: 8/25/2015 owner noted increased discharge.
- Treatments: OD: Ofloxacin; Remend corneal repair gel.
- Systemic: Clavamox 125 mg until gone; Rimadyl 25 mg; tramadol 50 mg for pain.
- Recheck: 9/2/2015 OD smaller erosion of temporal quadrants with partially adherent margins on its superonasal margin, 1+ edema, 9:00 previously observed pigmentation.
- Treatments: OD: Clerapliq® 1 drop q3d; Ofloxacin; Remand corneal repair gel.
- Systemic: Tramadol 50 mg as needed for pain.
- Recheck: 9/8/2015 OD adherent epithelium challenged with cotton tip applicator, 1+ edema, 9:00 pigmentation, 3+ paracentral superficial vascularization.
- Diagnostics: Healed persistent erosion.
- Treatments: OD: Neopolydex suspension.
- Systemic: Collar.
- Recheck: 10/1/2015 OD 1+ edema most prominent in temporal quadrants, temporal superficial pigmentation, attenuated paracentral superficial vascularization.
- 9 years old, Griffon, male/intact.
- History: Presented with persistent ulcer OD diagnosed by referring veterinarian on 10/21,
- OU endothelial dystrophy with cloudiness that owner had noticed for the past 3 months.
- Prior treatments: OD: BNP; ketorolac.
- OS: Rimadyl; cefpodoxime;
- OU: Schirmer tear test: 25 mm/min
- Findings: OU: 2+ diffuse edema;
- OD: Multiple erosion across palpebral fissure with poorly adherent epithelium in those regions, superonasal paracentral superficial vascularization, temporal paracentral focal superficial to deep scar, Seidel-negative.
- Diagnostics: 7/27/2015 OD: persistent erosion.
- OU: Corneal endothelial dystrophy.
- Treatments: OD: Diamond burr debridement; ofloxacin; 5% NaCl solution.
- Systemic: Tramadol 3 tabs for pain; Rimadyl; cefpodoxime as instructed until gone.
- Recheck: 8/10/2015 OU: 2+ diffuse edema.
- OD: Temporal paracentral erosion with poorly adherent epithelium margins, multifocal erosions at 1:00 periphery, 3+ paracentral superficial vascularization, temporal paracentral focal superficial to deep scar.
- Treatments: OD: Repeated diamond burr debridement; ofloxacin; 5% NaCl solution.
- Systemic: Tramadol 3 tabs for pain.
- Recheck: 8/26/2015 OU: 2+ diffuse edema.
- OD: Diffuse superficial vascularization and haze, temporal paracentral focal superficial to deep scar.
- OS: Nasal paracentral 3 mm erosion with nonadherent epithelium.
- Treatments: OS diamond burr debridement; E-collar; neopolydex suspension; ofloxacin; 5% NaCl solution.
- Systemic: Tramadol 3 tabs for pain.
- Recheck: 9/14/2015 OU: 2+ diffuse edema.
- OD: Attenuating diffuse superficial vascularization and haze, temporal paracentral focal superficial to deep scar.
- OS: Epithelial erosion of the superior quadrants with nonadherent epithelial margins on the superior periphery only (debrided to adherent margins with sterile cotton applicator), 3+ peripheral vascularization.
- Treatments: OD: Neopolydex suspension; ofloxacin; tobramycin.
- OS: 5% NaCl solution; Clerapliq® 1 drop q3d.
- Systemic: Tramadol 50 mg 3 for pain.
- Recheck: 9/14/2015 OU: 2+ diffuse edema.
- OD: Attenuated diffuse superficial vascularization and haze, temporal paracentral focal superficial to deep scar.
- OS: Epithelial erosion of the superotemporal quadrants with nonadherent epithelial margins, debrided to adherent margins with sterile cotton applicators, 3+ peripheral vascularization with raised vascularization in superotemporal periphery.
- Treatments: OU: neopolydex suspension.
- OS: Tobramycin; 5% NaCl solution; Clerapliq® 1 drop q3d.
- Systemic: Tramadol 50 mg 3 tabs for pain.
- Recheck: 9/24/2015 OU: 2+ diffuse edema.
- OD: Attenuating diffuse superficial vascularization and haze.
- OS: Focal epithelial erosion of the superotemporal quadrant with more adherent epithelial margins, attenuating raised vascularization, erosions in palpebral fissure with nonadherent margins.
- Treatments: OU: Neopolydex susp 1 drop OU q24h.
- OS: Diamond burr debridement; tobramycin; 5% NaCl solution; Clerapliq® 1 drop q3d.
- Systemic: Tramadol 50 mg 3 tabs for pain.
- Recheck: 10/5/2015 OU: 2+ diffuse edema.
- OD: Attenuating diffuse superficial vascularization and haze.
- OS: Attenuating vascularization, no bullae, diffuse superficial haze.
- Treatments: OD: Neopolydex suspension.
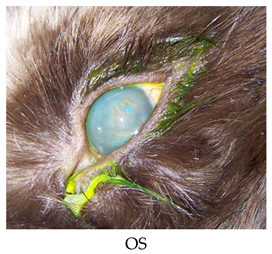
- Recheck: 11/3/2015 OU: 2+ diffuse edema, no bullae, attenuated diffuse superficial haze, and vascularization (mostly ghost vessels).
- Treatments: None.
- Diagnostics: OU: Healed persistent epithelial erosion, post-op diamond burr debridement, corneal endothelial dystrophy with predisposition to recurrent erosions.
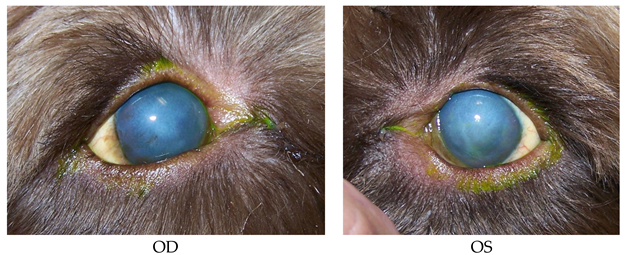
- 15 years old, Chihuahua, female/spayed.
