Long-Term Survival of a Cat with Primary Leiomyosarcoma of the Urinary Bladder
Abstract
:1. Introduction
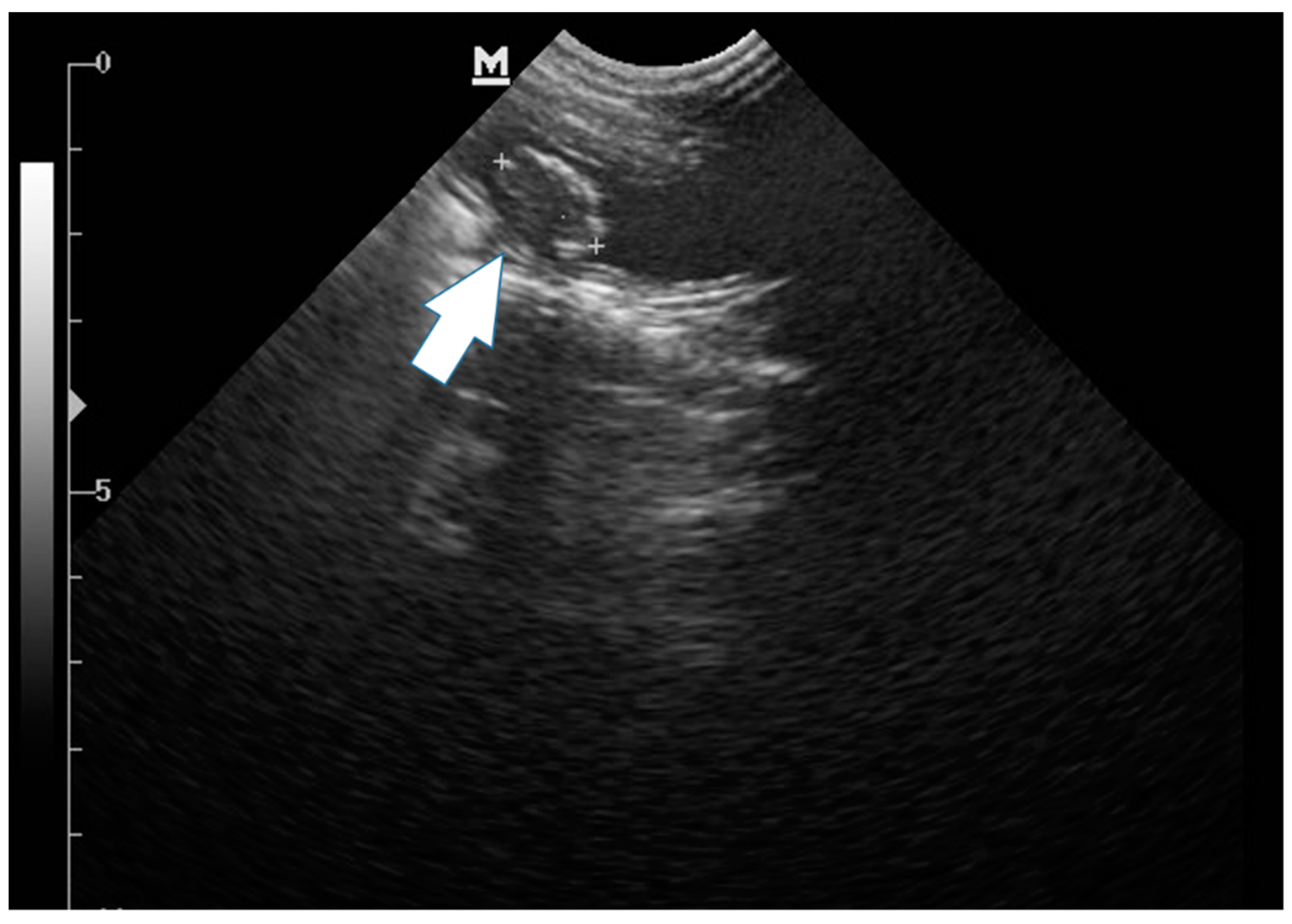
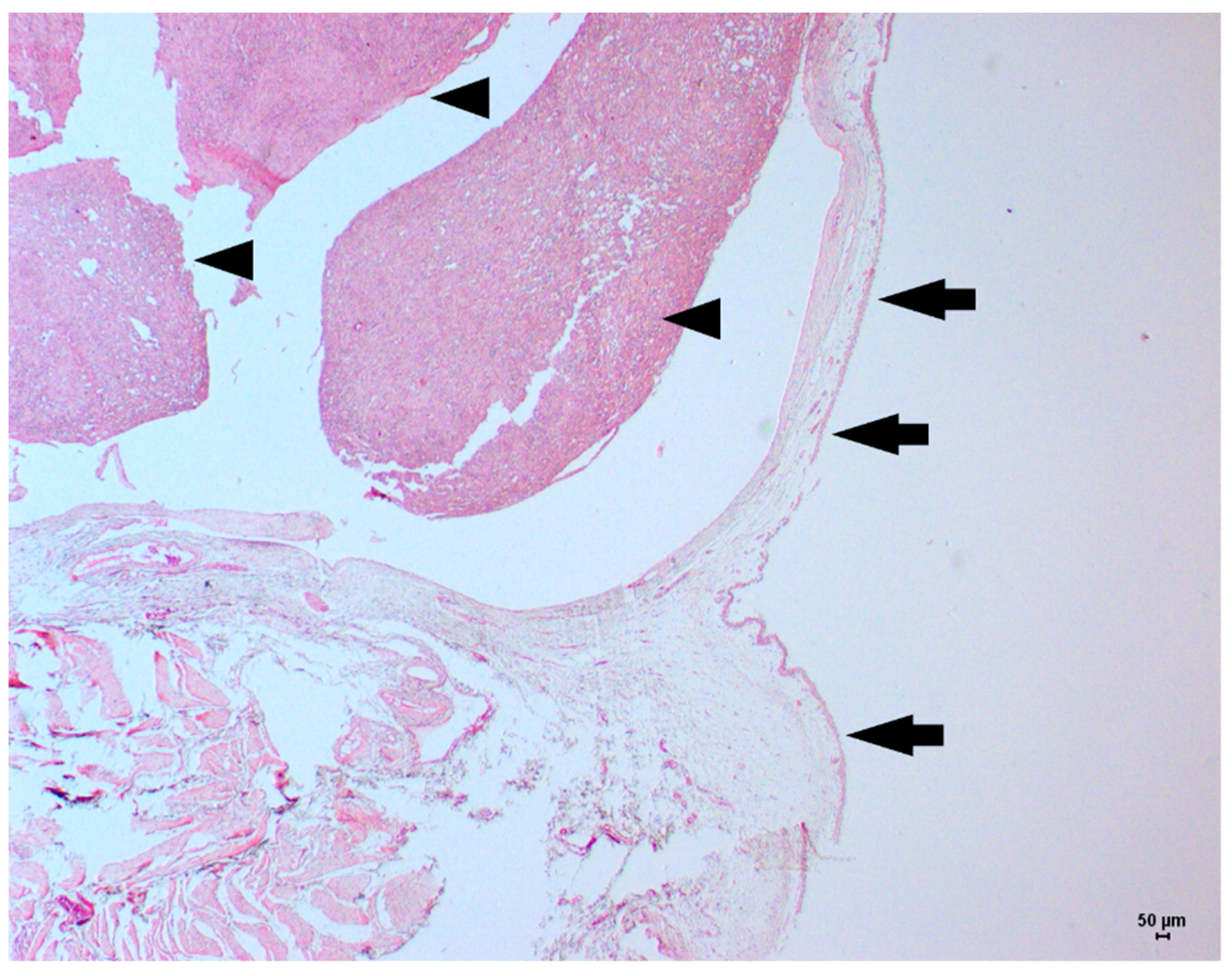
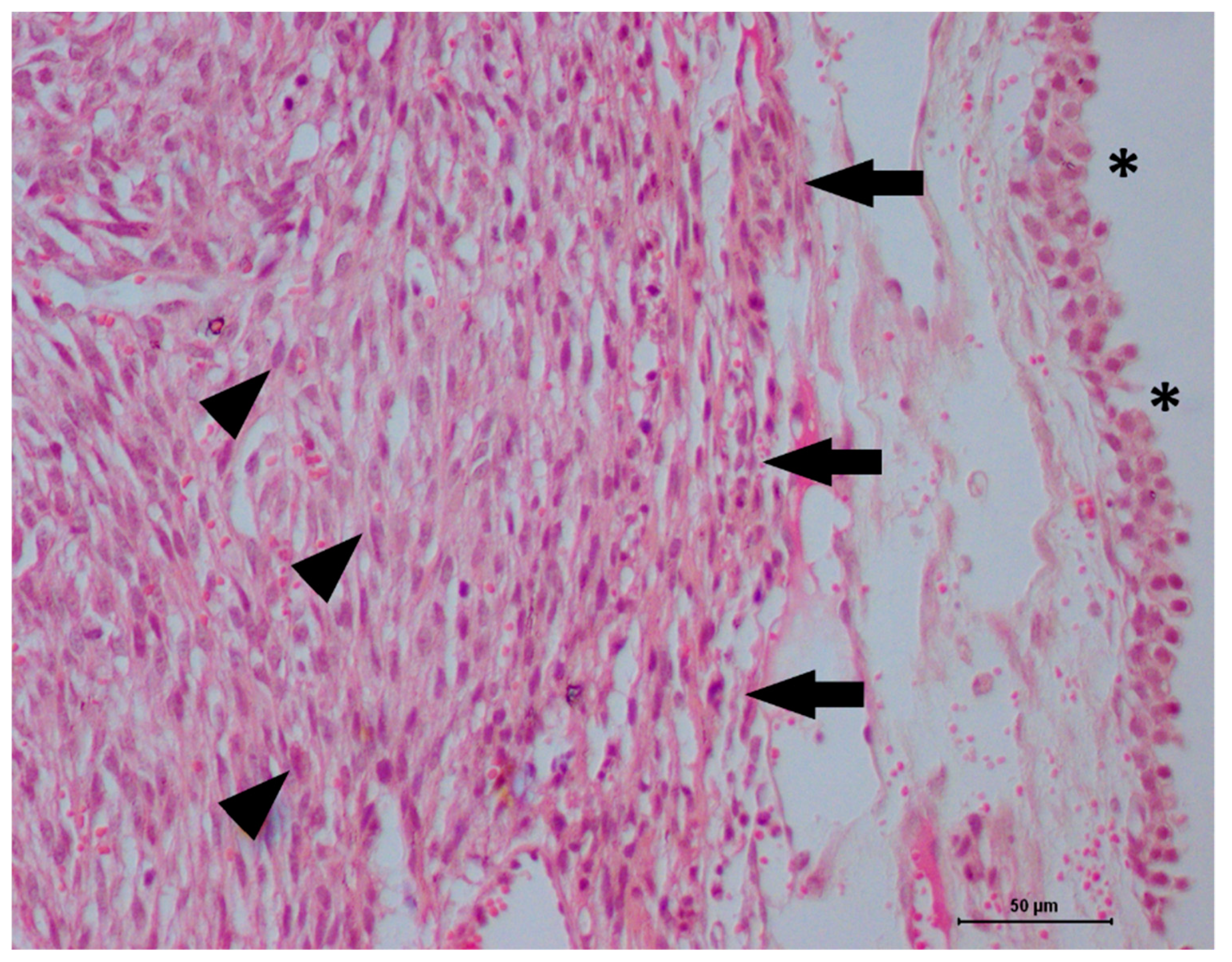
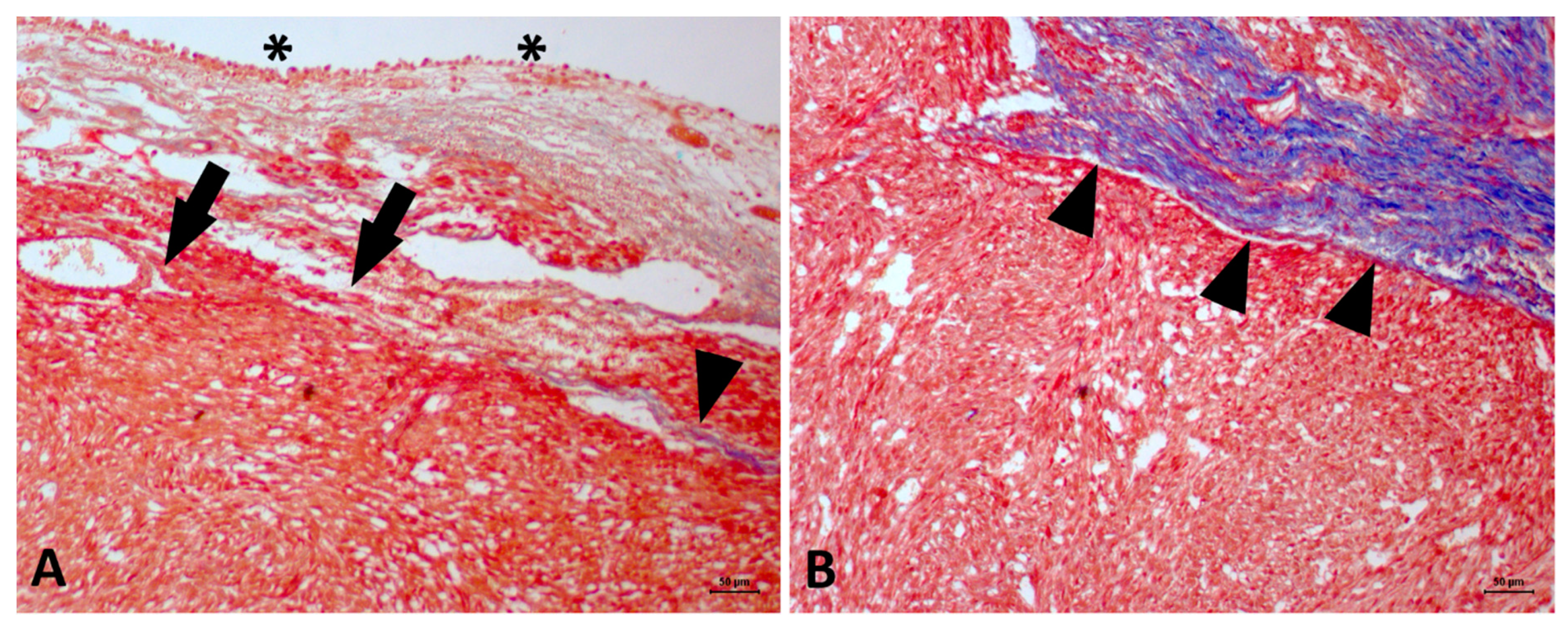
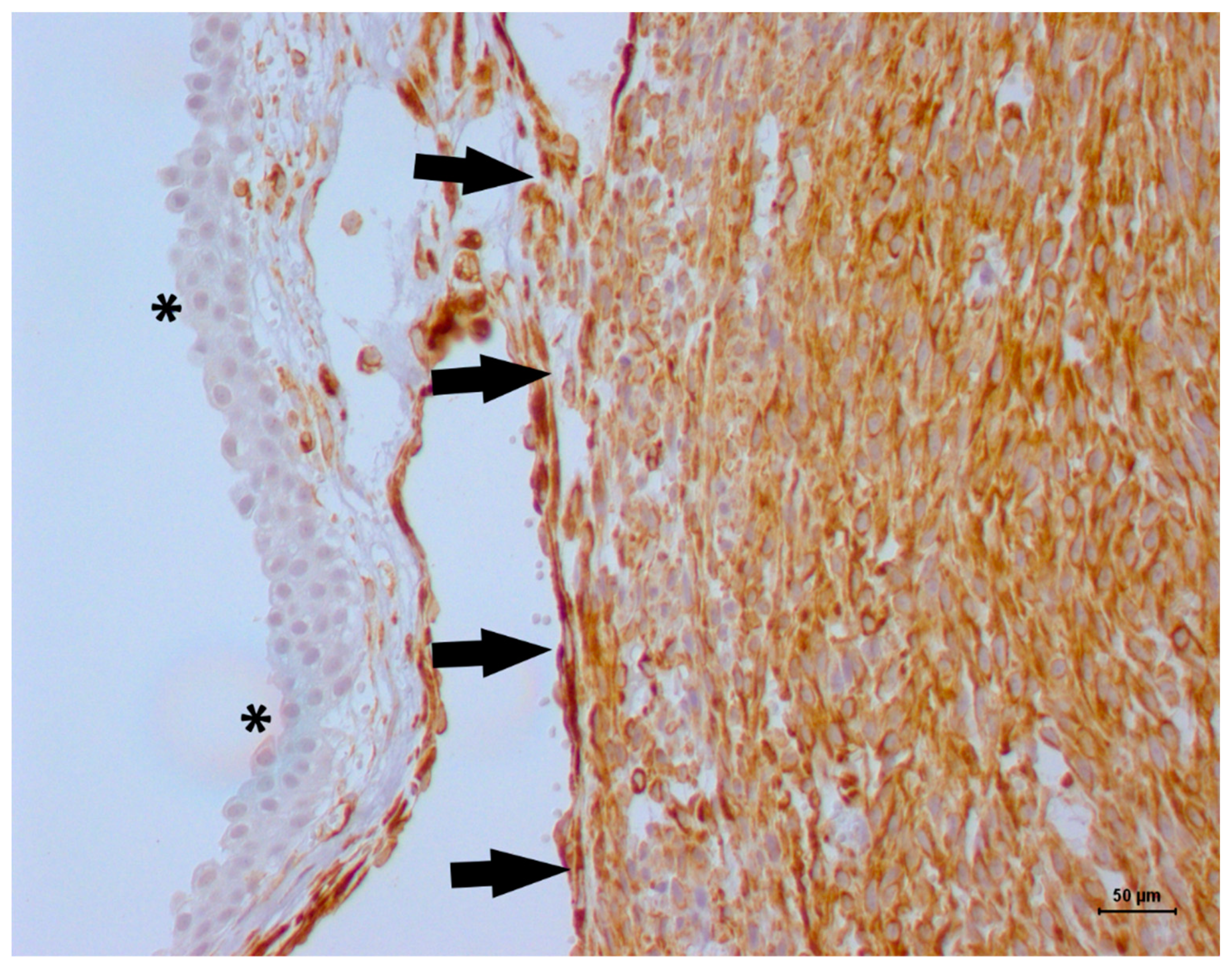
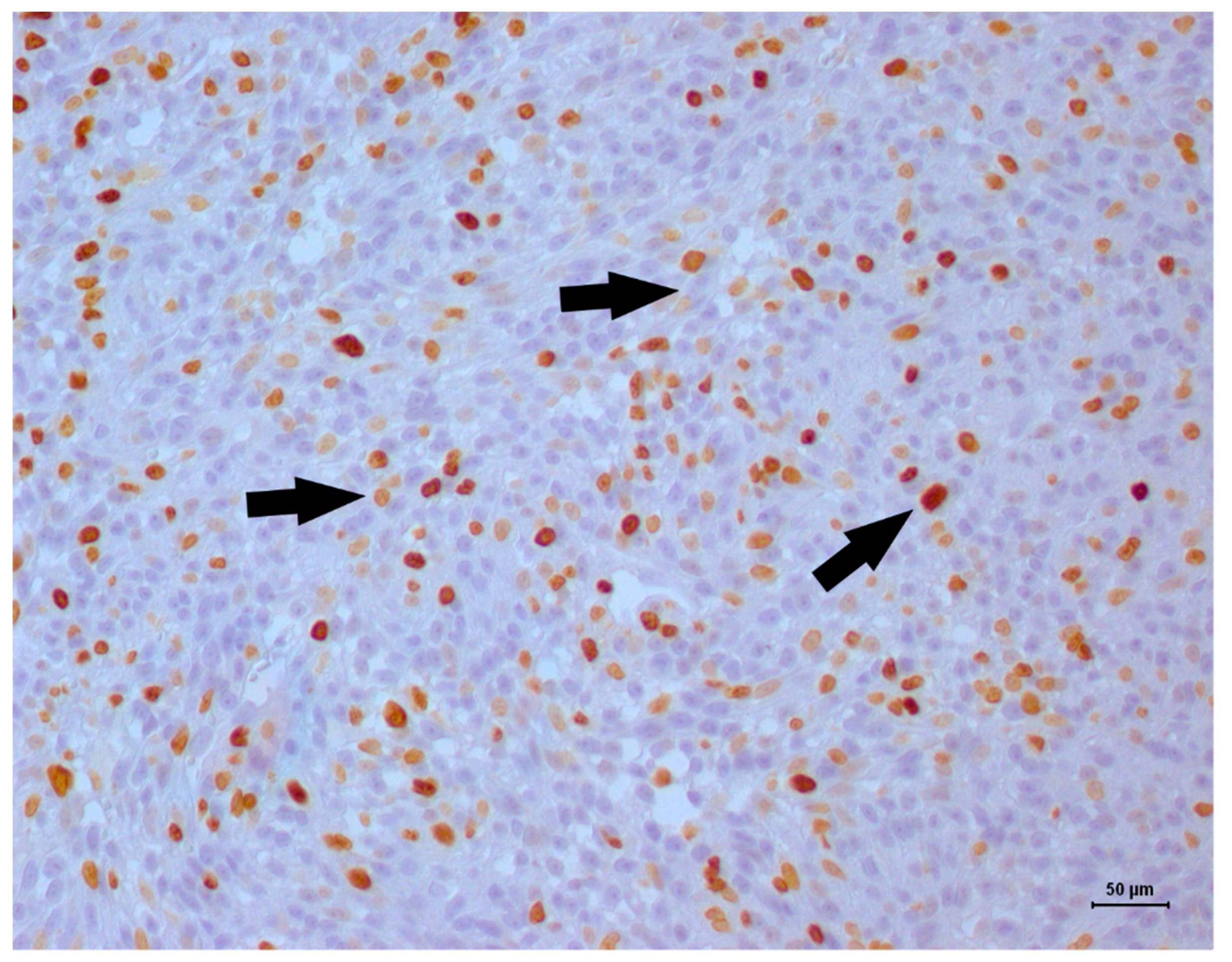
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wilson, H.M.; Chun, R.; Larson, V.S.; Kurzman, I.D.; Vail, D.M. Clinical signs, treatments, and outcome in cats with transitional cell carcinoma of the urinary bladder: 20 cases (1990–2004). J. Am. Vet. Med. Assoc. 2007, 231, 101–106, Erratum in: J. Am. Vet. Med. Assoc. 2007, 231, 1533. [Google Scholar] [CrossRef] [PubMed]
