Parasite Specific Antibody Levels, Interferon-γ and TLR2 and TLR4 Transcripts in Blood from Dogs with Different Clinical Stages of Leishmaniosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Dogs
2.2. Dogs with Clinical Leishmaniosis
2.3. Control Healthy Dogs
2.4. Whole Blood Cytokine Release Assay and Determination of Canine IFN-γ
2.5. RNA Extraction, RNA Concentration and Quality and cDNA Synthesis
2.6. TLR2, TLR4 and Reference Housekeeping Genes qPCR
2.7. Statistical Analysis
3. Results
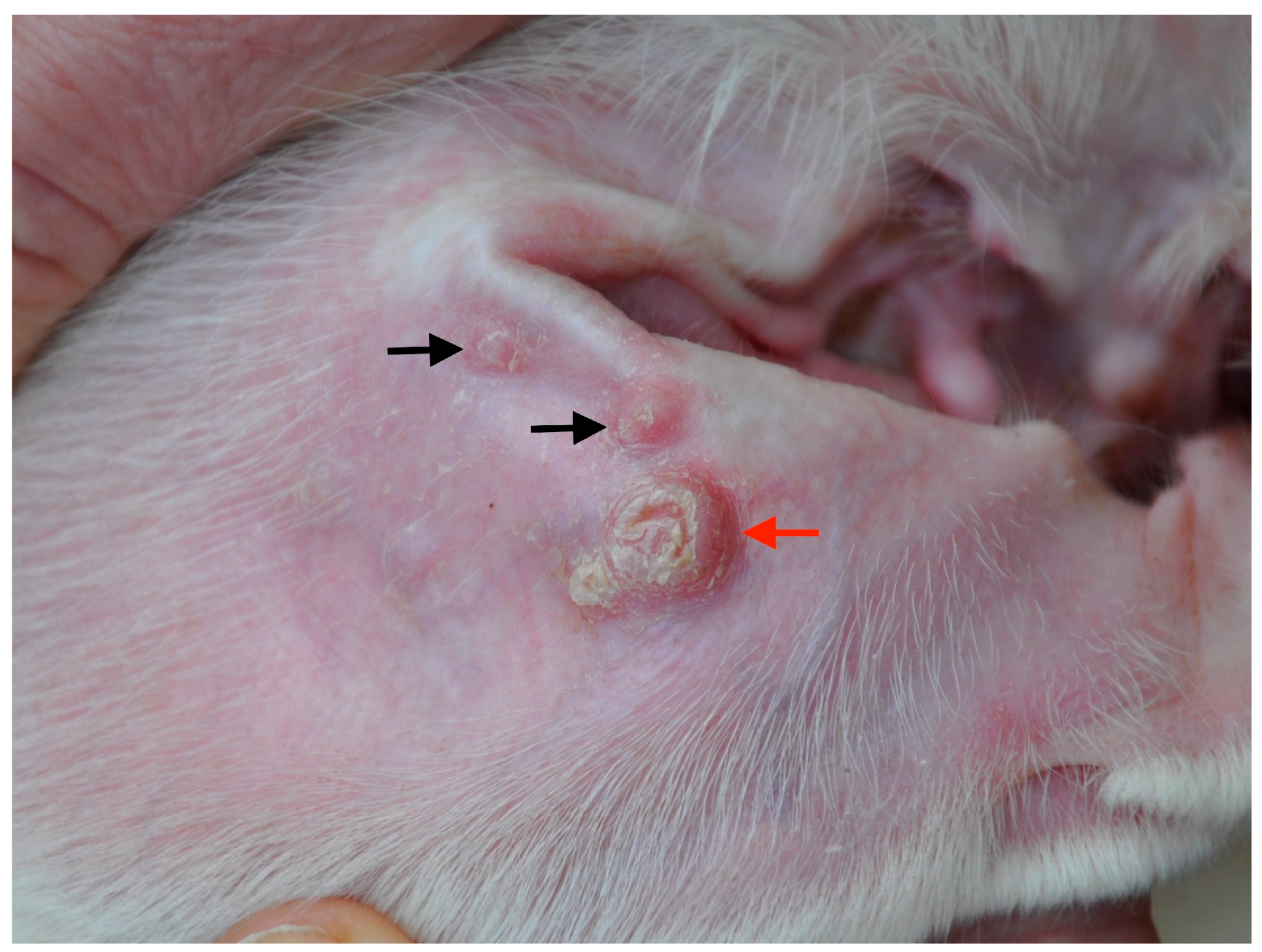
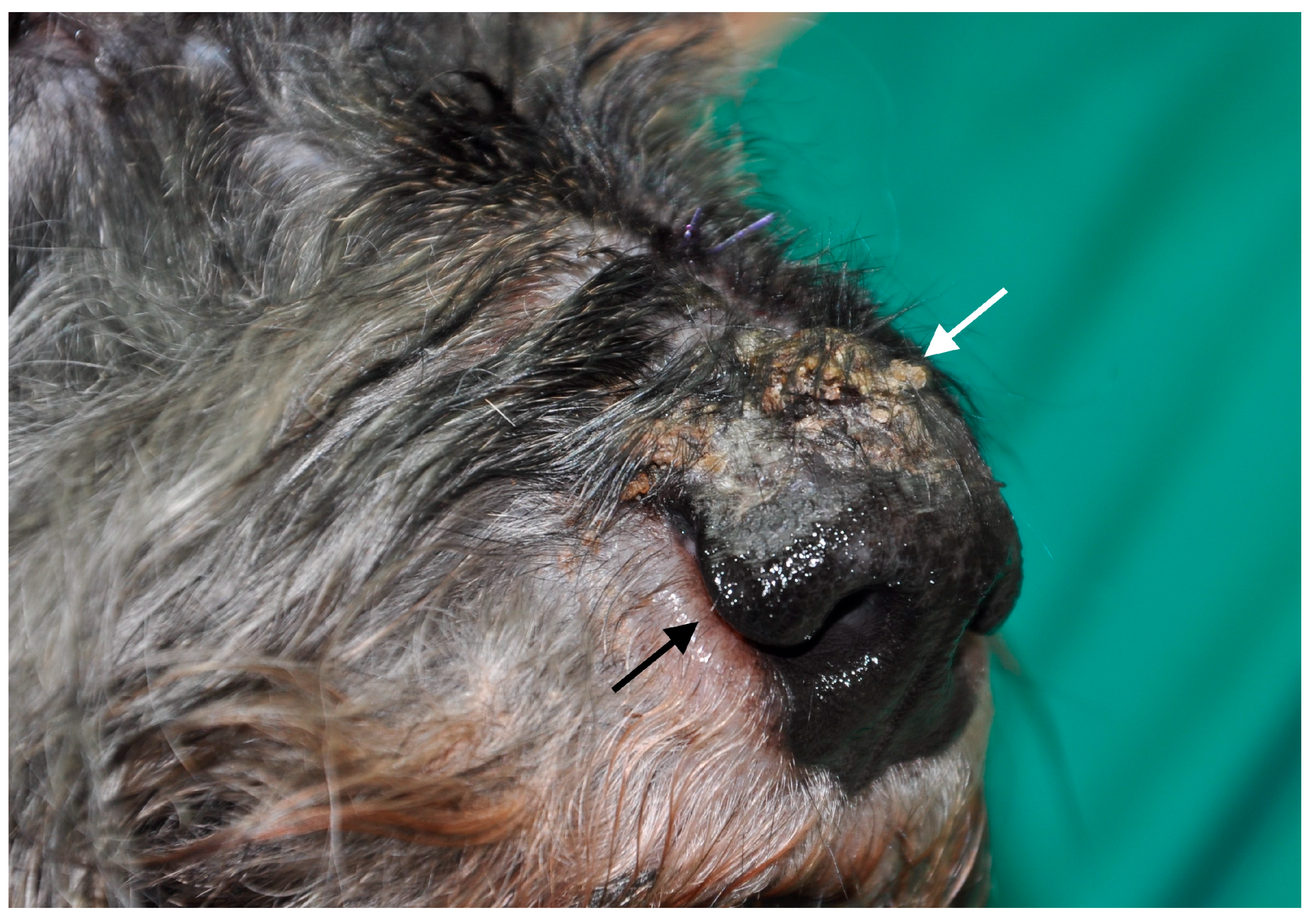
3.1. Dogs with Clinical Leishmaniosis
3.2. Blood Leishmania qPCR
3.3. Leishmania infantum Specific Antibody Levels
3.4. Leishmania infantum Specific IFN-γ Production after Blood Stimulation
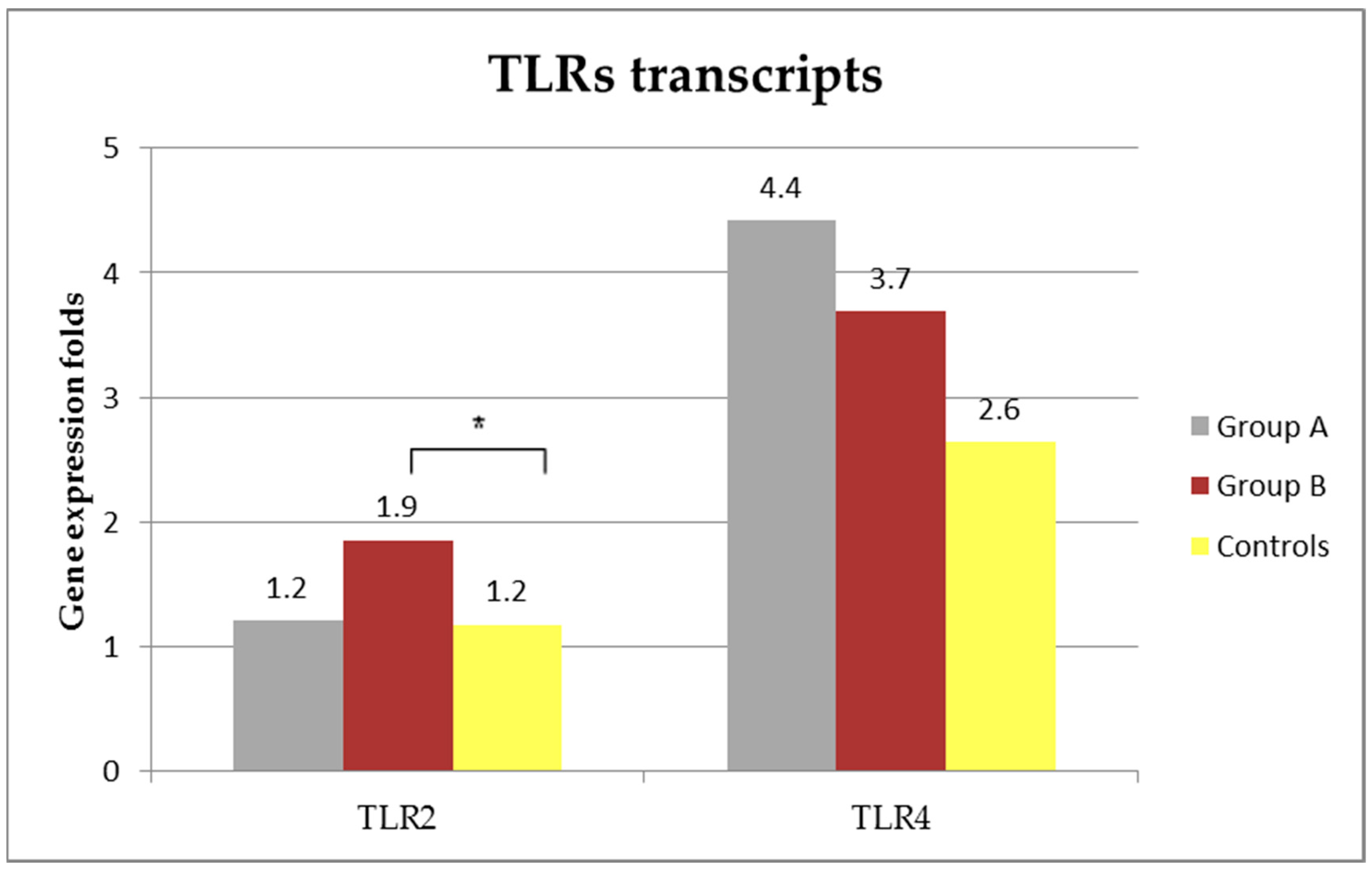
3.5. TLRs Transcripts
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Solano-Gallego, L.; Koutinas, A.; Miro, G.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G. Directions for the diagnosis, clinical staging, treatment and prevention of canine leishmaniosis. Vet. Parasitol. 2009, 165, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Hosein, S.; Blake, D.P.; Solano-Gallego, L. Insights on adaptive and innate immunity in canine leishmaniosis. Parasitology 2017, 144, 95–115. [Google Scholar] [CrossRef] [PubMed]
- Papadogiannakis, E.I.; Koutinas, A.F. Cutaneous immune mechanisms in canine leishmaniosis due to Leishmania infantum. Vet. Immunol. Immunopathol. 2015, 163, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Pinelli, E.; Killick-Kendrick, R.; Wagenaar, J.; Bernadina, W.; del Real, G.; Ruitenberg, J. Cellular and humoral immune responses in dogs experimentally and naturally infected with Leishmania infantum. Infect. Immun. 1994, 62, 229–235. [Google Scholar] [PubMed]