- History: Cataract Sx OD on 8/26/2015. OD TPA intracamaral injection on 9/8/2015.
- Prior diagnostics: OD: Post-operation of phacoemulsification and lens implant.
- OS: Mature cataract.
- Prior treatments: OD: Restart dorzolamide/timolol; tropicamide 1%; Genteal Gel drops; ketorolac 0.5%.
- OS: Neopolydex suspension.
- Systemic: Metacam; tramadol 50 mg 1/4 tab for pain; E-collar.
- Findings on 9/9/2015: OD: Seidel-negative incision, central erosion.
- OS: Central erosion.
- Diagnostics: OU: Nocturnal exposure keratitis and erosions.
- OD: Post-op phacoemulsification and lens implant, transient ocular hypertension.
- OS: Mature cataract.
- Treatments: OU: Start Clerapliq® 1 drop q3d; GenTeal Gel drops; artificial tear ointment.
- OD: Dorzolamide/timolol; tropicamide 1%; ketorolac 0.5%, neopolydex suspension.
- OS: Ofloxacin; ketorolac 0.5%.
- Systemic: Metacam; tramadol 50 mg 1/4 tab for pain.
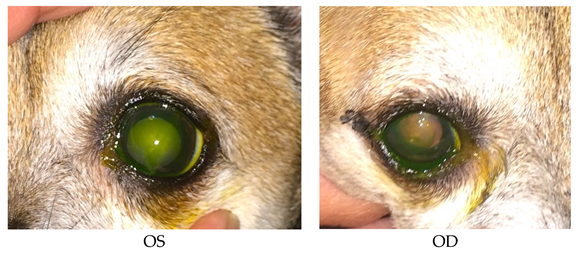
- Recheck: 9/15/2015 OD: Seidel-negative incision, central erosion with adherent margins.
- OS: Healed central erosion with 1+ haze.
- Treatments: OU: Clerapliq® 1 drop q3days; GenTeal Gel drops; artificial tear ointment.
- OD: Dorzolamide/timolol; tropicamide 1%; ketorolac 0.5%; neopolydex suspension.
- OS: Ofloxacin; ketorolac 0.5%.
- Systemic: Metacam; tramadol 50 mg for pain; E-collar.
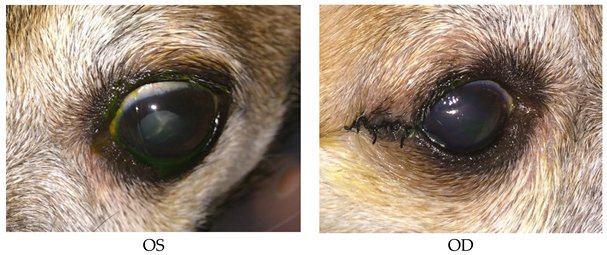
- Recheck: 9/24/15 OD: Seidel-negative vascularized incision, central erosion with adherent margins and edema.
- OS: Healed central erosion with 1+ haze.
- Treatments: OU: Artificial tear ointment.
- OD: Clerapliq® 1 drop OD q3days; ofloxacin; dorzolamide/timolol; tropicamide 1%; Genteal Gel drops; ketorolac 0.5%; neopolydex suspension.
- OS: Ketorolac 0.5%.
- Systemic: Metacam; tramadol 50 mg 1/4 tab for pain; E-collar.
- Diagnostics: OU: Nocturnal exposure keratitis and erosions.
- OD: Post-op phacoemulsification and lens implant, transient ocular hypertension.
- OS: Mature cataract.
- Recheck: 10/2/15 OD: Seidel-negative vascularized incision with suture remnants, smaller 2 mm central erosion with surrounding haze and reduced edema.
- OS: Healed central erosion with 1+ haze.
- Treatments: OU: Artificial tear ointment 1/2 inch strip at bed time;
- OD: Clerapliq® 1 dropq3days; ofloxacin; dorzolamide/timolol; tropicamide 1%; GenTeal Gel drops; ketorolac 0.5%, neopolydex suspension.
- OS: Ketorolac 0.5%.
- Systemic: Metacam; tramadol 50 mg 1/4 tab; E-collar.
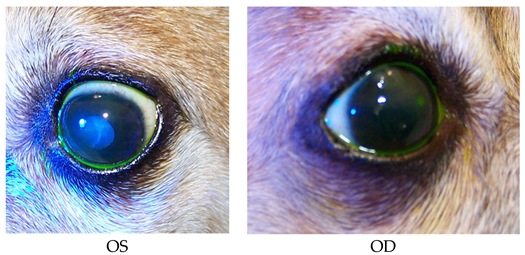
- Recheck: 10/20/15 OD: Seidel-negative vascularized incision scar with superficial pigment, 2 mm central erosion with pinpoint leukoma and surrounding adherent epithelial margins.
- OS: Central 1+ superficial haze with thin epithelium.
- Treatments: OU: Artificial tear ointment.
- OD: Clerapliq® 1 drop q3days until gone; ofloxacin tobramycin; dorzolamide/timolol; tropicamide 1%; Genteal Gel drops; ketorolac 0.5%; neopolydex suspension.
- OS: Ketorolac 0.5%.
- Systemic: Tramadol 50 mg 1/4 tab for pain; E-collar.
- Recheck: 11/3/2015 OD: Seidel-negative vascularized incision scar with superficial pigment, 2 mm central superficial ulcer with pinpoint leukoma and surrounding marginally adherent epithelial margins.
- OS: Central 1+ superficial haze with multifocal subepithelial fluorescein uptake.
- Treatments: OU: tobramycin; Genteal Gel drops; artificial tear ointment.
- OD: Central to paracentral diamond burr debridement; ketorolac 0.5%.
- OS: Geographic diamond burr debridement.; ketorolac 0.5%; neopolydex suspension.
- Systemic: Tramadol 50 mg 1/4 tab for pain; E-collar.
- Ran out of Clerapliq® samples.
