In Vitro Anthelmintic Activity of Saponins Derived from Medicago spp. Plants against Donkey Gastrointestinal Nematodes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material Extraction, Purification, and Characterization of Saponins
2.2. Nematode Egg Collection, Purification, and Suspension
2.3. Evaluation of the In Vitro Anthelmintic Activity of Medicago Saponin Extracts
2.4. Statistical Analysis
2.5. Ethical Declaration
3. Results
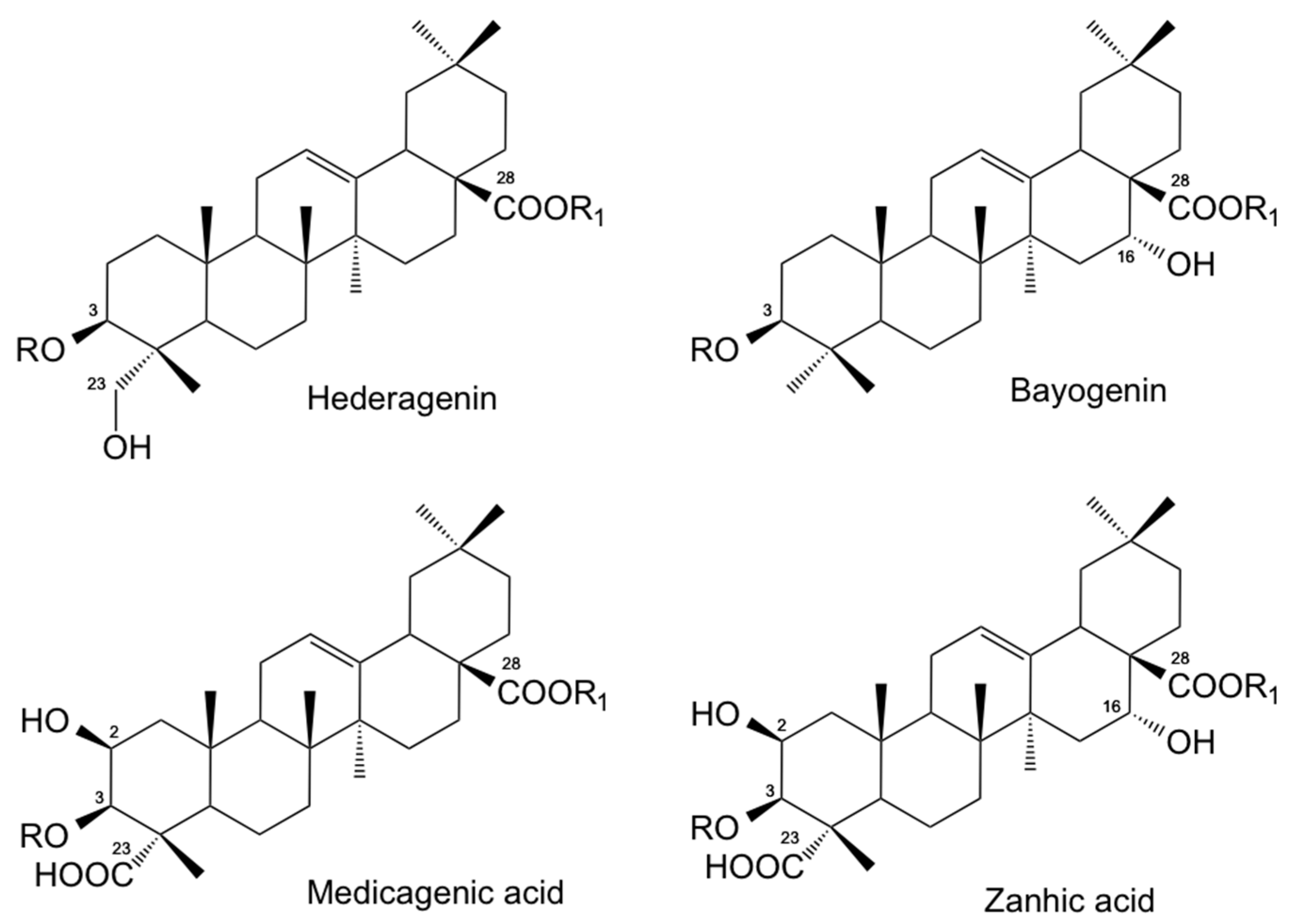
3.1. Composition of Medicago Saponins
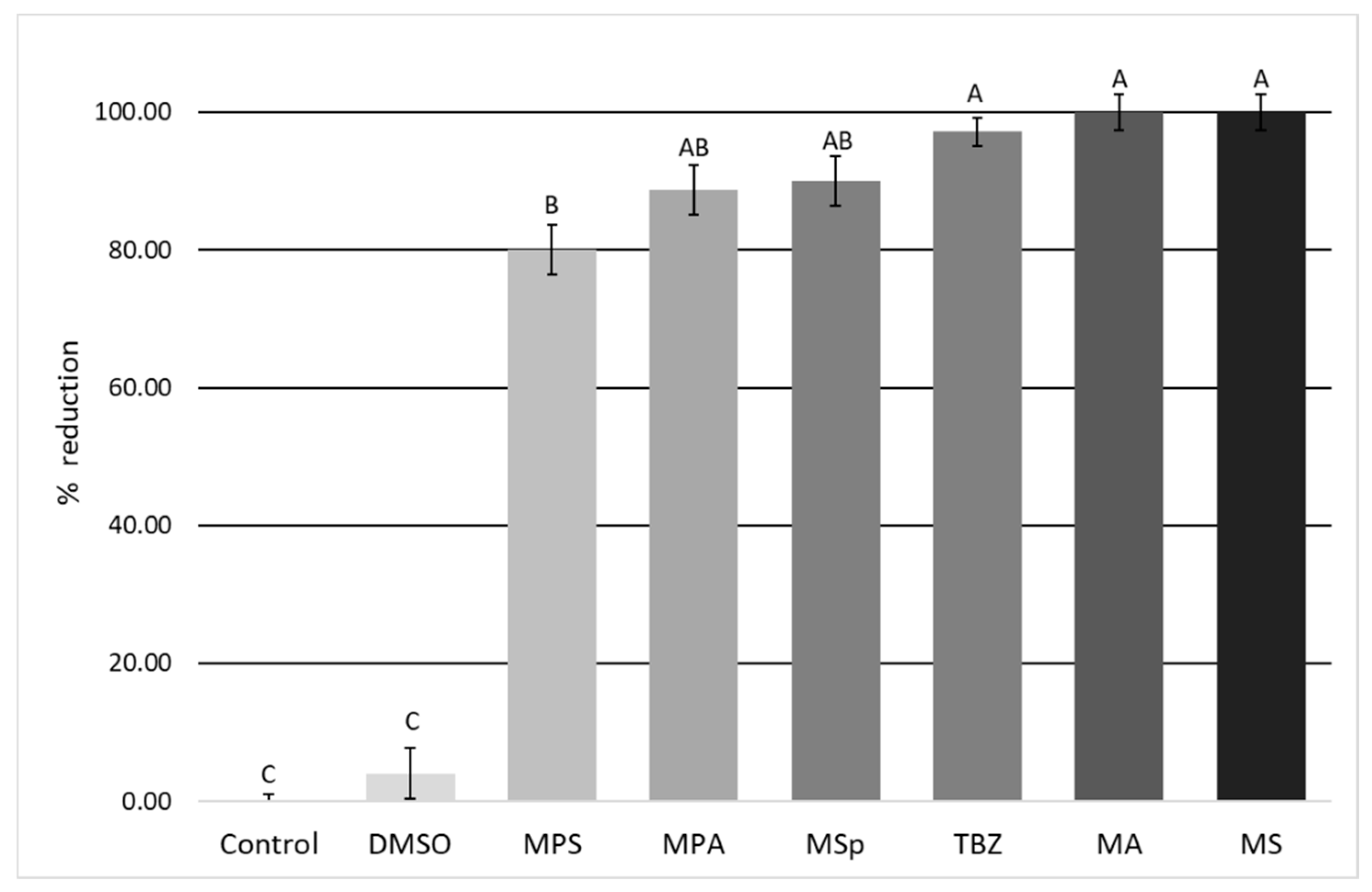
3.2. Evaluation of the In Vitro Anthelmintic Activity of Plant Extracts
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Camillo, F.; Rota, A.; Biagini, L.; Tesi, M.; Fanelli, D.; Panzani, D. The current situation and trend of donkey industry in Europe. J. Equine Vet. Sci. 2018, 65, 44–49. [Google Scholar] [CrossRef]
- Matthews, J.B.; Burden, F.A. Common helminth infections of donkeys and their control in temperate regions. Equine Vet. Educ. 2013, 25, 461–467. [Google Scholar] [CrossRef]
- Traversa, D.; von Samson-Himmelstjerna, G.; Demeler, J.; Milillo, P.; Schürmann, S.; Barnes, H.; Otranto, D.; Perrucci, S.; Frangipane di Regalbono, A.; Beraldo, P.; et al. Anthelmintic resistance in cyathostomin populations from horse yards in Italy, United Kingdom and Germany. Parasit. Vectors 2009, 2, S2. [Google Scholar] [CrossRef]
- Whelan, M.; Kinsella, B.; Furey, A.; Moloney, M.; Cantwell, H.; Lehotay, S.J.; Danaher, M. Determination of anthelmintic drug residues in milk using ultra high performance liquid chromatography-tandem mass spectrometry with rapid polarity switching. J. Chromatogr. A. 2010, 1217, 4612–4622. [Google Scholar] [CrossRef]
- Fernandes, M.A.M.; Gilaverte, S.; Bianchi, M.D.; da Silva, C.J.A.; Molento, B.M.; Reyes, F.G.R.; Monteiro, A.L.G. Moxidectin residues in tissues of lambs submitted to three endoparasite control programs. Res. Vet. Sci. 2017, 114, 406–411. [Google Scholar] [CrossRef]