- Wimberly, H.C.; Lewis, R.M. Transitional cell carcinoma in the domestic cat. Vet. Pathol. 1979, 16, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, A.K.; Schwarz, P.D.; Greene, R.W. A histopathologic study of twenty urinary bladder neoplasms in the cat. J. Small Anim. Pract. 1986, 27, 433–445. [Google Scholar] [CrossRef]
- Khodakaram-Tafti, A.; Shirian, S.; Vesal, N.; Hadadi, S. Lipoma of the urinary bladder in a cat. J. Comp. Pathol. 2011, 144, 212–213. [Google Scholar] [CrossRef] [PubMed]
- Geigy, C.A.; Dandrieux, J.; Miclard, J.; Kircher, P.; Howard, J. Extranodal B-cell lymphoma in the urinary bladder with cytological evidence of concurrent involvement of the gall bladder in a cat. J. Small Anim. Pract. 2010, 51, 280–287. [Google Scholar] [CrossRef] [PubMed]
- House, H.B.; Specht, A.J.; Johnson, V.S. What is your diagnosis? Lymphoma of the urinary bladder. J. Am. Vet. Med. Assoc. 2010, 236, 291–292. [Google Scholar] [CrossRef] [PubMed]
- Greci, V.; Rocchi, P.M.; Sontuoso, A.F.; Olivero, D.; Capasso, A.; Raiano, V. Primary fibrosarcoma of the urinary bladder in a cat: Follow-up after incomplete surgical excision. JFMS Open Rep. 2017, 3. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, A.K.; Greene, R.W. Intravenous leiomyoma of the bladder in a cat. J. Am. Vet. Med. Assoc. 1979, 175, 381–383. [Google Scholar] [PubMed]
