Characterisation of the First Bovine Parainfluenza Virus 3 Isolate Detected in Cattle in Turkey
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Material
2.2. Preparation of Lung Homogenate
2.3. Viral and Bacterial Culture
2.4. Antigen ELISA
2.5. RT-PCR Detection
2.6. Sequencing and Phylogenetic Analysis
3. Results
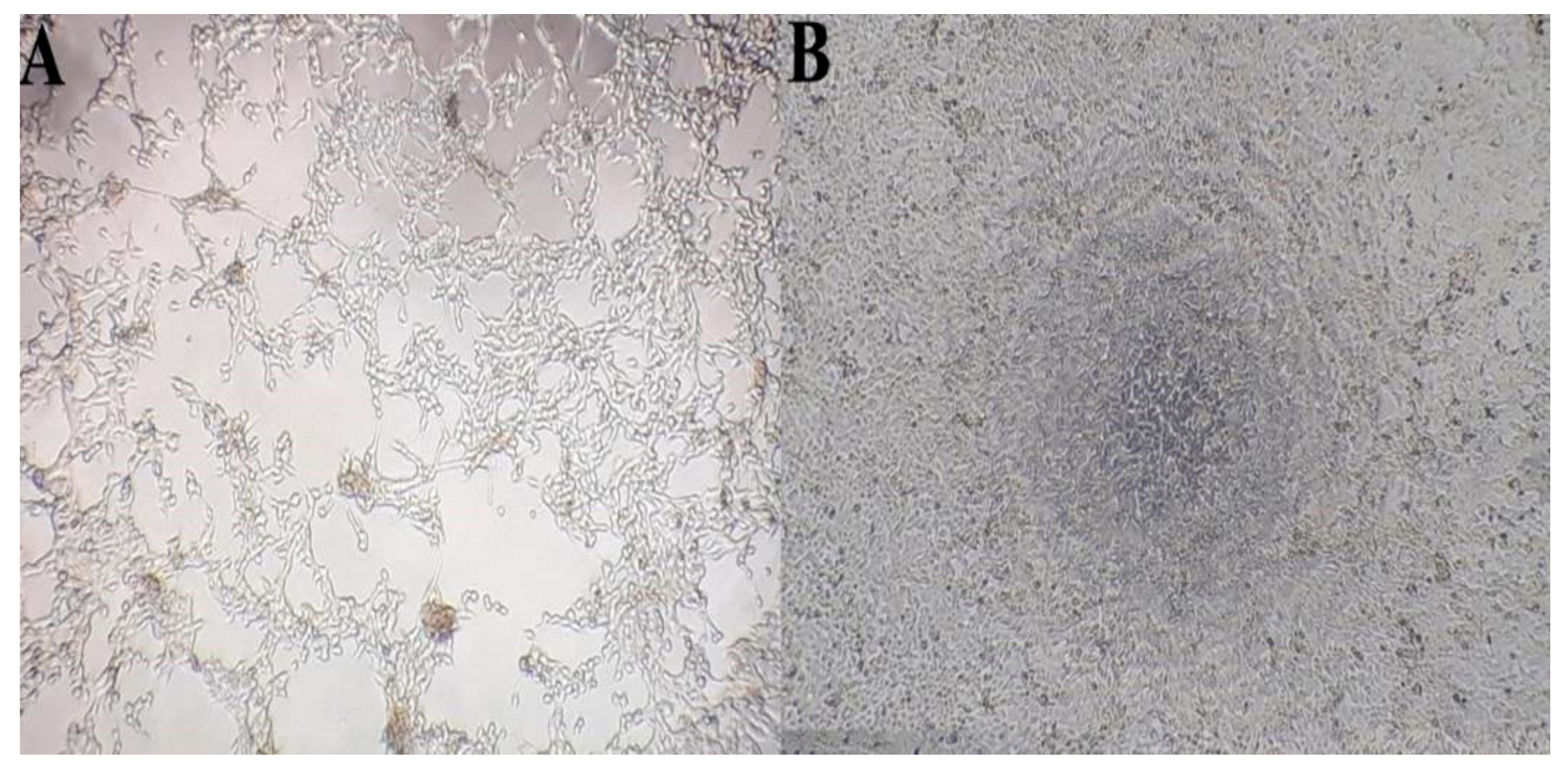
3.1. Laboratory Results
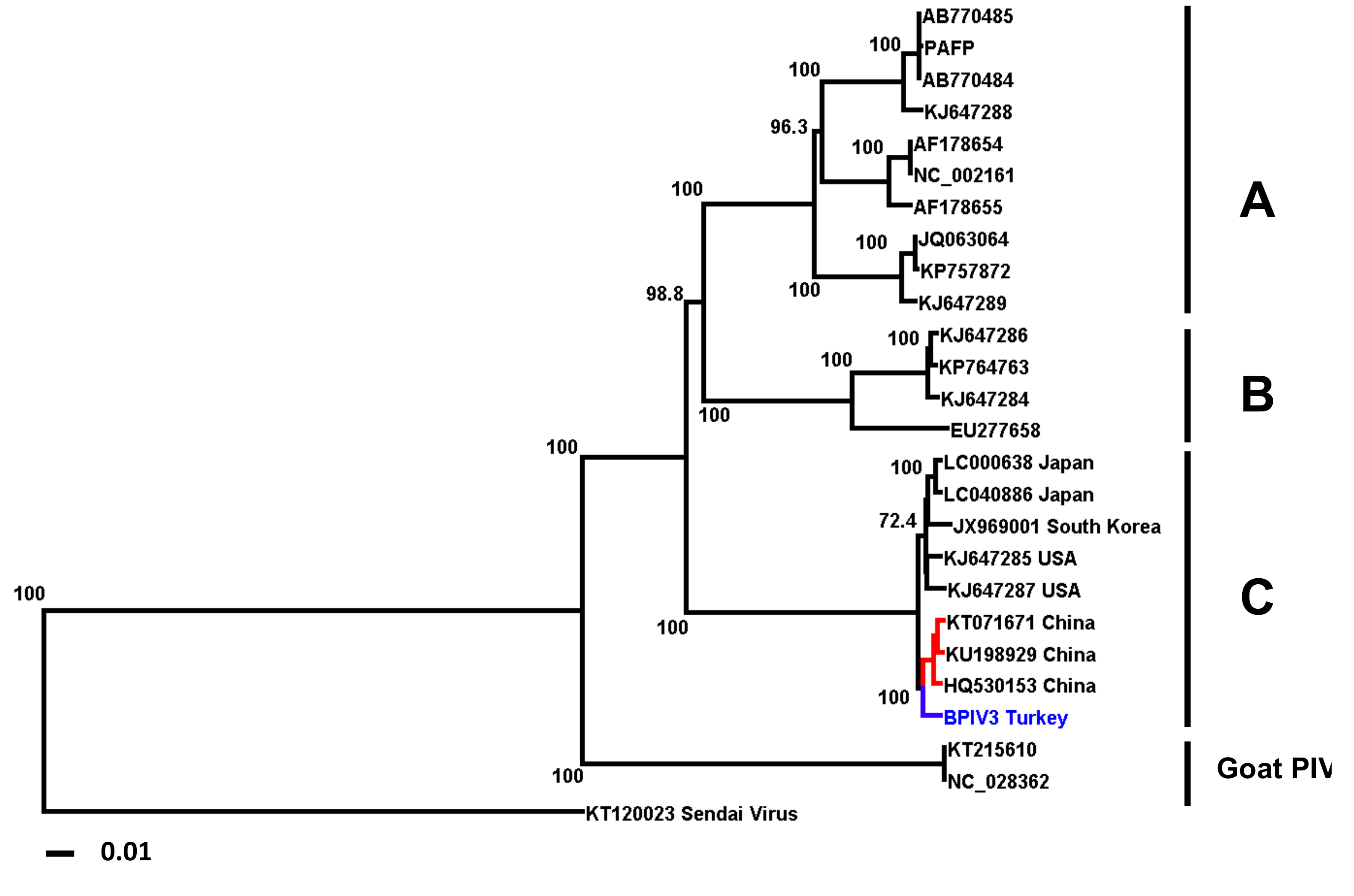
3.2. Sequencing and Phylogenetic Analysis
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Fulton, R.W.; Neill, J.D.; Saliki, J.T.; Landis, C.; Burge, L.J.; Payton, M.E. Genomic and antigenic characterization of bovine parainfluenza-3 viruses in the United States including modified live virus vaccine (MLV) strains and field strains from cattle. Virus Res. 2017, 235, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.M.; Shi, H.F.; Gao, Y.R.; Xin, J.Q.; Liu, N.H.; Xiang, W.H.; Ren, X.G.; Feng, J.K.; Zhao, L.P.; Xue, F. Isolation and genetic characterization of bovine parainfluenza virus type 3 from cattle in China. Vet. Microbiol. 2011, 149, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Oem, J.K.; Lee, E.Y.; Lee, K.K.; Kim, S.H.; Lee, M.H.; Hyun, B.H. Molecular characterization of a Korean bovine parainfluenza virus type 3 isolate. Vet. Microbiol. 2013, 162, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Horwood, P.F.; Gravel, J.L.; Mahony, T.J. Identification of two distinct bovine parainfluenza virus type 3 genotypes. J. Gen. Virol. 2008, 89, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Newcomer, B.W.; Neill, J.D.; Galik, P.K.; Riddell, K.P.; Zhang, Y.; Passler, T.; Velayudhan, B.T.; Walz, P.H. Serologic survey for antibodies against three genotypes of bovine parainfluenza 3 virus in unvaccinated ungulates in Alabama. Am. J. Vet. Res. 2017, 78, 239–243. [Google Scholar] [CrossRef]