- Patient 5 developed melting ulcers in both eyes after diamond burr debridement.
- 13 years old, Poodle mix, male/neutered.
- History: Presented OD squinting/epiphora x2d; coughing for 2 months.
- Prior treatments: OD: ofloxacin, doxycycline, hydroxyzine, and cough tabs (dextromethorphane/guafesesin).
- Findings: OD: Diffuse edema, temporal paracentral superficial vascularization, and erosion with poorly adherent margins debrided to adherent margins with sterile cotton applicators.
- OS: 2+ diffuse edema most prominent in temporal quadrants with multifocal bullae and paracentral superficial vascularization and traces of pigment.
- Diagnostics: OU: Corneal endothelial dystrophy with risk of recurrent erosions.
- OD: Corneal epithelial erosion.
- Treatments: OD: Ofloxacin; 5% NaCl solution.
- Systemic: E-collar; other medicines as per referring veterinarian.
- Recheck: 8/25/2015 OD: diffuse edema, temporal paracentral superficial vascularization, and erosion with nonadherent margins.
- OS: 2+ diffuse edema most prominent in temporal quadrants with multifocal bullae and paracentral superficial vascularization and traces of pigment.
- Treatments: OD: Geographic diamond burr debridement; ofloxacin; 5% NaCl solution ointment;
- Systemic: Tramadol 50 mg 1 tab for pain; E-collar; other medicines as per referring veterinarian.
- Recheck: 8/27/2015 OD: diffuse edema, epithelialization of entire cornea, except for temporal quadrant with superficial vascularization.
- OS: 2+ diffuse edema most prominent in temporal quadrants with multifocal bullae and paracentral superficial vascularization and traces of pigment.
- Treatments: OD: Ofloxacin; 5% NaCl solution ointment.
- Systemic: Tramadol 50 mg 1 tab for pain; E-collar; other medicines as per referring veterinarian.
- Recheck: 9/9/2015 OD: diffuse edema, central to paracentral erosion with nonadherent margins, temporal superficial vascularization.
- OS: 2+ diffuse edema most prominent in temporal quadrants, no bullae and paracentral superficial vascularization and traces of pigment.
- Treatments: OD: Clerapliq® 1 drop q3d; ofloxacin; 5% NaCl solution ointment.
- Systemic: Tramadol 50 mg 1 tab for pain; E-collar; other medicines as per referring veterinarian.
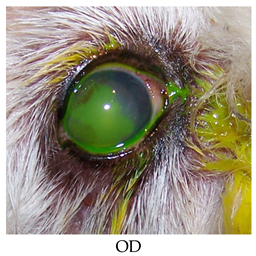
- Recheck: 9/21/2015 OD: Diffuse edema, temporal paracentral large bullae with fluorescein stipple and 2 focal erosions in inferior quadrant, diffuse superficial vascularization.
- OS: 2+ diffuse edema most prominent in temporal quadrants, nasal paracentral bullae with fluorescein stipple and paracentral superficial vascularization and traces of pigment.
- Treatments: OU: Clerapliq® 1 drop q3d.
- OD: Ofloxacin; 5% NaCl solution ointment.
- Systemic: E-collar.
- Recheck: 10/19/2015 OD: Diffuse edema, temporal paracentral erosion with nonadherent margins, diffuse superficial vascularization.
- OS: 2+ diffuse edema most prominent in temporal quadrants, nasal paracentral linear break in epithelium with subepithelial fluorescein uptake, paracentral superficial vascularization and traces of pigment.
- Treatments: OU: Ofloxacin; 5% NaCl solution ointment; no more Clerapliq® samples.
- Systemic: E-collar PRN for rubbing.
- Follow-up: Some erosions recurred, and some were persisting OU for which I discussed thermo-keratoplasty or conjunctival pedicle grafting (keratoleptnysis), which may help minimize progressive corneal edema.
- 12 years old Boston Terrier, male/neutered.
- History: OD cloudy more than a year; OD red and squinting early last week
- Prior treatments: OD: With BNPH and changed on 9/5/2015 to ofloxacin and atropine, Remand; corneal repair gel; rimadyl and tramadol.
- Findings: OD: Central to paracentral 3+ edema, central bullae with fairly adherent epithelial cells challenged with sterile cotton applicator.
- OS: Within normal limits.
- Diagnostics on 9/15/2015: OU: Iris cysts.
- OD: Corneal endothelial dystrophy with predisposition to recurrent erosions, emerging Meibomian mass.
- OS: Zonulysis.
- Treatments: OD: Clerapliq® 1 drop q3d; ofloxacin; 5% NaCl solution ointment; Remand; corneal repair gel;
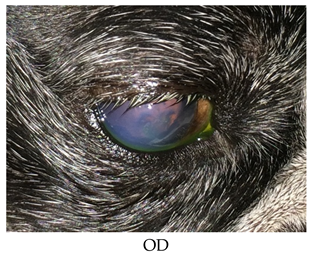
- Recheck on 9/28/2015: OD: Central to paracentral 3+ edema, no bullae, 2 curvilinear epithelial breaks where lid mass apposes with fairly adherent epithelial cells challenged with sterile cotton applicator;
- OS: Within normal limits.
- Treatments: OD: Clerapliq® 1 drop q3d; ofloxacin; 5% NaCl solution ointment; Remand; corneal repair gel
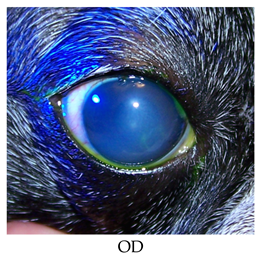
- Lost to follow up.
- 14 years old, miniature Poodle, female/spayed, diabetic.
- History: Bilateral cataract section on 8/18/2015.
- Since 8/31/2015: OU: Seidel-negative incision, 1+ diffuse edema, central erosion.
- Prior treatments: OU: ketorolac 0.5%; tropicamide 1%; dorzolamide/timolol; GenTeal Gel drops; neopolydex suspension.
