Effect of Beta-Carotene Supplementation on the Serum Oxidative Stress Biomarker and Antibody Titer against Live Bovine Respiratory Syncytial Virus Vaccination in Japanese Black Calves
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Measurements and Analysis
2.3. Statistical Analysis
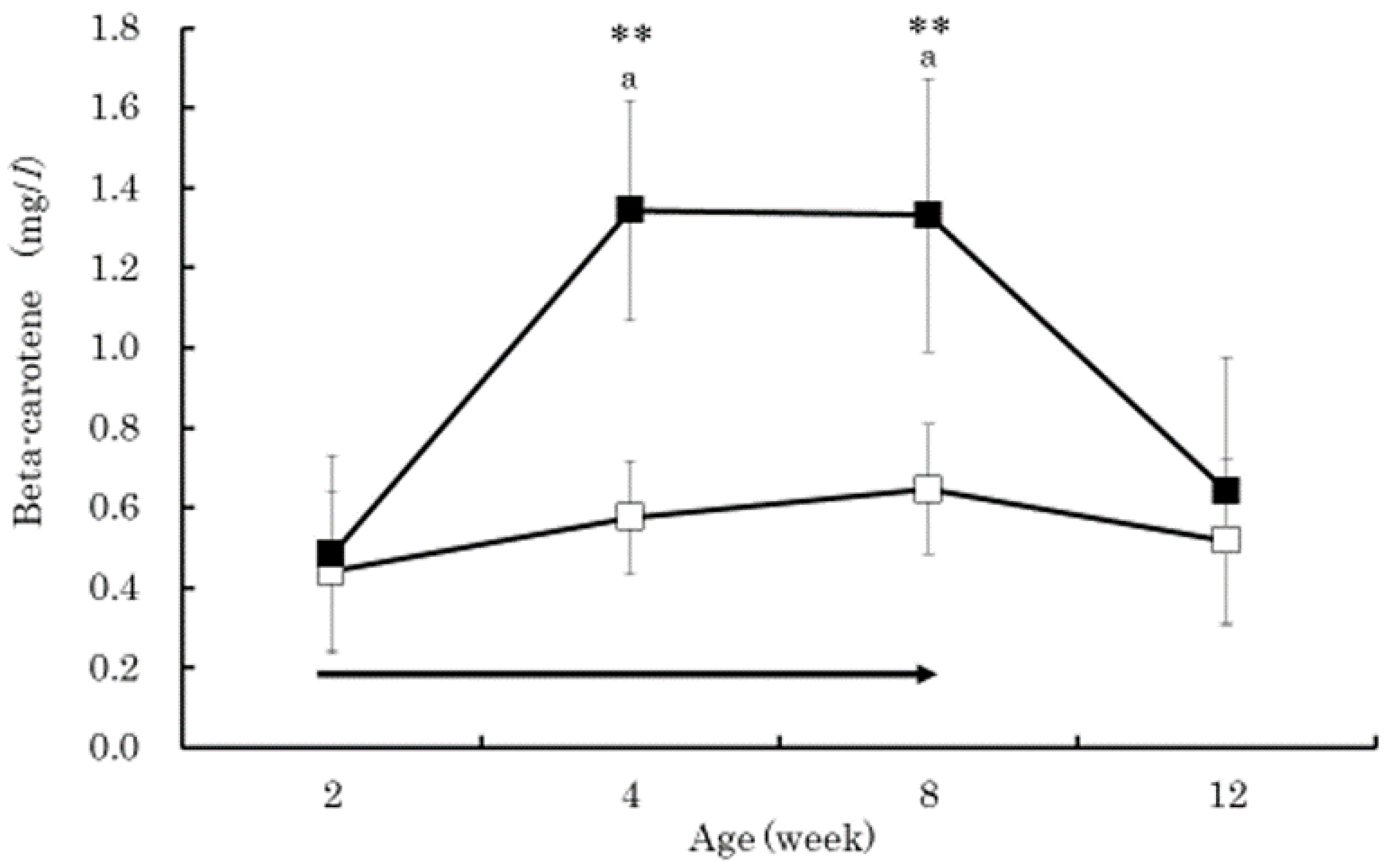
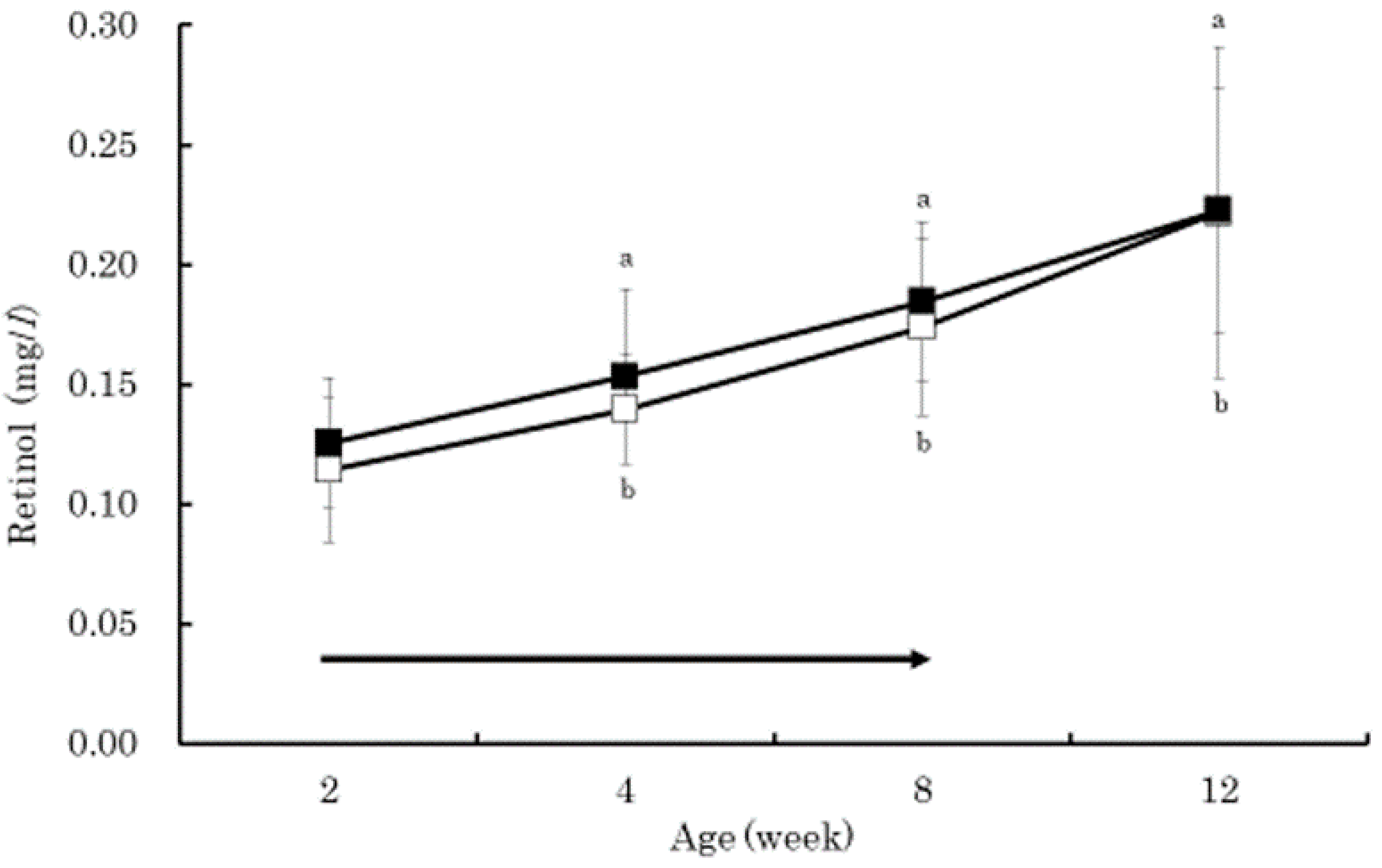
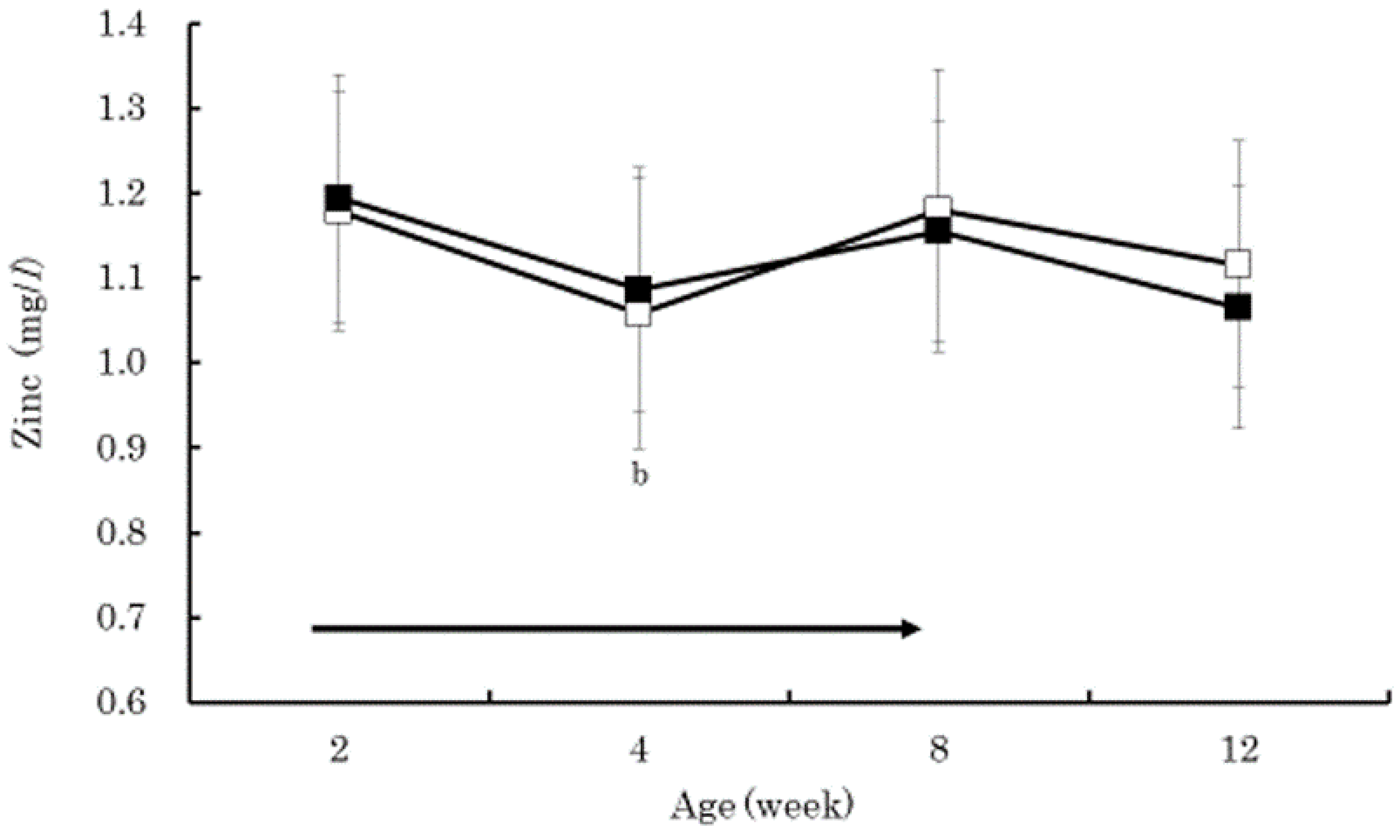
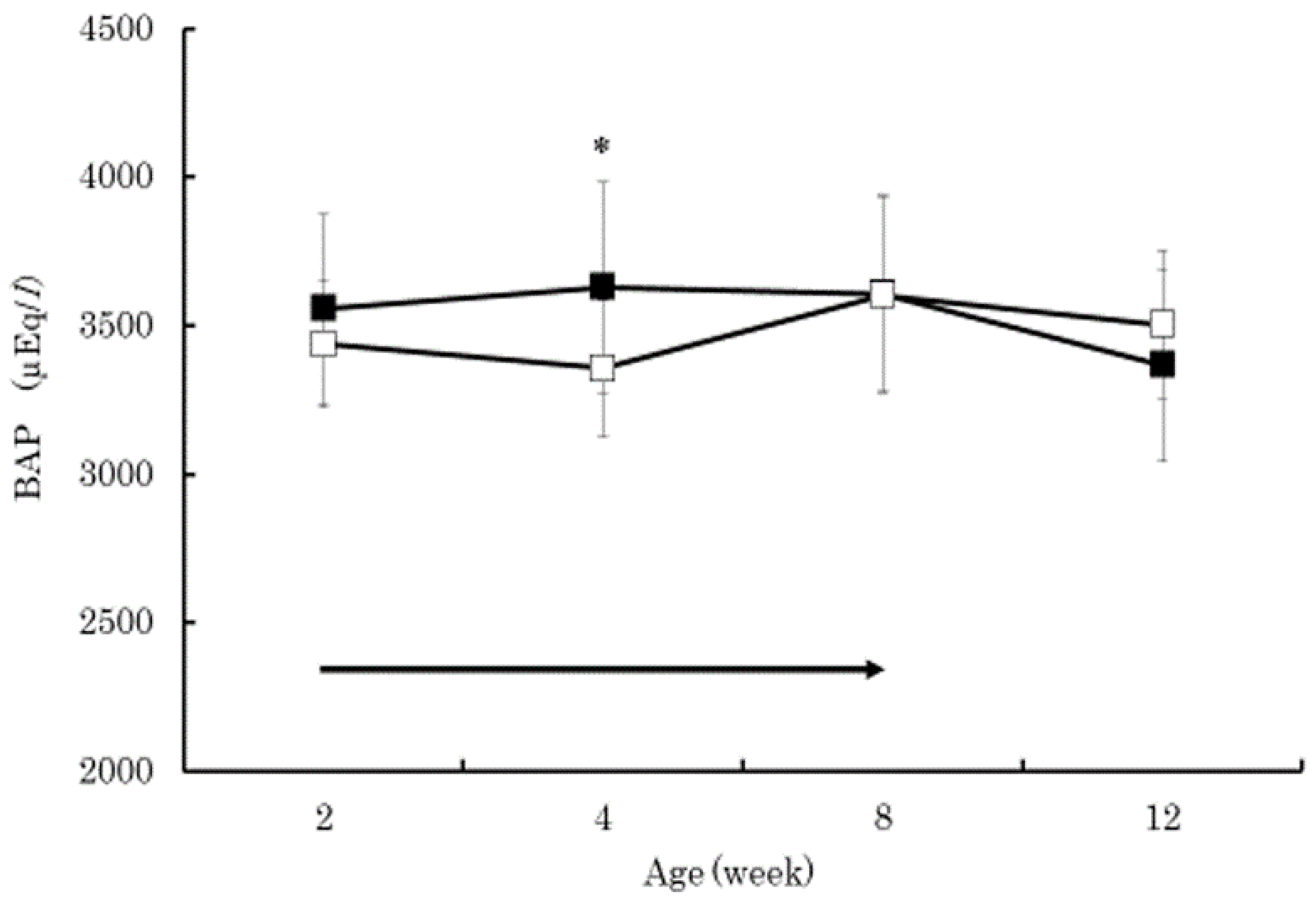
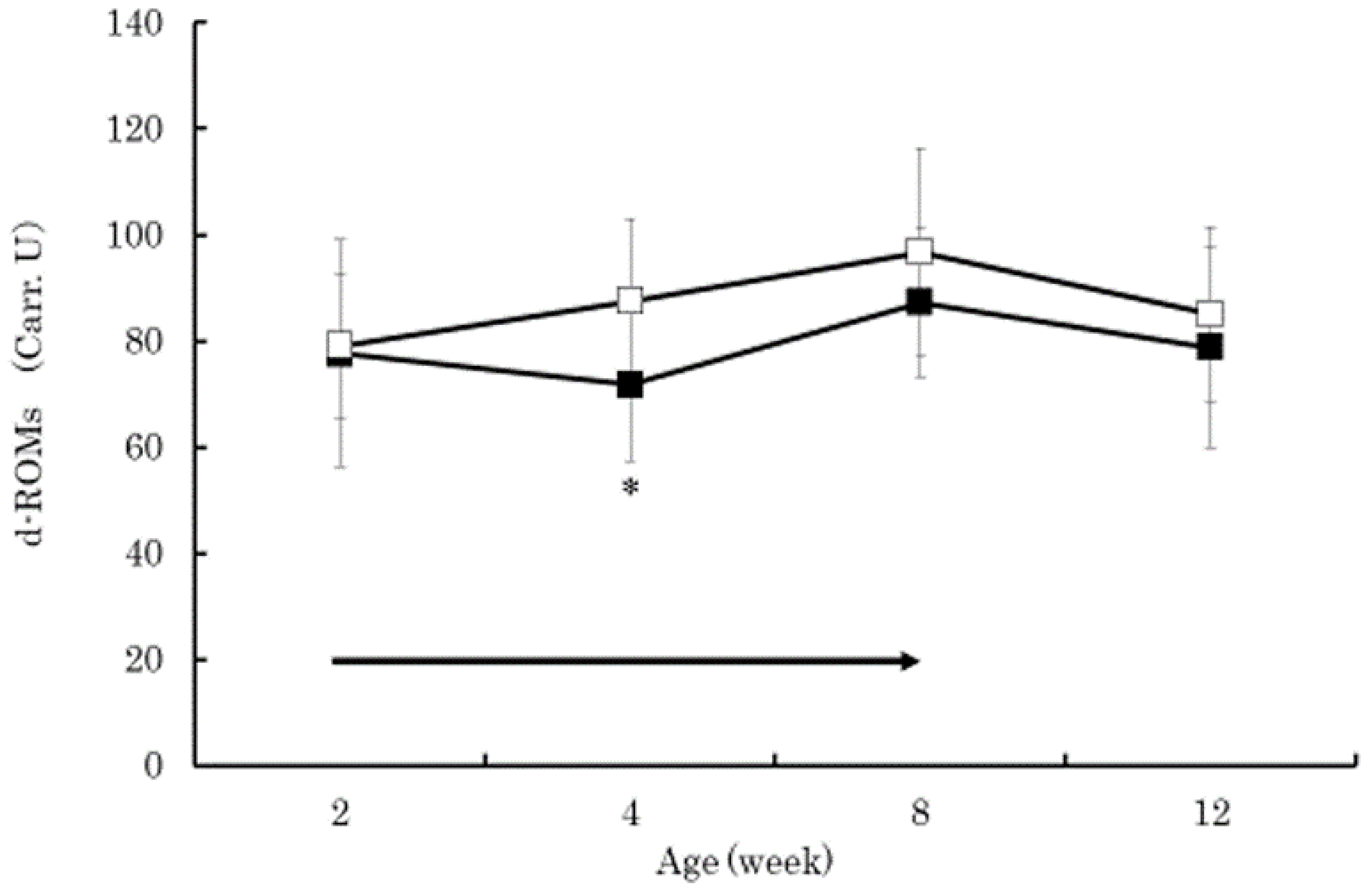
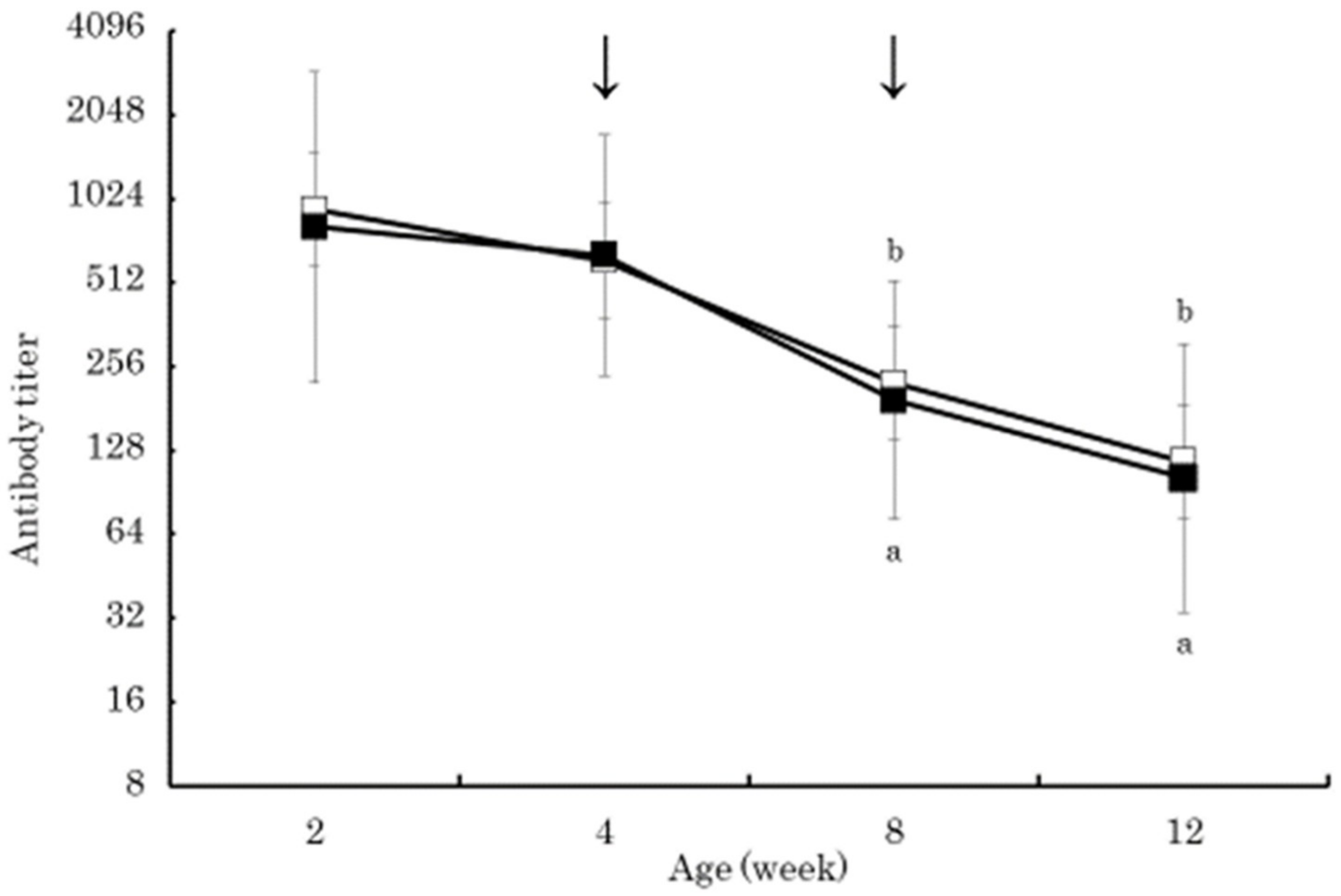
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kampen, A.H.; Olsen, I.; Tollersrud, T.; Storset, A.K.; Lund, A. Lymphocyte subpopulations and neutrophil function in calves during the first 6 months of life. Vet. Immunol. Immunopathol. 2006, 113, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Menanteau-Horta, A.M.; Ames, T.R.; Johnson, D.W.; Meiske, J.C. Effect of maternal antibody upon vaccination with infectious bovine rhinotracheitis and bovine virus diarrhea vaccines. Can. J. Comp. Med. 1985, 49, 10–14. [Google Scholar] [PubMed]