- Burk, R.L.; Meierhenry, E.F.; Schaubhut, C.W., Jr. Leiomyosarcoma of the urinary bladder in a cat. J. Am. Vet. Med. Assoc. 1975, 167, 749–751. [Google Scholar] [PubMed]
- Alves, R.C.C.; Batista, T.L.; Laufer-Amorim, R.; Elias, F.; Calazans, S.G.; Fonseca-Alves, C.E. Clinicopathological and immunohistochemical evaluation of oesophageal leiomyosarcoma in a dog. Ciênc. Rural. 2015, 45, 1644–1647. [Google Scholar] [CrossRef] [Green Version]
- Gakis, G. The role of inflammation in bladder cancer. Adv. Exp. Med. Biol. 2014, 816, 183–196. [Google Scholar] [PubMed]
- Lippi, I.; Mannucci, T.; Santa, D.D.; Barella, G.; Oranges, M.; Citi, S. Emphysematous cystitis: Retrospective evaluation of predisposing factors and ultrasound features in 36 dogs and 2 cats. Can. Vet. J. 2019, 60, 514–518. [Google Scholar] [PubMed]
- Capasso, A.; Raiano, V.; Sontuoso, A.; Olivero, D.; Greci, V. Fibrosarcoma of the urinary bladder in a cat. JFMS Open Rep. 2015, 1. [Google Scholar] [CrossRef] [PubMed]
| Antibody | Dilution | Clone | Manufacturer |
|---|---|---|---|
| S100 | 1:400 | Polyclonal | Dako Cytomation (Carpinteria, CA, USA) |
| MyoD1 | 1:200 | 5.8A | Novocastra (Wetzlar, Germany) |
| Pan-cytokeratin | 1:300 | AE1/AE3 | Invitrogen (Carlsbad, CA, USA) |
| Vimentin | 1:300 | V3 | Invitrogen (Carlsbad, CA, USA) |
| Desmin | 1:100 | D33 | Dako Cytomation (Carpinteria, CA, USA) |