- Systemic: Metacam after surgery; Ocu-Glo; Tramadol 50 mg 1/2 tab for pain.
- Recheck: 9/9/2015 OU: Seidel-negative incision, 1+ diffuse edema.
- OD: Central erosion with nonadherent margins debrided to adherent margins with sterile cotton applicator; Schirmer tear test OD: 11 mm/min; anterior chamber: Trace flare, no cells, no lipid-laden aqueous.
- OS: Central linear erosion with adherent epithelium challenged with sterile cotton applicator. Schirmer tear test OS: 12 mm/min; anterior chamber: Trace flare, no cells/lipid.
- Treatments: OU: Ketorolac 0.5%; tropicamide 1%; dorzolamide/timolol; GenTeal Gel drops 1; neopolydex; ofloxacin; prednisolone acetate suspension 1%; start cyclosporine 2%.
- Systemic: Tramadol 50 mg 1/2 tab for pain; Ocu-Glo.
- Recheck: 9/17/2015 OU: Seidel-negative incision, 2+ diffuse edema.
- OD: Central erosion with nonadherent margins debrided to adherent margins with sterile cotton applicator.
- OS: 1+ central edema, central focal erosion with fairly adherent epithelium challenged with sterile cotton applicator.
- Treatments: OU: Clerapliq® 1 drop q3d; ketorolac 0.5%; tropicamide 1%; GenTeal Gel drops; ofloxacin; prednisolone acetate suspension 1%; cyclosporine 2%.
- Systemic: Tramadol 50 mg 1/2 tab for pain; Ocu-Glo.
- Recheck: 10/1/2015 OU: Seidel-negative vascularized incision with suture remnants, 1-2+ diffuse edema.
- OD: Deep stromal ulcer with deepest portion epithelialized and inferior portion with keratomalacia; Schirmer tear test: 18 mm/min.
- OS: 1+ central edema, central superficial haze; Schirmer tear test: 15 mm/min.
- 10/1/2015: Corneal culture and cytology: Septic neutrophilic inflammation with epithelial dysplasia. Enterococcus species 4+.
- Treatments: OU: Cyclosporine 2%; ketorolac 0.5%; tropicamide 1%.
- OD: Clerapliq® 1 drop q3d; gentamicin 20 mg/0.2 mL sub-conjunctival; cefazolin 50 mg/mL; 1.2% gentamicin; GenTeal Gel drops 1 drop OD tid.
- OS: Ofloxacin; prednisolone acetate suspension 1% only.
- Systemic: E-collar; tramadol 50 mg 1/2 tab for pain; Ocu-Glo.
- Comments: The erosion OS healed, but the erosion OD became infected with a deep melting ulcer that is partially epithelialized.
- Recheck: 10/5/2015 OU: Seidel-negative vascularized incision scar, 1+ diffuse edema.
- OD: Deep stromal ulcer with deepest portion epithelialized and inferior portion with reduced keratomalacia and increased epithelialization.
- OS: 1+ central edema, central superficial haze.
- Treatments: OU: Ketorolac 0.5%; tropicamide 1%; cyclosporine 2%; tobramycin.
- OD: Clerapliq® 1 drop q3d; cefazolin; 1.2% gentamicin; ofloxacin.
- OS: GenTeal Gel drops; prednisolone acetate suspension 1%.
- Systemic: E-collar; Ocu-Glo; tramadol 50 mg 1/2 tab for pain.
- Comments: The melting ulcer is healing well and will probably not need a conjunctival graft. The cytology was negative for fungus, and the culture revealed enterococcus resistant to fluoroquinolones and cephalosporins.
- Recheck: 10/12/15 OU: Seidel-negative vascularized incision scar, reduced diffuse edema.
- OD: Deep stromal scar complete and adherent epithelium challenged with sterile cotton applicator; Schirmer tear test: 18 mm/min.
- OS: 1+ central edema, central superficial haze with adherent epithelium challenged with sterile cotton applicator. Schirmer tear test: 14 mm/min.
- Diagnostics: OU: Post-op phacoemulsification and lens implant for diabetic cataract, healed persistent corneal erosion, KCS (i.e., dry eye syndrome).
- OD: Healed melting ulcer.
- Treatments: OU: Serum; tobramycin; ketorolac 0.5%; tropicamide 1%; GenTeal Gel drops; cyclosporine 2%;
- OS: Prednisolone acetate suspension 1%.
- Systemic: E-collar; Ocu-Glo.
- Comments: Both corneas have epithelialized with adherent but thin central epithelium. The infection appears cleared.
- Recheck: 10/19/2015. OU: Seidel-negative vascularized incision scar, reduced diffuse edema.
- OD: Deep stromal scar complete and adherent epithelium challenged with sterile cotton applicator.
- OS: 1+ central edema, central superficial haze with adherent epithelium challenged with sterile cotton applicator.
- Treatments: OU: Tobramycin; gentamicin ointment; ketorolac 0.5%; tropicamide 1%; GenTeal Gel drops; d/c prednisolone acetate; cyclosporine 2%;.
- Systemic: E-collar; Ocu-Glo.
- Recheck: 10/26/2015 OU: Seidel-negative vascularized incision scar, reduced diffuse edema.
- OD: Deep stromal scar complete and adherent epithelium.
- OS: 1+ central edema, 11:00 paracentral anterior stromal ulcer with adherent margins, central superficial haze with adherent epithelium challenged with sterile cotton applicator.
- Treatments: OU: Ketorolac 0.5%; tropicamide 1%; GenTeal Gel drops; cyclosporine 2%.
- OD: Restart prednisolone acetate suspension 1%.
- OS: Tobramycin; gentamicin ointment 1.
- Systemic: E-collar; Ocu-Glo.
- Recheck: 11/2/2015 OU: Seidel-negative vascularized incision scar, reduced diffuse edema.
- OD: Deep stromal scar complete but thin epithelium.
- OS: 1+ central edema, 11:00 paracentral anterior stromal ulcer site with thin epithelium, central superficial haze.