- Neill, J.D.; Ridpath, J.F.; Valayudhan, B.T. Identification and genome characterization of genotype B and genotype C bovine parainfluenza type 3 viruses isolated in the United States. BMC Vet. Res. 2015, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Sobhy, N.M.; Mor, S.K.; Bastawecy, I.M.; Fakhry, H.M.; Youssef, C.R.B.; Goyal, S.M. Surveillance, isolation and complete genome sequence of bovine parainfluenza virus type 3 in Egyptian cattle. Int. J. Vet. Sci. Med. 2017, 5, 8–13. [Google Scholar] [CrossRef]
- Konishi, M.; Ohkura, T.; Shimizu, M.; Akiyama, M.; Kameyama, K.; Takeuchi, K. Complete genome sequence of the first isolate of genotype C bovine parainfluenza virus type 3 in Japan. Genome Announc. 2014, 2. [Google Scholar] [CrossRef]
- Maidana, S.S.; Lomonaco, P.M.; Combessies, G.; Craig, M.I.; Diodati, J.; Rodriguez, D.; Parreno, V.; Zabal, O.; Konrad, J.L.; Crudelli, G.; et al. Isolation and characterization of bovine parainfluenza virus type 3 from water buffaloes (Bubalus bubalis) in Argentina. BMC Vet. Res. 2012, 8. [Google Scholar] [CrossRef]
- Stevenson, R.G.; Hore, D.E. Comparative Pathology of Lambs and Calves Infected with Parainfluenza Virus Type-3. J. Comp. Pathol. 1970, 80, 613. [Google Scholar] [CrossRef]
- Ben-Ishai, Z.; Naftali, V.; Avram, A.; Yatziv, S. Human infection by a bovine strain of parainfluenza virus type 3. J. Med. Virol. 1980, 6, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Alkan, F.; Ozkul, A.; Bilge-Dagalp, S.; Yesilbag, K.; Oguzoglu, T.C.; Akca, Y.; Burgu, I. Virological and serological studies on the role of PI-3 virus, BRSV, BVDV and BHV-1 on respiratory infections of cattle. I. The detection of etiological agents by direct immunofluorescence technique. Deut. Tierarztl. Woch. 2000, 107, 193–195. [Google Scholar]