- Baker, J.C.; Werdin, R.E.; Ames, T.R.; Markham, R.J.; Larson, V.L. Study on the etiologic role of bovine respiratory syncytial virus in pneumonia of dairy calves. J. Am. Vet. Med. Assoc. 1986, 189, 66–70. [Google Scholar]
- Frye, T.M.; Williams, S.N.; Graham, T.W. Vitamin deficiencies in cattle. Vet. Clin. North. Am. Food. Anim. Pract. 1991, 7, 217–275. [Google Scholar] [CrossRef]
- Herdt, T.H.; Stowe, H.D. Fat-soluble vitamin nutrition for dairy cattle. Vet. Clin. North Am. Food Anim. Pract. 1991, 7, 391–415. [Google Scholar] [CrossRef]
- Chew, B.P.; Park, J.S.; Wong, T.S.; Kim, H.W.; Weng, B.B.; Byrne, K.M.; Hayek, M.G.; Reinhart, G.A. Dietary beta-carotene stimulates cell-mediated and humoral immune response in dogs. J. Nutr. 2000, 130, 1910–1913. [Google Scholar] [CrossRef]
- Chew, B.P.; Park, J.S. Carotenoid action on the immune response. J. Nutr. 2004, 134, 257S–261S. [Google Scholar] [CrossRef]
- Lombardi, P.; Musco, N.; Cutrignelli, M.I.; Mollica, M.P.; Trinchese, G.; Calabro, S.; Tudisco, R.; Grossi, M.; Mastellone, V.; Vassalotti, G.; et al. The association of aloe and β-carotene supplementation improves oxidative stress and inflammatory state in pregnant buffalo cows. Buffalo Bull. 2017, 36, 497–503. [Google Scholar]