- Diagnostics: OU: Post-op phacoemulsification and lens implant for diabetic cataract, persistent corneal erosion, KCS (i.e., dry eye syndrome).
- OD: Healed melting ulcer.
- OS: Healing superficial ulcer.
- Treatments: OU: Chloramphenicol 1% ointment; d/c prednisolone acetate suspension; ketorolac 0.5%; tropicamide 1%; GenTeal Gel drops; cyclosporine 2%.
- OS: Ulcer healed and rechecks continue for cataracterisation.
- Systemic: E-collar; Ocu-Glo.
- 15 years old, miniature Poodle, male/neutered.
- History: Cataract Surgery OD on 9/9/2015. On 9/22/2015, both corneas have persistent epithelial erosions which may eventually need diamond burr debridement.
- Prior treatments: OU: Cyclosporine 2%; Genteal Gel drops; neopolydex suspension.
- OD: Ketorolac 0.5%.
- OS: Ofloxacin.
- Systemic: Rimadyl 25 mg 1 tab; E-collar.
- Findings: OU: Attenuated superior paracentral superficial vascularization.
- OD: Seidel-negative incision, reduced edema, faint central superficial haze/lipid/mineral, central 3 mm erosion with nonadherent margins.
- OS: Central focal erosion with poorly adherent epithelium.
- Diagnostics: OD: Post-op phacoemulsification and lens implantation.
- OS: Hypermature cataract and lens induced uveitis.
- OU: Severe iris atrophy, suspected nocturnal lagophthalmia.
- Treatments: OU: Clerapliq® 1 drop q3d; cyclosporine 2%; GenTeal Gel drops; neopolydex suspension.
- OD: Ketorolac 0.5%.
- OS: Ofloxacin 1 drop.
- Sytsemic: Rimadyl 25 mg 1 tab; E-collar.
- Recheck: 10/5/2015 OU: Central fluorescein stipple in palpebral fissure with little subepithelial uptake and more adherent margins, attenuated superior paracentral superficial vascularization.
- OD: Seidel-negative partially vascularized incision, reduced edema, faint central superficial haze/lipid/mineral.
- Treatments: OU: Cyclosporine 2%; GenTeal Gel drops; neopolydex suspension.
- OD: Ketorolac 0.5%.
- OS: Ofloxacin 1 drop.
- Systemic: Rimadyl 25 mg 1 tab; E-collar.
- Recheck: 10/20/15 OD: Seidel-negative partially vascularized incision scar, reduced edema, faint central superficial haze/lipid/mineral, central thin epithelium, attenuated superior paracentral superficial vascularization.
- OS: Central thin epithelium, attenuated superior paracentral superficial vascularization.
- Diagnostics: OU: Severe iris atrophy, suspected nocturnal lagophthalmia.
- OD: Post-op phacoemulsification and lens implantation.
- OS: Hypermature cataract and lens induced uveitis.
- Treatment: OU: Cyclosporine 2%; GenTeal Gel drops; ketorolac 0.5%.
- OD: Ofloxacin 1 drop.
- OS: Neopolydex suspension.
- Systemic: E-collar.
- Recheck on 11/3/2015: OD: Seidel-negative partially vascularized incision scar, reduced edema, central anterior stromal ulcer with superficial haze/lipid/mineral, attenuated superior paracentral superficial vascularization.
- OS: Central thin epithelium, attenuated superior paracentral superficial vascularization.
- Diagnostics: OU: Severe iris atrophy, suspected nocturnal lagophthalmia.
- OD: Post-op phacoemulsification and lens implantation.
- OS: Hypermature cataract and lens induced uveitis.
- Treatment: OU: Cyclosporine 2%; GenTeal Gel drops; ketorolac 0.5%.
- OD: Gentamicin 20 mg/0.2 mL subconjunctival; ofloxacin.
- OS: Neopolydex suspension.
- Systemic: E-collar.
- Comments: Patient 9 is doing well, almost being 2 months after cataract surgery, but the right cornea re-ulcerated for which a culture is pending. Patient 9 melting ulcer was healed on antibiotics selected based on culture results.
- 12 years old, Pomeranian mix, female/intact.
- History: OD KCS (i.e., dry eye syndrome), post-op conjunctival pedicle graft for penetrating ulcer, mature cataract.
- OS: Immature cataract.
- Prior treatments: OU: ketorolac 0.5%.
- OD: Cyclosporine 2%.
- Systemic: Ocu-Glo.
- Findings on 9/23/2015: OD: Seidel-negative intact and viable conjunctival pedicle graft with surrounding vascularization, reduced edema in inferior quadrants.
- OS: Superotemporal paracentral pinpoint erosion, attenuated superficial scar/pigment/vascularization and edema most prominent temporally, reduced superficial lipid.
- Diagnostics: OD: KCS (i.e., dry eye syndrome), post-op conjunctival pedicle graft for penetrating ulcer, mature cataract.
- OS: Epithelial erosion, immature cataract.
- Treatments: OU: Ketorolac 0.5%.
- OD: Cyclosporine 2%.
- OS: Clerapliq® 1 drop q3d; ofloxacin.
- Systemic: Ocu-Glo.
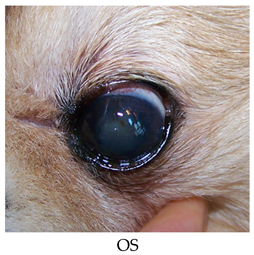
- Recheck on 10/1/2015: OD: Seidel-negative intact and viable conjunctival pedicle graft with surrounding vascularization, reduced edema in inferior quadrants.
- OS: Healed superotemporal paracentral pinpoint erosion, superior paracentral faint fluorescein stipple with adherent epithelium challenged with sterile cotton applicators, attenuated superficial scar/pigment/vascularization and edema most prominent temporally, reduced superficial lipid.
- Diagnostics: OD: KCS (i.e., dry eye syndrome), post-op conjunctival pedicle graft for penetrating ulcer, mature cataract.
- OS: Epithelial erosion, immature cataract.