- Gokbulut, C.; Aksit, D.; Santoro, M.; Roncoroni, C.; Mariani, U.; Buono, F.; Rufrano, D.; Fagiolo, A.; Veneziano, V. Plasma disposition, milk excretion and parasitological efficacy of mebendazole in donkeys naturally infected by Cyathostominae. Vet. Parasitol. 2016, 217, 95–100. [Google Scholar] [CrossRef]
- Nielsen, M.K.; Reinemeyer, C.R.; Donecker, J.M.; Leathwickd, D.M.; Marchiondo, A.A.; Kaplan, R.M. Anthelmintic resistance in equine parasites—Current evidence and knowledge gaps. Vet. Parasitol. 2014, 204, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Hernández, J.Á.; Sánchez-Andrade, R.; Cazapal-Monteiro, C.F.; Arroyo, F.L.; Sanchís, J.M.; Paz-Silva, A.; Arias, M.S. A combined effort to avoid strongyle infection in horses in an oceanic climate region: rotational grazing and parasiticidal fungi. Parasit. Vectors. 2018, 11, 240. [Google Scholar] [CrossRef]
- Tavassoli, M.; Jalilzadeh-Amin, G.; Besharati Fard, V.R.; Esfandiarpour, R. The in vitro effect of Ferula asafoetida and Allium sativum extracts on Strongylus spp. Ann. Parasitol. 2018, 64, 59–63. [Google Scholar] [PubMed]
- Oliveira, A.F.; Costa Junior, L.M.; Lima, A.S.; Silva, C.R.; Ribeiro, M.N.S.; Mesquista, J.W.C.; Rocha, C.Q.; Tangerina, M.M.P.; Vilegas, W. Anthelmintic activity of plant extracts from Brazilian savanna. Vet. Parasitol. 2017, 236, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Gomes, D.C.; de Lima, H.G.; Vaz, A.V.; Santos, N.S.; Santos, F.O.; Dias, Ê.R.; Botura, M.B.; Branco, A.; Batatinha, M.J. In vitro anthelmintic activity of the Zizyphus joazeiro bark against gastrointestinal nematodes of goats and its cytotoxicity on Vero cells. Vet. Parasitol. 2016, 226, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.C.V.; Santos, F.O.; Lima, H.G.; Silva, G.D.D.; Uzêda, R.S.; Dias, Ê.R.; Branco, A.; Cardoso, K.V.; David, J.M.; Botura, M.B.; et al. In vitro ovicidal and larvicidal activities of some saponins and flavonoids against parasitic nematodes of goats. Parasitol. 2018, 145, 1884–1889. [Google Scholar] [CrossRef]
- Coles, G.C.; Jackson, F.; Pomroy, W.E.; Prichard, R.K.; von Samson-Himmelstjerna, G.; Silvestre, A.; Taylor, M.A.; Vercruysse, J. The detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 2006, 136, 167–185. [Google Scholar] [CrossRef]