- Yesilbag, K.; Gungor, B. Seroprevalence of bovine respiratory viruses in North-Western Turkey. Trop. Anim. Health Prod. 2008, 40, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Ozkul, A.; Yesilbag, K.; Burgu, I. Comparison of four diagnostic techniques for detecting bovine virus diarrhoea virus (BVDV) in buffy coat samples after long-term storage. Turk. J. Vet. Anim. Sci. 2002, 26, 1043–1048. [Google Scholar]
- Lyon, M.; Leroux, C.; Greenland, T.; Chastang, J.; Patet, J.; Mornex, J.F. Presence of a unique parainfluenza virus 3 strain identified by RT-PCR in visna-maedi virus infected sheep. Vet. Microbiol. 1997, 57, 95–104. [Google Scholar] [CrossRef]
- Schmieder, R.; Edwards, R. Quality control and preprocessing of metagenomic datasets. Bioinformatics 2011, 27, 863–864. [Google Scholar] [CrossRef]
- Crusoe, M.R.; Alameldin, H.F.; Awad, S.; Boucher, E.; Caldwell, A.; Cartwright, R.; Charbonneau, A.; Constantinides, B.; Edvenson, G.; Fay, S.; et al. The khmer software package: Enabling efficient nucleotide sequence analysis. F1000Research 2015, 4, 900. [Google Scholar] [CrossRef] [PubMed]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. A J. Comput. Mol. Cell Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Huson, D.H.; Richter, D.C.; Rausch, C.; Dezulian, T.; Franz, M.; Rupp, R. Dendroscope: An interactive viewer for large phylogenetic trees. BMC Bioinform. 2007, 8, 460. [Google Scholar] [CrossRef]
- Ide, P.R. Developments in veterinary science. The etiology of enzootic pneumonia of calves. Can. Vet. J. 1970, 11, 194–202. [Google Scholar] [PubMed]