- Treatments: OU: Cyclosporine 2%; ketorolac 0.5%.
- OS: Clerapliq® 1 drop q3d; ofloxacin.
- Systemic: Ocu-Glo.
- Comments: The left corneal erosion is essentially healed.
- Lost to follow up
- 14 years old, Dachshund, female/spayed.
- History: Lost to follow-up since 2011, when there was good vision but diminished at night with small retinal vessels suspecting early progressive retinal atrophy; owner stopped topical NSAID and Ocu-Glo years ago.
- OU: Cloudy for a few months and red; no squinting; good navigation at home without bumping into things but hesitates in the dark.
- Prior treatments: OU: neopolydex suspension.
- OS: Latanoprost.
- Systemic: Ocu-Glo.
- Findings on 9/24/2015: OU: Seidel-negative incision scar, diffuse edema, traces of superficial pigment peripherally, reduced central cholesterol.
- OD: Central focal erosion with nonadherent margins.
- Diagnostics: OU: Post-op phacoemulsification and lens implant, corneal endothelial dystrophy; progressive retinal atrophy.
- OD: Persistent epithelial erosion.
- OS: Suspected glaucoma.
- Treatments: OU: Ketorolac 0.5%.
- OD: Diamond burr debridement centrally and nasally; Clerapliq® 1 drop q3d; neopolydex; ofloxacin.
- OS: Latanoprost.
- Systemic: Ocu-Glo; E-collar.
- Recheck: 10/1/2015 OU: Seidel-negative incision scar, diffuse edema, traces of superficial pigment peripherally, reduced central cholesterol.
- OD: Central multifocal stipple with bullae.
- Diagnostics: OU: Post-op phacoemulsification and lens implant, corneal endothelial dystrophy; progressive retinal atrophy.
- OD: Healing persistent epithelial erosion.
- OS: Suspected glaucoma.
- Treatment: OU: Ketorolac 0.5%.
- OD: Clerapliq® 1 drop q3d; ofloxacin.
- OS: Latanoprost.
- Systemic: Ocu-Glo; E-collar.
- Comments: The central erosion OD is almost healed.
- Lost to follow up!
References
- Groah, S.L.; Libin, A.; Spungen, M.; Nguyen, K.L.; Woods, E.; Nabili, M.; Ramella-Roman, J.; Barritault, D. Regenerating matrix-based therapy for chronic wound healing: A prospective within-subject pilot study. Int. Wound J. 2011, 8, 85–95. [Google Scholar] [CrossRef]
- Garcia-Filipe, S.; Barbier-Chassefiere, V.; Alexakis, C.; Huet, E.; Ledoux, D.; Kerros, M.E.; Petit, E.; Barritault, D.; Caruelle, J.P.; Kern, P. RGTA OTR4120, a heparan sulfate mimetic, is a possible long-term active agent to heal burned skin. J. Biomed. Mater. Res. A 2007, 80, 75–84. [Google Scholar] [CrossRef]
- Barritault, D.; Desgranges, P.; Meddahi-Pelle, A.; Denoix, J.M.; Saffar, J.L. RGTA((R))-based matrix therapy —A new branch of regenerative medicine in locomotion. Jt. Bone Spine 2017, 84, 283–292. [Google Scholar] [CrossRef]
- Barritault, D.; Gilbert-Sirieix, M.; Rice, K.L.; Sineriz, F.; Papy-Garcia, D.; Baudouin, C.; Desgranges, P.; Zakine, G.; Saffar, J.L.; van Neck, J. RGTA((R)) or ReGeneraTing Agents mimic heparan sulfate in regenerative medicine: From concept to curing patients. Glycoconj. J. 2017, 34, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Jacquet-Guibon, S.; Dupays, A.G.; Coudry, V.; Crevier-Denoix, N.; Leroy, S.; Sineriz, F.; Chiappini, F.; Barritault, D.; Denoix, J.M. Randomized controlled trial demonstrates the benefit of RGTA(R) based matrix therapy to treat tendinopathies in racing horses. PLoS ONE 2018, 13, e0191796. [Google Scholar] [CrossRef] [PubMed]
- Ouidja, M.O.; Petit, E.; Kerros, M.E.; Ikeda, Y.; Morin, C.; Carpentier, G.; Barritault, D.; Brugere-Picoux, J.; Deslys, J.P.; Adjou, K.; et al. Structure-activity studies of heparan mimetic polyanions for anti-prion therapies. Biochem. Biophys. Res. Commun. 2007, 363, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Hayek, S.; Dibo, S.; Baroud, J.; Ibrahim, A.; Barritault, D. Refractory sickle cell leg ulcer: Is heparan sulphate a new hope? Int. Wound J. 2016, 13, 35–38. [Google Scholar] [CrossRef]
- Aifa, A.; Gueudry, J.; Portmann, A.; Delcampe, A.; Muraine, M. Topical treatment with a new matrix therapy agent (RGTA) for the treatment of corneal neurotrophic ulcers. Investig. Ophthalmol. Vis. Sci. 2012, 53, 8181–8185. [Google Scholar] [CrossRef]
- Bata, A.M.; Witkowska, K.J.; Wozniak, P.A.; Fondi, K.; Schmidinger, G.; Pircher, N.; Szegedi, S.; Aranha Dos Santos, V.; Pantalon, A.; Werkmeister, R.M.; et al. Effect of a Matrix Therapy Agent on Corneal Epithelial Healing After Standard Collagen Cross-linking in Patients With Keratoconus: A Randomized Clinical Trial. JAMA Ophthalmol. 2016, 134, 1169–1176. [Google Scholar] [CrossRef]
- Brignole-Baudouin, F.; Warnet, J.M.; Barritault, D.; Baudouin, C. RGTA-based matrix therapy in severe experimental corneal lesions: Safety and efficacy studies. J. Fr. Ophtalmol. 2013, 36, 740–747. [Google Scholar] [CrossRef]
- Kirschner, S.E. Persistent corneal ulcers. What to do when ulcers won’t heal. Vet. Clin. N. Am. Small Anim. Pract. 1990, 20, 627–642. [Google Scholar] [CrossRef]