| Alpha-actin | 1:100 | 1A4 | Dako Cytomation (Carpinteria, CA, USA) |
| Ki67 | 01:50 | MIB1 | Dako Cytomation (Carpinteria, CA, USA) |
| Reference | Primary Diagnosis | Number of Cases | Treatment | Overall Survival |
|---|---|---|---|---|
| Patnaik and Greene [8] | Leiomyoma | 1 | Surgery | 25 months |
| Burk et al. [9] | Leiomyosarcoma | 1 | Proteolytic enzymes | 1 month |
| Patnaik et al. [3] | Leiomyosarcoma | 2 | ND | ND |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buzatto, A.B.; Elias, F.; Franzoni, M.S.; Fonseca-Alves, C.E. Long-Term Survival of a Cat with Primary Leiomyosarcoma of the Urinary Bladder. Vet. Sci. 2019, 6, 60. https://doi.org/10.3390/vetsci6030060
Buzatto AB, Elias F, Franzoni MS, Fonseca-Alves CE. Long-Term Survival of a Cat with Primary Leiomyosarcoma of the Urinary Bladder. Veterinary Sciences. 2019; 6(3):60. https://doi.org/10.3390/vetsci6030060
Chicago/Turabian StyleBuzatto, Anneliese Baetz, Fabiana Elias, Mayara Simão Franzoni, and Carlos Eduardo Fonseca-Alves. 2019. "Long-Term Survival of a Cat with Primary Leiomyosarcoma of the Urinary Bladder" Veterinary Sciences 6, no. 3: 60. https://doi.org/10.3390/vetsci6030060
APA StyleBuzatto, A. B., Elias, F., Franzoni, M. S., & Fonseca-Alves, C. E. (2019). Long-Term Survival of a Cat with Primary Leiomyosarcoma of the Urinary Bladder. Veterinary Sciences, 6(3), 60. https://doi.org/10.3390/vetsci6030060






