Immunostaining for p53 and p16CDKN2A Protein Is Not Predictive of Prognosis for Dogs with Malignant Mammary Gland Neoplasms
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Cases Included and Histological Evaluation of MGTs
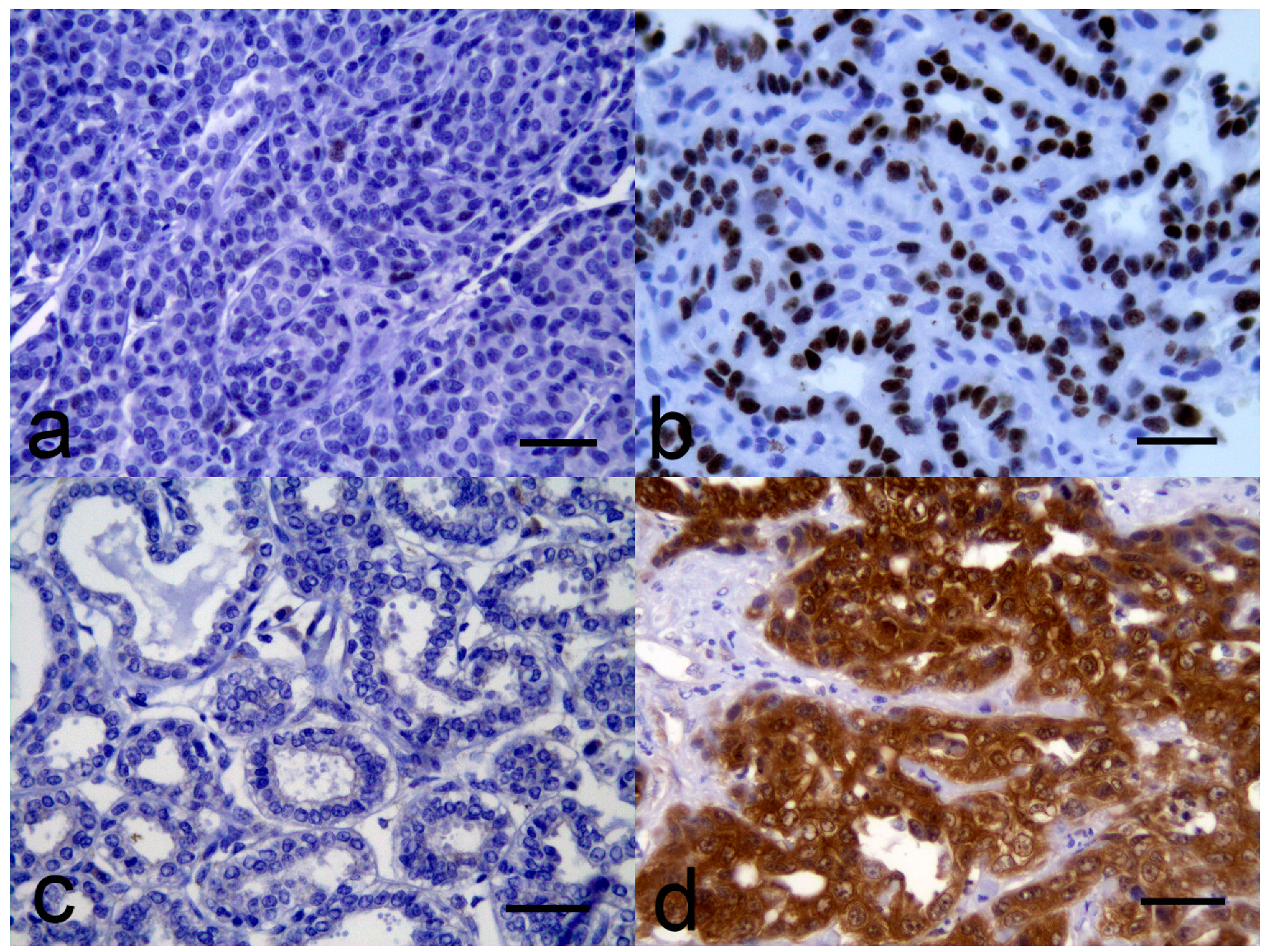
3.2. Immunohistochemistry Evaluation
3.3. Survival of Dogs with MGTs
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Im, K.S.; Kim, N.H.; Lim, H.Y.; Kim, H.W.; Shin, J.I.; Sur, J.H. Analysis of a new histological and molecular-based classification of canine mammary neoplasia. Vet. Pathol. 2014, 51, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Rasotto, R.; Berlato, D.; Goldschmidt, M.H.; Zappulli, V. Prognostic significance of canine mammary tumor histologic subtypes: An observational cohort study of 229 cases. Vet. Pathol. 2017, 54, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Park, K.; Jung, H.H.; Lee, E.; Cho, E.Y.; Lee, K.H.; Bae, S.Y.; Lee, S.K.; Kim, S.W.; Lee, J.E.; et al. Association between mutation and expression of TP53 as a potential prognostic marker of triple-negative breast cancer. Cancer Res. Treat. 2016, 48, 1338–1350. [Google Scholar] [CrossRef]
- Morris, J.S.; Nixon, C.; King, O.J.; Morgan, I.M.; Philbey, A.W. Expression of TopBP1 in canine mammary neoplasia in relation to histological type, Ki67, ERalpha and p53. Vet. J. 2009, 179, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Kim, W.H.; Lim, J.H.; Kang, M.S.; Kim, D.Y.; Kweon, O.K. Mutation and overexpression of p53 as a prognostic factor in canine mammary tumors. J. Vet. Sci. 2004, 5, 63–69. [Google Scholar] [CrossRef]
- Dolka, I.; Krol, M.; Sapierzynski, R. Evaluation of apoptosis-associated protein (Bcl-2, Bax, cleaved caspase-3 and p53) expression in canine mammary tumors: An immunohistochemical and prognostic study. Res. Vet. Sci. 2016, 105, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Karpathiou, G.; Monaya, A.; Forest, F.; Froudarakis, M.; Casteillo, F.; Marc Dumollard, J.; Prades, J.M.; Peoc’h, M. p16 and p53 expression status in head and neck squamous cell carcinoma: A correlation with histological, histoprognostic and clinical parameters. Pathology 2016, 48, 341–348. [Google Scholar] [CrossRef]
- Foulkes, W.D.; Flanders, T.Y.; Pollock, P.M.; Hayward, N.K. The CDKN2A (p16) gene and human cancer. Mol. Med. 1997, 3, 5–20. [Google Scholar] [CrossRef]
- Parry, D.; Bates, S.; Mann, D.J.; Peters, G. Lack of cyclin D-Cdk complexes in Rb-negative cells correlates with high levels of p16INK4/MTS1 tumour suppressor gene product. EMBO J. 1995, 14, 503–511. [Google Scholar] [CrossRef]