- Bentley, E.; Abrams, G.A.; Covitz, D.; Cook, C.S.; Fischer, C.A.; Hacker, D.; Stuhr, C.M.; Reid, T.W.; Murphy, C.J. Morphology and immunohistochemistry of spontaneous chronic corneal epithelial defects (SCCED) in dogs. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2262–2269. [Google Scholar]
- Da Silva, E.G.; Powell, C.C.; Gionfriddo, J.R.; Ehrhart, E.J.; Hill, A.E. Histologic evaluation of the immediate effects of diamond burr debridement in experimental superficial corneal wounds in dogs. Vet. Ophthalmol. 2011, 14, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.T.; Kambampati, R.; Murphy, C.J.; Bentley, E. Expression of matrix metalloproteinase 2 and 9 in experimentally wounded canine corneas and spontaneous chronic corneal epithelial defects. Cornea 2007, 26, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Bentley, E. Spontaneous chronic corneal epithelial defects in dogs: A review. J. Am. Anim. Hosp. Assoc. 2005, 41, 158–165. [Google Scholar] [CrossRef]
- Cooley, P.L.; Dice, P.F., II. Corneal dystrophy in the dog and cat. Vet. Clin. N. Am. Small. Anim. Pract. 1990, 20, 681–692. [Google Scholar] [CrossRef]
- Rosenthal, J.J. Bullous keratopathy: A latent complication of chronic corneal disease. Vet. Med. Small Anim. Clin. 1974, 69, 181–182. [Google Scholar]
- Torricelli, A.A.; Singh, V.; Santhiago, M.R.; Wilson, S.E. The corneal epithelial basement membrane: Structure, function, and disease. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6390–6400. [Google Scholar] [CrossRef]
- Wu, D.; Smith, S.M.; M. Stine, J.; M. Michau, T.; Miller, T.R.; Pederson, S.L.; Freeman, K.S. Treatment of spontaneous chronic corneal epithelial defects (SCCEDs) with diamond burr debridement vs. combination diamond burr debridement and superficial grid keratotomy. Vet. Ophthalmol. 2018, 21, 622–631. [Google Scholar] [CrossRef]
- Miller, W.W. Use of polysulfated glycosaminoglycans in treatment of persistent corneal erosions in dogs—A pilot clinical study. Vet. Med. 1996, 916–922. [Google Scholar]
- Famose, F. Evaluation of accelerated corneal collagen cross-linking for the treatment of bullous keratopathy in eight dogs (10 eyes). Vet. Ophthalmol. 2016, 19, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Pot, S.A.; Gallhofer, N.S.; Walser-Reinhardt, L.; Hafezi, F.; Spiess, B.M. Treatment of bullous keratopathy with corneal collagen cross-linking in two dogs. Vet. Ophthalmol. 2015, 18, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Spiess, B.M.; Pot, S.A.; Florin, M.; Hafezi, F. Corneal collagen cross-linking (CXL) for the treatment of melting keratitis in cats and dogs: A pilot study. Vet. Ophthalmol. 2014, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ledbetter, E.C.; Munger, R.J.; Ring, R.D.; Scarlett, J.M. Efficacy of two chondroitin sulfate ophthalmic solutions in the therapy of spontaneous chronic corneal epithelial defects and ulcerative keratitis associated with bullous keratopathy in dogs. Vet. Ophthalmol. 2006, 9, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Chebbi, C.K.; Kichenin, K.; Amar, N.; Nourry, H.; Warnet, J.M.; Barritault, D.; Baudouin, C. Pilot study of a new matrix therapy agent (RGTA OTR4120) in treatment-resistant corneal ulcers and corneal dystrophy. J. Fr. Ophtalmol. 2008, 31, 465–471. [Google Scholar]
- De Monchy, I.; Labbe, A.; Pogorzalek, N.; Gendron, G.; M’Garrech, M.; Kaswin, G.; Labetoulle, M. Management of herpes zoster neurotrophic ulcer using a new matrix therapy agent (RGTA): A case report. J. Fr. Ophtalmol. 2012, 35, 187. [Google Scholar] [CrossRef]
- Michaud, B. Medical management of corneal ulcers: Use of RGTA Prise en charge médicale des ulcères cornéens: Utilisation des RGTA(r). L’essentiel Vétérinaire 2013, 285, 16–24. [Google Scholar]
- Kymionis, G.D.; Liakopoulos, D.A.; Grentzelos, M.A.; Tsoulnaras, K.I.; Detorakis, E.T.; Cochener, B.; Tsilimbaris, M.K. Effect of the Regenerative Agent Poly(Carboxymethylglucose Sulfate) on Corneal Wound Healing After Corneal Cross-Linking for Keratoconus. Cornea 2015, 34, 928–931. [Google Scholar] [CrossRef]
- Xeroudaki, M.; Peebo, B.; Germundsson, J.; Fagerholm, P.; Lagali, N. RGTA in corneal wound healing after transepithelial laser ablation in a rabbit model: A randomized, blinded, placebo-controlled study. Acta. Ophthalmol. 2016, 94, 685–691. [Google Scholar] [CrossRef]
- Pison, A.; Feumi, C.; Bourges, J.L. Healing of a resistant neurotrophic corneal ulcer using a new matrix therapy agent. J. Fr. Ophtalmol. 2014, 37, e101–e104. [Google Scholar] [CrossRef]