- Ondrejkova, A.; Suli, J.; Harvanova, J.; Ondrejka, R.; Prokes, M. Antioxidative protection of squalene adjuvant and rabies vaccine with adjuvant. Biol. Pharm. Bull. 2017, 40, 1029–1034. [Google Scholar] [CrossRef]
- Rust, P.; Eichler, I.; Renner, S.; Elmadfa, I. Long term oral beta-carotene supplementation in patients with cystic fibrosis, effects on antioxidative status and pulmonary function. Ann. Nutr. Metab. 2000, 44, 30–37. [Google Scholar] [CrossRef]
- Mutinati, M.; Pantaleo, M.; Roncetti, M.; Piccinno, M.; Rizzo, A.; Sciorsci, R.L. Oxidative stress in neonatology: A review. Reprod. Domest. Anim. 2014, 49, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Trotti, R.; Carratelli, M.; Barbieri, M. Performance and clinical application of a new, fast method for the detection of hydroperoxides in serum. Panminerva Med. 2002, 44, 37–40. [Google Scholar] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.A.; Hassard, L.E.; Morley, P.S. Bovine respiratory syncytial virus-specific immune responses in calves after inoculation with commercially available vaccines. J. Am. Vet. Med. Assoc. 1995, 206, 354–361. [Google Scholar] [PubMed]