- Barbosa, M.A.; Alexandre-Pires, G.; Soares-Clemente, M.; Marques, C.; Rodrigues, O.R.; De Brito, T.V.; Da Fonseca, I.P.; Alves, L.C.; Santos-Gomes, G.M. Cytokine gene expression in the tissues of dogs infected by Leishmania infantum. J. Comp. Pathol. 2011, 145, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Bellon, H.; Solano-Gallego, L.; Rodriguez, A.; Rutten, V.P.; Hoek, A.; Ramis, A.; Alberola, J.; Ferrer, L. Comparison of three assays for the evaluation of specific cellular immunity to Leishmania infantum in dogs. Vet. Immunol. Immunopathol. 2005, 107, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Vidya, M.K.; Kumar, V.G.; Sejian, V.; Bagath, M.; Krishnan, G.; Bhatta, R. Toll-like receptors: Significance, ligands, signaling pathways, and functions in mammals. Int. Rev. Immunol. 2017, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Montserrat-Sangrà, S.; Alborch, L.; Ordeix, L.; Solano-Gallego, L. TLR-2 and TLR-4 transcriptions in unstimulated blood from dogs with leishmaniosis due to Leishmania infantum at the time of diagnosis and during follow-up treatment. Vet. Parasitol. 2016, 228, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Esteve, L.O.; Saz, S.V.; Hosein, S.; Solano-Gallego, L. Histopathological findings and detection of Toll-like receptor 2 in cutaneous lesions of canine leishmaniosis. Vet. Parasitol. 2015, 209, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Melo, G.D.; Silva, J.E.; Grano, F.G.; Homem, C.G.; Machado, G.F. Compartmentalized gene expression of Toll-like receptors 2, 4 and 9 in the brain and peripheral lymphoid organs during canine visceral leishmaniasis. Parasite. Immunol. 