- Mateo, A.; Abadia, B.; Calvo, P.; Minguez, E.; Pablo, L.; Del Castillo, J.M. Treatment of Acanthamoeba neurotrophic corneal ulcer with topical matrix therapy. J. Ophthalmic Inflamm. Infect. 2015, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Ledbetter, E.C. Canine herpesvirus-1 ocular diseases of mature dogs. N. Z. Vet. J. 2013, 61, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Ledbetter, E.C.; Kim, S.G.; Dubovi, E.J. Outbreak of ocular disease associated with naturally-acquired canine herpesvirus-1 infection in a closed domestic dog colony. Vet. Ophthalmol. 2009, 12, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Ledbetter, E.C.; Riis, R.C.; Kern, T.J.; Haley, N.J.; Schatzberg, S.J. Corneal ulceration associated with naturally occurring canine herpesvirus-1 infection in two adult dogs. J. Am. Vet. Med. Assoc. 2006, 229, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Deback, C.; Rousseau, A.; Breckler, M.; Molet, L.; Boutolleau, D.; Burrel, S.; Roque-Afonso, A.M.; Labetoulle, M. Antiviral effects of Cacicol((R)), a heparan sulfate biomimetic for corneal regeneration therapy, for herpes simplex virus type-1 and varicella zoster virus infection. Antivir. Ther. 2018, 23, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Sebbag, L.; Allbaugh, R.; Strong, T.; Strauss, R.; Wehrman, R.; Foote, B.; Peterson, C.; Ben-Shlomo, G. Lack of effect of a topical regenerative agent on re-epithelialization rate of canine spontaneous chronic corneal epithelial defects: A randomized, double-masked, placebo-controlled study. Vet. J. 2018, 233, 63–65. [Google Scholar] [CrossRef]
- Gumus, K.; Guerra, M.G.; de Melo Marques, S.H.; Karakucuk, S.; Barritault, D. A New Matrix Therapy Agent for Faster Corneal Healing and Less Ocular Discomfort Following Epi-off Accelerated Corneal Cross-linking in Progressive Keratoconus. J. Refract. Surg. 2017, 33, 163–170. [Google Scholar] [CrossRef]
- Robciuc, A.; Arvola, R.P.J.; Jauhiainen, M.; Holopainen, J.M. Matrix regeneration agents improve wound healing in non-stressed human corneal epithelial cells. Eye 2018, 32, 813–819. [Google Scholar] [CrossRef]
- Utine, C.A.; Engin Durmaz, C.; Kocak, N. Corneal matrix repair therapy with the regenerating agent in neurotrophic persistent epithelial defects. Int. J. Ophthalmol. 2017, 10, 1935–1939. [Google Scholar] [CrossRef]
| Dog # | ID# | Age (years) | Breed | Diagnostic | Eye Treated | Duration of Clerapliq® Treatment (day) | Frequency | Failure (Y/N) | Lost to Follow-up (Y/N) | DBD (Y/N) | Clerapliq® after DBD (day) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 12285 | 12.8 | Shih-Tzu | OS healed anterior stromal ulcer | OS | 17 | 1 drop q3d | 0 | 0 | 0 | 0 |
| 2 | 13204 | 10.3 | Boston Terrier | Corneal endothelial dystrophy and persistent epithelial erosion | OD | 35 | 1 drop q3d | 0 | 0 | 1 | 11 |
| 3 | 13256 | 7.2 | Canine | Bullae in temporal paracentral region/persistent epithelial erosion | OD | 6 | 1 drop q3d | 0 | 0 | 1 | 9 |
| 4 | 13206 | 9.3 | Griffon | Corneal endothelial dystrophy OU/persistent epithelial erosion | OS | 27 | 1 drop q3d | 0 | 0 | 1 | 13 |
| 5 | 13201 | 15.4 | Chihuahua | OU corneal endothelial dystrophy prognosis: good keratitis and erosions | OU | 55 | 1 drop q3d | 1 | 0 | 0 | 0 |
| 6 | 13250 | 13.7 | Mix | OU corneal endothelial dystrophy with risk of recurrent erosion; OD corneal epithelial erosion | OD | 40 | 1 drop q3d | 1 | 0 | 1 | 15 |
| 7 | 13266 | 11.6 | Boston Terrier | Corneal endothelial dystrophy with predisposition to persistent erosion | OD | 13 | 1 drop q3d | 0 | 1 | 0 | 0 |
| 8 | 13194 | 13.8 | Canine | Post-op phacoemulsification and lens implant for diabetic cataract and persistent corneal erosion | OU | 14 | 1 drop q3d | 0 | 0 | 0 | 0 |
| 9 | 13241 | 15.3 | Miniature Poodle | OD post-op phacoemulsification and lens implantation; OS hyper-mature cataract and lens induced uveitis; OU severe iris atrophy, suspected nocturnal lagophthalmia and epithelial erosion | OU | 13 | 1 drop q3d | 0 | 0 | 0 | 0 |
| 10 | 12840 | 12.1 | Pomeranian Mix | OS epithelial erosion, immature cataract | OS | 8 | 1 drop q3d | 0 | 1 | 0 | 0 |
| 11 | 11031 | 14.3 | Dachshund | OU post-op phacoemulsification and lens implant, corneal endothelial dystrophy, PRA; OD persistent epithelial erosion | OD | 7 | 1 drop q3d | 0 | 1 | 1 | 7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez, J.A.; Chiappini, F.; Barritault, D. Case Reports for Topical Treatment of Corneal Ulcers with a New Matrix Therapy Agent or RGTA® in Dogs. Vet. Sci. 2019, 6, 103. https://doi.org/10.3390/vetsci6040103
Martinez JA, Chiappini F, Barritault D. Case Reports for Topical Treatment of Corneal Ulcers with a New Matrix Therapy Agent or RGTA® in Dogs. Veterinary Sciences. 2019; 6(4):103. https://doi.org/10.3390/vetsci6040103
Chicago/Turabian StyleMartinez, Jessica A., Franck Chiappini, and Denis Barritault. 2019. "Case Reports for Topical Treatment of Corneal Ulcers with a New Matrix Therapy Agent or RGTA® in Dogs" Veterinary Sciences 6, no. 4: 103. https://doi.org/10.3390/vetsci6040103
APA StyleMartinez, J. A., Chiappini, F., & Barritault, D. (2019). Case Reports for Topical Treatment of Corneal Ulcers with a New Matrix Therapy Agent or RGTA® in Dogs. Veterinary Sciences, 6(4), 103. https://doi.org/10.3390/vetsci6040103