- Lewis, J.S., Jr.; Thorstad, W.L.; Chernock, R.D.; Haughey, B.H.; Yip, J.H.; Zhang, Q.; El-Mofty, S.K. p16 positive oropharyngeal squamous cell carcinoma:an entity with a favorable prognosis regardless of tumor HPV status. Am. J. Surg. Pathol. 2010, 34, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Pare, R.; Shin, J.S.; Lee, C.S. Increased expression of senescence markers p14(ARF) and p16(INK4a) in breast cancer is associated with an increased risk of disease recurrence and poor survival outcome. Histopathology 2016, 69, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Milde-Langosch, K.; Bamberger, A.M.; Rieck, G.; Kelp, B.; Loning, T. Overexpression of the p16 cell cycle inhibitor in breast cancer is associated with a more malignant phenotype. Breast Cancer Res. Treat. 2001, 67, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Ju, J.H.; Shin, J.I.; Seung, B.J.; Sur, J.H. Differential and correlated expressions of p16/p21/p27/p38 in mammary gland tumors of aged dogs. J. Vet. Sci. 2017, 18, 479–485. [Google Scholar] [CrossRef]
- Goldschmidt, M.; Pena, L.; Rasotto, R.; Zappulli, V. Classification and grading of canine mammary tumors. Vet. Pathol. 2011, 48, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Munday, J.S.; Aberdein, D. Loss of retinoblastoma protein, but not p53, is associated with the presence of papillomaviral DNA in feline viral plaques, bowenoid in situ carcinomas, and squamous cell carcinomas. Vet. Pathol. 2012, 49, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Munday, J.S.; Tucker, R.S.; Kiupel, M.; Harvey, C.J. Multiple oral carcinomas associated with a novel papillomavirus in a dog. J. Vet. Diagn. Investig. 2015, 27, 221–225. [Google Scholar] [CrossRef]
- Wakui, S.; Muto, T.; Yokoo, K.; Yokoo, R.; Takahashi, H.; Masaoka, T.; Hano, H.; Furusato, M. Prognostic status of p53 gene mutation in canine mammary carcinoma. Anticancer Res. 2001, 21, 611–616. [Google Scholar] [PubMed]
- Klopfleisch, R.; Gruber, A.D. Differential expression of cell cycle regulators p21, p27 and p53 in metastasizing canine mammary adenocarcinomas versus normal mammary glands. Res. Vet. Sci. 2009, 87, 91–96. [Google Scholar] [CrossRef]
- Oliveira, T.F.; Maues, T.; Ramundo, M.S.; Figureueiredo, A.M.S.; de Mello, M.F.V.; El-Jaick, K.B.; Ferreira, M.L.G.; Ferreira, A.M.R. TP53 gene expression levels and tumor aggressiveness in canine mammary carcinomas. J. Vet. Diagn. Investig. 2017, 29, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Munday, J.S.; French, A.F.; Gibson, I.R.; Knight, C.G. The presence of p16 CDKN2A protein immunostaining within feline nasal planum squamous cell carcinomas is associated with an increased survival time and the presence of papillomaviral DNA. Vet. Pathol. 2013, 50, 269–273. [Google Scholar] [CrossRef]