2014, 36, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Saridomichelakis, M.N.; Koutinas, A.F. Cutaneous involvement in canine leishmaniosis due to Leishmania infantum (syn. L. Chagasi). Vet. Dermatol. 2014, 25, 61–71, e22. [Google Scholar] [CrossRef] [PubMed]
- Ordeix, L.; Fondati, A. Manifestaciones clínicas cutáneas en Leishmaniosis Canina. Una Revisión Actualizada; Solano-Gallego, L., Ed.; Servet: Zaragoza, Spain, 2013; pp. 69–95. [Google Scholar]
- Ordeix, L.; Solano-Gallego, L.; Fondevila, D.; Ferrer, L.; Fondati, A. Papular dermatitis due to Leishmania spp. infection in dogs with parasite-specific cellular immune responses. Vet. Dermatol. 2005, 16, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Ordeix, L.; Dalmau, A.; Osso, M.; Llull, J.; Montserrat-Sangrà, S.; Solano-Gallego, L. Histological and parasitological distinctive findings in clinically-lesioned and normal-looking skin of dogs with different clinical stages of leishmaniosis. Parasit.Vect. 2017, 10, 121. [Google Scholar] [CrossRef] [PubMed]
- Ordeix, L.; Rodríguez, A.; Martínez-Orellana, P.; Montserrat-Sangrà, S.; Solano-Gallego, L. Clinical follow-up of a series of dogs with papular dermatitis due to Leishmania infantum. Vet. Dermatol. 2017, 28, 549. [Google Scholar]
- Bottero, E.; Poggi, M.; Viglione, M. Lesioni papulari indotte da Leishmania spp. in 8 cani giovani. Veterinaria 2006, 1, 33–36. [Google Scholar]
- Lombardo, G.; Pennisi, M.G.; Lupo, T.; Chicharro, C.; Solano-Gallego, L. Papular dermatitis due to Leishmania infantum infection in seventeen dogs: Diagnostic features, extent of the infection and treatment outcome. Parasites Vectors 2014, 7, 120. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Orellana, P.; Montserrat-Sangrà, S.; Ordeix, L.; Solano-Gallego, L. Leishmania infantum-specific IFN-γ production in stimulated blood from dogs with clinical leishmaniosis at diagnosis and during treatment. Vet. Parasitol. 2017, 248, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gallego, L.; Di Filippo, L.; Ordeix, L.; Planellas, M.; Roura, X.; Altet, L.; Martínez-Orellana, P.; Montserrat, S. Early reduction of Leishmania infantum-specific antibodies and blood parasitemia during treatment in dogs with moderate or severe disease. Parasites Vectors 2016, 9, 235. [Google Scholar] [CrossRef] [PubMed]
- Martinez, V.; Quilez, J.; Sanchez, A.; Roura, X.; Francino, O.; Altet, L. Canine leishmaniasis: The key points for qpcr result interpretation. Parasites Vectors 2011, 4, 57. [Google Scholar] [CrossRef] [PubMed]
- Tabar, M.D.; Roura, X.; Francino, O.; Altet, L.; Ruiz de Gopegui, R. Detection of Leishmania infantum by real-time PCR in a canine blood bank. J. Small Anim. Pract. 2008, 49, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gallego, L.; Montserrrat-Sangrà, S.; Ordeix, L.; Martínez-Orellana, P. Leishmania infantum-specific production of IFN-gamma and IL-10 in stimulated blood from dogs with clinical leishmaniosis. Parasites Vectors 2016, 9, 317. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gallego, L.; Cardoso, L.; Pennisi, M.G.; Petersen, C.; Bourdeau, P.; Oliva, G.; Miró, G.; Ferrer, L.; Baneth, G. Diagnostic challenges in the era of canine Leishmania infantum vaccines. Trends. Parasitol. 