Metastatic Cardiac Hemangiosarcoma in a 6 Year Old Wheaten Terrier Mix
Abstract
1. Introduction
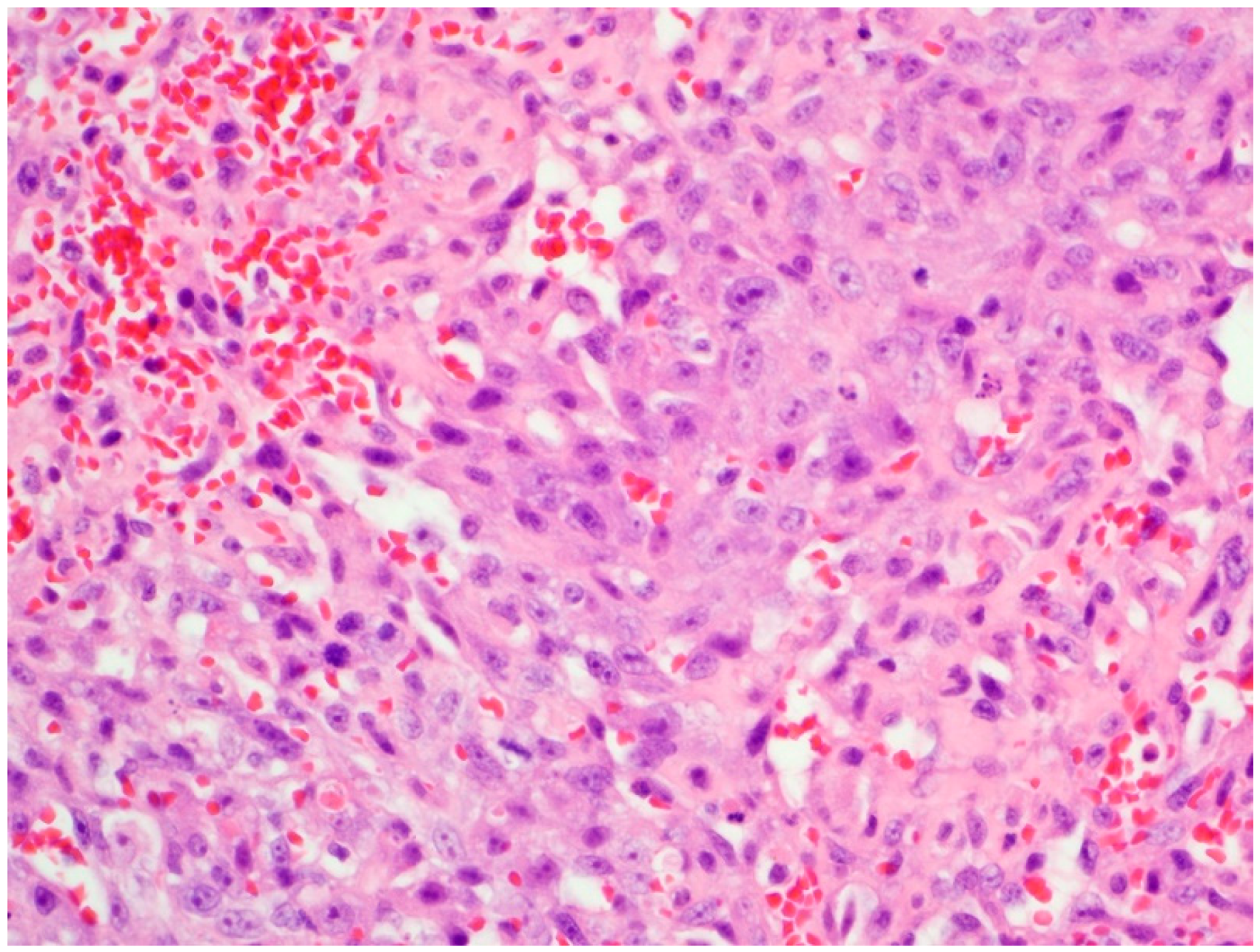
2. Case Presentation
3. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aronsohn, M. Cardiac hemangiosarcoma in the dog: A review of 38 cases. J. Am. Vet. Med. Assoc. 1985, 187, 922–926. [Google Scholar] [PubMed]
- Ware, W.A.; Hopper, D.L. Cardiac tumors in dogs: 1982–1995. J. Vet. Intern. Med. 1999, 13, 95–103. [Google Scholar] [PubMed]
- Aupperle, H.; März, I.; Ellenberger, C.; Buschatz, S.; Reischauer, A.; Schoon, H.A. Primary and secondary heart tumours in dogs and cats. J. Comp. Pathol. 2007, 136, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, V.; Jesty, S.A.; Craig, L.E.; Gompf, R. Comparison of presumptive echocardiographic and definitive diagnoses of cardiac tumors in dogs. J. Vet. Intern. Med. 2013, 27, 1092–1096. [Google Scholar] [CrossRef] [PubMed]
- Treggiari, E.; Pedro, B.; Dukes-McEwan, J.; Gelzer, A.R.; Blackwood, L. A descriptive review of cardiac tumours in dogs and cats. Vet. Comp. Oncol. 2017, 15, 273–288. [Google Scholar] [CrossRef]
- MacDonald, K.A.; Cagney, O.; Magne, M.L. Echocardiographic and clinicopathologic characterization of pericardial effusion in dogs: 107 cases (1985–2006). J. Am. Vet. Med. Assoc. 2009, 235, 1456–1461. [Google Scholar] [CrossRef] [PubMed]
- Nolan, M.W.; Arkans, M.M.; LaVine, D.; DeFrancesco, T.; Myers, J.A.; Griffith, E.H.; Posner, L.P.; Keene, B.W.; Tou, S.P.; Gieger, T.L. Pilot study to determine the feasibility of radiation therapy for dogs with right atrial masses and hemorrhagic pericardial effusion. J. Vet. Cardiol. 2017, 19, 132–143. [Google Scholar] [CrossRef]
- Yamamoto, S.; Hoshi, K.; Hirakawa, A.; Chimura, S.; Kobayashi, M.; Machida, N. Epidemiological, clinical and pathological features of primary cardiac hemangiosarcoma in dogs: A review of 51 cases. J. Vet. Med. Sci. 2013, 75, 1433–1441. [Google Scholar] [CrossRef]
- Mullin, C.M.; Arkans, M.A.; Sammarco, C.D.; Vail, D.M.; Britton, B.M.; Vickery, K.R.; Risbon, R.E.; Lachowicz, J.; Burgess, K.E.; Manley, C.A.; et al. Doxorubicin chemotherapy for presumptive cardiac hemangiosarcoma in dogs (†). Vet. Comp. Oncol. 2016, 14, e171–e183. [Google Scholar] [CrossRef]
- Wykes, P.M.; Rouse, G.P.; Orton, E.C. Removal of five canine cardiac tumors using a stapling instrument. Vet. Surg. 1986, 15, 103–106. [Google Scholar] [CrossRef]