- Von Samson-Himmelstjerna, G.; Coles, G.C.; Jackson, F.; Bauer, C.; Borgsteede, F.; Cirak, V.Y.; Demeler, J.; Donnan, A.; Dorny, P.; Epe, C.; et al. Standardization of the egg hatch test for the detection of benzimidazole resistance in parasitic nematodes. Parasitol. Res. 2009, 105, 825–834. [Google Scholar] [CrossRef]
- Tava, A.; Avato, P. Chemical and biological activity of triterpene saponins from Medicago species. Nat. Prod. Commun. 2006, 1, 1159–1180. [Google Scholar] [CrossRef]
- Doligalska, M.; Jóźwicka, K.; Kiersnowska, M.; Mroczek, A.; Pączkowski, C.; Janiszowska, W. Triterpenoid saponins affect the function of P-glycoprotein and reduce the survival of the free-living stages of Heligmosomoides bakeri. Vet. Parasitol. 2011, 179, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Vo, N.N.Q.; Fukushima, E.O.; Muranaka, T. Structure and hemolytic activity relationships of triterpenoid saponins and sapogenins. J. Nat. Med. 2017, 71, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Piano, E.; Pecetti, L. Minor legume species. In Fodder Cops and Amenity Grasses. Handbook of Plant Breeding Series, 2nd ed.; Boller, B., Posselt, U.K., Veronesi, F., Eds.; Springer: New York, NY, USA, 2010; Volume 5, pp. 477–500. [Google Scholar]
- Stringi, L.; Sarno, R.; Amato, G.; Leto, G.; Pristina, L.; Corrao, A. Shrubs utilization in semi-arid environment: Bio-agronomic aspects and their use in sheep husbandry. World Rev. Anim. Prod. 1987, 23, 79–90. [Google Scholar]
- Jurzysta, M.; Bialy, Z. Antifungal and haemolytic activity of root of alfalfa (Medicago spp.) in relation to saponin content. In Modern fungicides and antifungal compounds II: 12th International Reinhardsbrunn Symposium, May 24th-29th 1998: Berg Hotel, Friedrichroda, Thuringia, Germany; Intercept Ltd: London, UK, 1999 24 May; pp. 445–451. [Google Scholar]