- Ranade, R.; Talukder, S.; Muscatello, G.; Celi, P. Assessment of oxidative stress biomarkers in exhaled breath condensate and blood of dairy heifer calves from birth to weaning. Vet. J. 2014, 202, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, T.; Albrecht, E.; Teuscher, F.; Kawabata, K.; Sakashita, K.; Kawamoto, H.; Wegner, J. Differences in muscle and fat accretion in Japanese Black and European cattle. Meat Sci. 2009, 82, 300–308. [Google Scholar] [CrossRef]
- Otomaru, K.; Wataya, K.; Uto, T.; Kasai, K. Blood biochemical values in Japanese Black calves in Kagoshima Prefecture, Japan. J. Vet. Med. Sci. 2016, 78, 301–303. [Google Scholar] [CrossRef]
- Bierer, T.L.; Merchen, N.R.; Nelson, D.R.; Erdman, J.W. Transport of newly-absorbed beta-carotene by the preruminant calf. Ann. N. Y. Acad. Sci. 1993, 691, 226–228. [Google Scholar] [CrossRef]
- Agriculture, Factory and Fisheries Research Council Secretariat (Ed.) Japanese Feeding Standard for Beef Cattle; Japan Livestock Industry Association: Tokyo, Japan, 2008. (In Japanese) [Google Scholar]
- Makino, T.; Saito, M.; Horiguchi, D.; Kina, K. A highly sensitive colorimetric determination of serum zinc using water-soluble pyridylazo dye. Clin. Chim. Acta 1982, 120, 127–135. [Google Scholar]
- Adachi, K.; Katsura, N.; Nomura, Y.; Arikawa, A.; Hidaka, M.; Onimaru, T. Serum vitamin A and vitamin E in Japanese black fattening cattle in Miyazaki prefecture as determined by automatic column-switching high performance liquid chromatography. J. Vet. Med. Sci. 1996, 58, 461–464. [Google Scholar] [CrossRef]
- Kubota, M.; Fukuyama, S.; Takamura, K.; Izumida, A.; Kodama, K. Field trials on a live bovine respiratory syncytial virus vaccine in calves. J. Vet. Med. Sci. 1992, 54, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Chew, B.P.; Wong, T.S.; Michal, J.J. Uptake of orally administered beta-carotene by blood plasma, leukocytes, and lipoproteins in calves. J. Anim. Sci. 1993, 71, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Cheng, H.; Wan, F.; Bi, Y.; Liu, G.; Liu, X.; Zhao, H.; You, W.; Liu, Y.; Tan, X. Effects