- Weisse, C.; Soares, N.; Beal, M.W.; Steffey, M.A.; Drobatz, K.J.; Henry, C.J. Survival times in dogs with right atrial hemangiosarcoma treated by means of surgical resection with or without adjuvant chemotherapy: 23 cases (1986–2000). J. Am. Vet. Med. Assoc. 2005, 226, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, S.; Pelio, D.C.; Lange, A.J.; Arndt, J.W.; Chretin, J.D.; Fiocchi, S.C.; Bianco, D.; Nakamura, R.K. A retrospective evaluation of doxorubicin-based chemotherapy for dogs with right atrial masses and pericardial effusion. J. Small Anim. Pract. 2014, 55, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Veterinary Co-operative Oncology Group (VCOG). Common terminology criteria for adverse events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.0. Vet. Comp. Oncol. 2004, 2, 195–213. [Google Scholar] [CrossRef] [PubMed]
- Ployart, S.; Libermann, S.; Doran, I.; Bomassi, E.; Monnet, E. Thoracoscopic resection of right auricular masses in dogs: 9 cases (2003–2011). J. Am. Vet. Med. Assoc. 2013, 242, 237–241. [Google Scholar] [CrossRef]
- Verbeke, F.; Binst, D.; Stegen, L.; Waelbers, T.; de Rooster, H.; Van Goethem, B. Total venous inflow occlusion and pericardial auto-graft reconstruction for right atrial hemangiosarcoma resection in a dog. Can. Vet. J. 2012, 53, 1114–1118. [Google Scholar] [PubMed]
- Higashiyama, M.; Tokunaga, T.; Nakagiri, T.; Ishida, D.; Kuno, H.; Okami, J. Pulmonary metastasectomy: Outcomes and issues according to the type of surgical resection. Gen. Thorac. Cardiovasc. Surg. 2015, 63, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Treasure, T.; Milošević, M.; Fiorentino, F.; Macbeth, F. Pulmonary metastasectomy: What is the practice and where is the evidence for effectiveness? Thorax 2014, 69, 946–949. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.G.; Straw, R.C.; Withrow, S.J.; Powers, B.E.; Jameson, V.J.; Lafferty, M.; Ogilvie, G.K.; LaRue, S.M. Resection of pulmonary metastases in canine osteosarcoma: 36 cases (1983–1992). Vet. Surg. 1993, 22, 105–109. [Google Scholar] [CrossRef]
- Salah, S.; Ardissone, F.; Gonzalez, M.; Gervaz, P.; Riquet, M.; Watanabe, K.; Zabaleta, J.; Al-Rimawi, D.; Toubasi, S.; Massad, E.; et al. Pulmonary metastasectomy in colorectal cancer patients with previously resected liver metastasis: Pooled analysis. Ann. Surg. Oncol. 2015, 22, 1844–1850. [Google Scholar] [CrossRef]
- Yu, X.; Wang, B.; Li, X.; Lin, G.; Zhang, C.; Yang, Y.; Fang, D.; Song, Y.; He, Z.; Zhou, L. The Significance of metastasectomy in patients with metastatic renal cell carcinoma in the era of targeted therapy. Biomed. Res. Int. 2015, 2015, 176373. [Google Scholar] [CrossRef]