- Avato, P.; Bucci, R.; Tava, A.; Vitali, C.; Rosato, A.; Bialy, Z.; Jurzysta, M. Antimicrobial activity of saponins from Medicago sp. structure–activity relationship. Phytother. Res. 2006, 20, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Houghton, P.; Patel, N.; Jurzysta, M.; Biely, Z.; Cheung, C. Anti-dermatophyte activity of Medicago extracts and contained saponins and their structure-activity relationships. Phytother. Res. 2006, 20, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Tava, A.; Odoardi, M. Saponins from Medicago spp: chemical characterization and biological activity against insects. In Saponins used in food and agriculture; Tava, A.; Odoardi, M. Plenum Press: New York, NY, USA, 1996; pp. 97–109. [Google Scholar]
- Avato, P.; Migoni, D.; Argentieri, M.; Fanizzi, F.P.; Tava, A. Activity of Saponins from Medicago species against HeLa and MCF-7 cell lines and their capacity to potentiate cisplatin effect. Anticancer Agents Med. Chem. 2017, 17, 1508–1518. [Google Scholar] [CrossRef]
- D’Addabbo, T.; Carbonara, T.; Leonetti, P.; Radicci, V.; Tava, A.; Avato, P. Control of plant parasitic nematodes with active saponins and biomass from Medicago sativa. Phytochem. Rev. 2011, 10, 305–519. [Google Scholar] [CrossRef]
- Tava, A.; Mella, M.; Avato, P.; Argentieri, M.P.; Bialy, Z.; Jurzysta, M. Triterpenoid glycosides from the leaves of Medicago arborea L. J. Agric. Food Chem. 2005, 53, 9954–9965. [Google Scholar] [CrossRef] [PubMed]
- Tava, A.; Pecetti, L.; Romani, M.; Mella, M.; Avato, P. Triterpenoid glycosides from the leaves of two cultivars of Medicago polymorpha L. J. Agric. Food Chem. 2011, 59, 6142–6149. [Google Scholar] [CrossRef] [PubMed]
- Tava, A.; Pecetti, L. Chemical investigation of saponins from twelve Medicago species and their bioassay with the brine shrimp Artemia salina. Nat. Prod. Commun. 2012, 7, 837–840. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Agriculture, Fisheries and Food (MAFF). Manual of Veterinary Parasitological Laboratory Techniques; Her Majesty’s Stationary Office (HMSO): London, UK, 1986; pp. 1–152. [Google Scholar]
- Bevilaqua, C.M.L.; Rodrigues, M.L.; Concordet, D. Identification of infective larvae of some common nematode strongylids of horses. Rev. Med. Vet. 1993, 144, 989–995. [Google Scholar]