2017, 33, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Carson, C.; Antoniou, M.; Ruiz-Arguello, M.B.; Alcami, A.; Christodoulou, V.; Messaritakis, I.; Blackwell, J.M.; Courtenay, O. A prime/boost DNA/modified vaccinia virus ankara vaccine expressing recombinant Leishmania DNA encoding tryp is safe and immunogenic in outbred dogs, the reservoir of zoonotic visceral leishmaniasis. Vaccine 2009, 27, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Singh, O.P.; Sundar, S. Whole blood assay and visceral leishmaniasis: Challenges and promises. Immunobiology 2014, 219, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Lichtman, A.H.; Pillai, S. Cellular and Molecular Immunology, 7th ed.; Elsevier: Philadelphia, PA, USA, 2012. [Google Scholar]
- Boggiatto, P.M.; Ramer-Tait, A.E.; Metz, K.; Kramer, E.E.; Gibson-Corley, K.; Mullin, K.; Hostetter, J.M.; Gallup, J.M.; Jones, D.E.; Petersen, C.A. Immunologic indicators of clinical progression during canine Leishmania infantum infection. Clin. Vaccine Immunol. 2010, 17, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Hosein, S.; Rodriguez-Cortes, A.; Blake, D.P.; Allenspach, K.; Alberola, J.; Solano-Gallego, L. Transcription of Toll-like receptors 2, 3, 4 and 9, Foxp3 and Th17 cytokines in a susceptible experimental model of canine Leishmania infantum infection. PLoS ONE 2015, 10, e0140325. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, M.M.; Amorim, I.F.; Pinto, A.J.; Barbosa, V.S.; Pinheiro Lde, J.; Deoti, B.; Faria, A.M.; Tafuri, W.L. Expression of Toll-like receptors 2 and 9 in cells of dog jejunum and colon naturally infected with Leishmania infantum. BMC Immunol. 2013, 14, 22. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Melo, L.M.; Perosso, J.; Almeida, B.F.; Silva, K.L.; Somenzari, M.A.; de Lima, V.M. Effects of p-MAPA immunomodulator on Toll-like receptor 2, ROS, Nitric oxide, MAPKp38 and IKK in PBMC and macrophages from dogs with visceral leishmaniasis. Int. Immunopharmacol. 