- Frankot, J.L.; Behrend, E.N.; Sebestyen, P.; Powers, B.E. Adrenocortical carcinoma in a dog with incomplete excision managed long-term with metastasectomy alone. J. Am. Anim. Hosp. Assoc. 2012, 48, 417–423. [Google Scholar] [CrossRef]
- Turner, H.; Seguin, B.; Worley, D.R.; Ehrhart, N.P.; Lafferty, M.H.; Withrow, S.J.; Selmic, L.E. Prognosis for dogs with stage III osteosarcoma following treatment with amputation and chemotherapy with and without metastasectomy. J. Am. Vet. Med. Assoc. 2017, 251, 1293–1305. [Google Scholar] [CrossRef] [PubMed]
- Biller, B. Metronomic chemotherapy in veterinary patients with cancer: Rethinking the targets and strategies of chemotherapy. Vet. Clin. North. Am. Small Anim. Pract. 2014, 44, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, E.; Kavallaris, M.; André, N. Metronomic chemotherapy: New rationale for new directions. Nat. Rev. Clin. Oncol. 2010, 7, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Lana, S.; U'ren, L.; Plaza, S.; Elmslie, R.; Gustafson, D.; Morley, P.; Dow, S. Continuous low-dose oral chemotherapy for adjuvant therapy of splenic hemangiosarcoma in dogs. J. Vet. Intern. Med. 2007, 21, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Finotello, R.; Henriques, J.; Sabattini, S.; Stefanello, D.; Felisberto, R.; Pizzoni, S.; Ferrari, R.; Marconato, L. A retrospective analysis of chemotherapy switch suggests improved outcome in surgically removed, biologically aggressive canine haemangiosarcoma (†). Vet. Comp. Oncol. 2016, 15, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, A.; Poirier, V.J.; Mantovani, F.; Foster, R.A.; Mutsaers, A.J. Adjuvant doxorubicin with or without metronomic cyclophosphamide for canine splenic hemangiosarcoma. J. Am. Anim. Hosp. Assoc. 2017, 53, 304–312. [Google Scholar] [CrossRef]
- Alexander, C.K.; Cronin, K.L.; Silver, M.; Gardner, H.L.; London, C. The addition of metronomic chemotherapy does not improve outcome for canine splenic haemangiosarcoma. J. Small Anim. Pract. 2018, 60, 32–37. [Google Scholar] [CrossRef]
- Wan, J.M.F.; Sit, W.H.; Yang, X.; Jiang, P.; Wong, L.L.Y. Polysaccharopeptides derived from Coriolus versicolor potentiate the S-phase specific cytotoxicity of Camptothecin (CPT) on human leukemia HL-60 cells. Chin. Med. 2010, 5, 16. [Google Scholar] [CrossRef]
- Wang, D.F.; Lou, N.; Li, X.D. Effect of coriolus versicolor polysaccharide-B on the biological characteristics of human esophageal carcinoma cell line eca109. Cancer Biol. Med. 2012, 9, 164–167. [Google Scholar]