- Ademola, I.O.; Eloff, J.N. In vitro anthelmintic activity of Combretum molle (R. Br. ex G. Don) (Combretaceae) against Haemonchus contortus ova and larvae. Vet. Parasitol. 2010, 169, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Copani, G.; Hall, J.O.; Miller, J.; Priolo, A.; Villalba, J.J. Plant secondary compounds as complementary resources: are they always complementary? Oecologia 2013, 172, 1041–1049. [Google Scholar] [CrossRef]
- Paparella, S.; Tava, A.; Avato, P.; Biazzi, E.; Macovei, A.; Biggiogera, M.; Carbonera, D.; Balestrazzi, A. Cell wall integrity, genotoxic injury and PCD dynamics in alfalfa saponin-treated white poplar cells highlight a complex link between molecule structure and activity. Phytochemistry 2015, 111, 114–123. [Google Scholar] [CrossRef]
- Vercruysse, J.; Holdsworth, P.; Letonja, T.; Conder, G.; Hamamoto, K.; Okano, K.; Rehbein, S. Veterinary International Co-operation on Harmonisation Working Group on anthelmintic guidelines. International harmonisation of anthelmintic efficacy guidelines (Part 2). Vet. Parasitol. 2001, 96, 171–193. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maestrini, M.; Tava, A.; Mancini, S.; Salari, F.; Perrucci, S. In Vitro Anthelmintic Activity of Saponins Derived from Medicago spp. Plants against Donkey Gastrointestinal Nematodes. Vet. Sci. 2019, 6, 35. https://doi.org/10.3390/vetsci6020035
Maestrini M, Tava A, Mancini S, Salari F, Perrucci S. In Vitro Anthelmintic Activity of Saponins Derived from Medicago spp. Plants against Donkey Gastrointestinal Nematodes. Veterinary Sciences. 2019; 6(2):35. https://doi.org/10.3390/vetsci6020035
Chicago/Turabian StyleMaestrini, Michela, Aldo Tava, Simone Mancini, Federica Salari, and Stefania Perrucci. 2019. "In Vitro Anthelmintic Activity of Saponins Derived from Medicago spp. Plants against Donkey Gastrointestinal Nematodes" Veterinary Sciences 6, no. 2: 35. https://doi.org/10.3390/vetsci6020035
APA StyleMaestrini, M., Tava, A., Mancini, S., Salari, F., & Perrucci, S. (2019). In Vitro Anthelmintic Activity of Saponins Derived from Medicago spp. Plants against Donkey Gastrointestinal Nematodes. Veterinary Sciences, 6(2), 35. https://doi.org/10.3390/vetsci6020035