of feeding β-carotene on levels of β-carotene and vitamin A in blood and tissues of beef cattle and the effects on beef quality. Meat Sci. 2015, 110, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Paiva, S.A.; Russell, R.M. Beta-carotene and other carotenoids as antioxidants. J. Am. Coll. Nutr. 1999, 18, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Spears, J.W. Micronutrients and immune function in cattle. Proc. Nutr. Soc. 2000, 59, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Ishida, M.; Nishijima, Y.; Ikeda, S.; Yoshitani, K.; Obata, A.; Sugie, Y.; Aoki, Y.; Yamaji, T.; Fujita, M.; Nakatsuji, Y.; et al. Effects of supplemental β-carotene on colostral immunoglobulin and plasma β-carotene and immunoglobulin in Japanese Black cows. Anim. Sci. J. 2018, 89, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Vangeel, I.; Antonis, A.F.; Fluess, M.; Riegler, L.; Peters, A.R.; Harmeyer, S.S. Efficacy of a modified live intranasal bovine respiratory syncytial virus vaccine in 3-week-old calves experimentally challenged with BRSV. Vet. J. 2007, 174, 627–635. [Google Scholar] [CrossRef]
| Item | Weeks of Age | ||||
|---|---|---|---|---|---|
| 2 | 4 | 8 | 12 | ||
| Amount (Dry Matter) | |||||
| Milk Replacer | (kg) | 0.72 | 0.92 | 0.92 | 0.00 |
| Concentrate | (kg) | 0.05 | 0.10 | 0.50 | 0.85 |
| Hey (Oats) | (kg) | 0.01 | 0.01 | 0.05 | 0.10 |
| Composition (Dry Basis) | |||||
| Crude Protein | (%) | 30.6 | 30.1 | 26.8 | 18.6 |
| Crude Fat | (%) | 15.7 | 15.2 | 11.6 | 2.6 |
| Total Digestible Nutrients | (%) | 101.1 | 100.1 | 92.9 | 74.8 |
| Zinc | mg/day | 71.8 | 94.0 | 124.4 | 61 |
| Retinol | mg/day | 18.9 | 18.3 | 14.1 | 3.5 |
| Beta-Carotene | mg/day | 0.0 | 0.0 | 0 | 0.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otomaru, K.; Ogawa, R.; Oishi, S.; Iwamoto, Y.; Hong, H.; Nagai, K.; Hyakutake, K.; Kubota, C.; Kaneshige, T. Effect of Beta-Carotene Supplementation on the Serum Oxidative Stress Biomarker and Antibody Titer against Live Bovine Respiratory Syncytial Virus Vaccination in Japanese Black Calves. Vet. Sci. 2018, 5, 102. https://doi.org/10.3390/vetsci5040102