- Kowalczewska, M.; Piotrowski, J.; Jędrzejewski, T.; Kozak, W. Polysaccharide peptides from Coriolus versicolor exert differential immunomodulatory effects on blood lymphocytes and breast cancer cell line MCF-7 in vitro. Immunol. Lett. 2016, 174, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Awadasseid, A.; Hou, J.; Gamallat, Y.; Xueqi, S.; Eugene, K.D.; Musa Hago, A.; Bamba, D.; Meyiah, A.; Gift, C.; Xin, Y. Purification, characterization, and antitumor activity of a novel glucan from the fruiting bodies of Coriolus Versicolor. PLoS ONE 2017, 12, e0171270. [Google Scholar] [CrossRef] [PubMed]
- Eliza, W.L.Y.; Fai, C.K.; Chung, L.P. Efficacy of Yun Zhi (Coriolus versicolor) on survival in cancer patients: Systematic review and meta-analysis. Recent Pat. Inflamm. Allergy Drug Discov. 2012, 6, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Fritz, H.; Kennedy, D.A.; Ishii, M.; Fergusson, D.; Fernandes, R.; Cooley, K.; Seely, D. Polysaccharide K and Coriolus versicolor extracts for lung cancer: A systematic review. Integr. Cancer Ther. 2015, 14, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.C.; Reetz, J. Single agent polysaccharopeptide delays metastases and improves survival in naturally occurring hemangiosarcoma. Evid. Based Complement. Altern. Med. 2012, 2012, 384301. [Google Scholar] [CrossRef] [PubMed]
- Chaikin, P.; Welihozkiy, A. Hemangiosarcoma in a dog: Unusual presentation and increased survival using a complementary/holistic approach combined with metronomic chemotherapy. Case Rep. Vet. Med. 2018, 2018, 6160980. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arai, S.; Milley, E.P.; Lichtenberger, J.; Savidge, C.; Lawrence, J.; Côté, E. Metastatic Cardiac Hemangiosarcoma in a 6 Year Old Wheaten Terrier Mix. Vet. Sci. 2019, 6, 65. https://doi.org/10.3390/vetsci6030065
Arai S, Milley EP, Lichtenberger J, Savidge C, Lawrence J, Côté E. Metastatic Cardiac Hemangiosarcoma in a 6 Year Old Wheaten Terrier Mix. Veterinary Sciences. 2019; 6(3):65. https://doi.org/10.3390/vetsci6030065
Chicago/Turabian StyleArai, Shiori, Ellen P Milley, Jonathan Lichtenberger, Christine Savidge, Jessica Lawrence, and Etienne Côté. 2019. "Metastatic Cardiac Hemangiosarcoma in a 6 Year Old Wheaten Terrier Mix" Veterinary Sciences 6, no. 3: 65. https://doi.org/10.3390/vetsci6030065
APA StyleArai, S., Milley, E. P., Lichtenberger, J., Savidge, C., Lawrence, J., & Côté, E. (2019). Metastatic Cardiac Hemangiosarcoma in a 6 Year Old Wheaten Terrier Mix. Veterinary Sciences, 6(3), 65. https://doi.org/10.3390/vetsci6030065