- Haanes, E.J.; Guimond, P.; Wardley, R. The bovine parainfluenza virus type-3 (BPIV-3) hemagglutinin/neuraminidase glycoprotein expressed in baculovirus protects calves against experimental BPIV-3 challenge. Vaccine 1997, 15, 730. [Google Scholar] [CrossRef]
- Snowder, G.D.; Van Vleck, L.D.; Cundiff, L.V.; Bennett, G.L. Bovine respiratory disease in feedlot cattle: Environmental, genetic, and economic factors. J. Anim. Sci. 2006, 84, 1999–2008. [Google Scholar] [CrossRef] [PubMed]
- Timurkan, M.O.; Aydin, H.; Sait, A. Identification and molecular characterisation of bovine parainfluenza virus-3 and bovine respiratory syncytial virus: First report from Turkey. J. Vet. Res. 2019, 63. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albayrak, H.; Yazici, Z.; Ozan, E.; Tamer, C.; Abd El Wahed, A.; Wehner, S.; Ulrich, K.; Weidmann, M. Characterisation of the First Bovine Parainfluenza Virus 3 Isolate Detected in Cattle in Turkey. Vet. Sci. 2019, 6, 56. https://doi.org/10.3390/vetsci6020056
Albayrak H, Yazici Z, Ozan E, Tamer C, Abd El Wahed A, Wehner S, Ulrich K, Weidmann M. Characterisation of the First Bovine Parainfluenza Virus 3 Isolate Detected in Cattle in Turkey. Veterinary Sciences. 2019; 6(2):56. https://doi.org/10.3390/vetsci6020056
Chicago/Turabian StyleAlbayrak, Harun, Zafer Yazici, Emre Ozan, Cuneyt Tamer, Ahmed Abd El Wahed, Stefanie Wehner, Kristina Ulrich, and Manfred Weidmann. 2019. "Characterisation of the First Bovine Parainfluenza Virus 3 Isolate Detected in Cattle in Turkey" Veterinary Sciences 6, no. 2: 56. https://doi.org/10.3390/vetsci6020056
APA StyleAlbayrak, H., Yazici, Z., Ozan, E., Tamer, C., Abd El Wahed, A., Wehner, S., Ulrich, K., & Weidmann, M. (2019). Characterisation of the First Bovine Parainfluenza Virus 3 Isolate Detected in Cattle in Turkey. Veterinary Sciences, 6(2), 56. https://doi.org/10.3390/vetsci6020056