Otomaru K, Ogawa R, Oishi S, Iwamoto Y, Hong H, Nagai K, Hyakutake K, Kubota C, Kaneshige T. Effect of Beta-Carotene Supplementation on the Serum Oxidative Stress Biomarker and Antibody Titer against Live Bovine Respiratory Syncytial Virus Vaccination in Japanese Black Calves. Veterinary Sciences. 2018; 5(4):102. https://doi.org/10.3390/vetsci5040102
Chicago/Turabian StyleOtomaru, Konosuke, Rei Ogawa, Shoko Oishi, Yuki Iwamoto, Hyeyoung Hong, Kathuhisa Nagai, Koji Hyakutake, Chikara Kubota, and Takahiro Kaneshige. 2018. "Effect of Beta-Carotene Supplementation on the Serum Oxidative Stress Biomarker and Antibody Titer against Live Bovine Respiratory Syncytial Virus Vaccination in Japanese Black Calves" Veterinary Sciences 5, no. 4: 102. https://doi.org/10.3390/vetsci5040102
APA StyleOtomaru, K., Ogawa, R., Oishi, S., Iwamoto, Y., Hong, H., Nagai, K., Hyakutake, K., Kubota, C., & Kaneshige, T. (2018). Effect of Beta-Carotene Supplementation on the Serum Oxidative Stress Biomarker and Antibody Titer against Live Bovine Respiratory Syncytial Virus Vaccination in Japanese Black Calves. Veterinary Sciences, 5(4), 102. https://doi.org/10.3390/vetsci5040102