- Meuten, D.; Munday, J.S.; Hauck, M. Time to standardize? Time to validate? Vet. Pathol. 2018, 55, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Northrup, N.C.; Howerth, E.W.; Harmon, B.G.; Brown, C.A.; Carmicheal, K.P.; Garcia, A.P.; Latimer, K.S.; Munday, J.S.; Rakich, P.M.; Richey, L.J.; et al. Variation among pathologists in the histologic grading of canine cutaneous mast cell tumors with uniform use of a single grading reference. J. Vet. Diagn. Investig. 2005, 17, 561–564. [Google Scholar] [CrossRef] [PubMed]
| Histological Subtype | Total | p53-pos | p53-neg | p16-pos | p16-int | p16-neg |
|---|---|---|---|---|---|---|
| Simple carcinomas (total) | 13 | 3 | 10 | 1 | 9 | 3 |
| Tubular carcinoma | 6 | 2 | 4 | 0 | 4 | 2 |
| Tubulopapillary carcinoma | 3 | 1 | 2 | 1 | 1 | 1 |
| Cribriform carcinoma | 2 | 0 | 2 | 0 | 2 | 0 |
| Cystic papillary carcinoma | 2 | 0 | 2 | 0 | 2 | 0 |
| Mixed mammary carcinoma | 6 | 0 | 6 | 0 | 6 | 0 |
| Intraductular papillary carcinoma | 6 | 2 | 4 | 1 | 5 | 0 |
| Complex carcinoma | 3 | 1 | 2 | 0 | 3 | 0 |
| Ductular carcinoma | 3 | 0 | 3 | 0 | 3 | 0 |
| Solid carcinoma | 2 | 1 | 1 | 0 | 0 | 2 |
| Comedocarcinoma | 1 | 0 | 1 | 0 | 1 | 0 |
| Adenosquamous carcinoma | 1 | 0 | 1 | 0 | 1 | 0 |
| All types | 35 | 7 | 28 | 2 | 28 | 5 |
| Number | Estimated Mean Survival Time (95% CI) Days | p Value | |
|---|---|---|---|
| All tumors | 35 | 882 (694–1071) | |
| p53 status | 0.57 | ||
| Positive | 7 | 670 (355–986) | |
| Negative | 28 | 934 (723–1144) | |
| p16CDKN2A status | 0.31 | ||
| Intermediate | 28 | 927 (704–1150) | |
| Negative | 5 | 683 (441–926) | |
| Positive | 2 | 307 (63–551) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munday, J.S.; Ariyarathna, H.; Aberdein, D.; Thomson, N.A. Immunostaining for p53 and p16CDKN2A Protein Is Not Predictive of Prognosis for Dogs with Malignant Mammary Gland Neoplasms. Vet. Sci. 2019, 6, 34. https://doi.org/10.3390/vetsci6010034
Munday JS, Ariyarathna H, Aberdein D, Thomson NA. Immunostaining for p53 and p16CDKN2A Protein Is Not Predictive of Prognosis for Dogs with Malignant Mammary Gland Neoplasms. Veterinary Sciences. 2019; 6(1):34. https://doi.org/10.3390/vetsci6010034
Chicago/Turabian StyleMunday, John S, Harsha Ariyarathna, Danielle Aberdein, and Neroli A Thomson. 2019. "Immunostaining for p53 and p16CDKN2A Protein Is Not Predictive of Prognosis for Dogs with Malignant Mammary Gland Neoplasms" Veterinary Sciences 6, no. 1: 34. https://doi.org/10.3390/vetsci6010034
APA StyleMunday, J. S., Ariyarathna, H., Aberdein, D., & Thomson, N. A. (2019). Immunostaining for p53 and p16CDKN2A Protein Is Not Predictive of Prognosis for Dogs with Malignant Mammary Gland Neoplasms. Veterinary Sciences, 6(1), 34. https://doi.org/10.3390/vetsci6010034