2014, 18, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Amorim, I.F.; Silva, S.M.; Figueiredo, M.M.; Moura, E.P.; Castro, R.S.; Lima, T.K.; Gontijo Nde, F.; Michalick, M.S.; Gollob, K.J.; Tafuri, W.L. Toll receptors type-2 and Cr3 expression of canine monocytes and its correlation with immunohistochemistry and xenodiagnosis in visceral leishmaniasis. PLoS ONE 2011, 6, e27679. [Google Scholar] [CrossRef] [PubMed]
- Bazzocchi, C.; Mortarino, M.; Comazzi, S.; Bandi, C.; Franceschi, A.; Genchi, C. Expression and function of Toll-like receptor 2 in canine blood phagocytes. Vet. Immunol. Immunopathol. 2005, 104, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Flo, T.H.; Halaas, O.; Torp, S.; Ryan, L.; Lien, E.; Dybdahl, B.; Sundan, A.; Espevik, T. Differential expression of Toll-like receptor 2 in human cells. J. Leukoc. Biol. 2001, 69, 474–481. [Google Scholar] [PubMed]
- Reis, A.B.; Teixeira-Carvalho, A.; Vale, A.M.; Marques, M.J.; Giunchetti, R.C.; Mayrink, W.; Guerra, L.L.; Andrade, R.A.; Correa-Oliveira, R.; Martins-Filho, O.A. Isotype patterns of immunoglobulins: Hallmarks for clinical status and tissue parasite density in brazilian dogs naturally infected by Leishmania (Leishmania) chagasi. Vet. Immunol. Immunopathol. 2006, 112, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.B.; Martins-Filho, O.A.; Teixeira-Carvalho, A.; Giunchetti, R.C.; Carneiro, C.M.; Mayrink, W.; Tafuri, W.L.; Correa-Oliveira, R. Systemic and compartmentalized immune response in canine visceral leishmaniasis. Vet. Immunol. Immunopathol. 2009, 128, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Becker, I.; Salaiza, N.; Aguirre, M.; Delgado, J.; Carrillo-Carrasco, N.; Kobeh, L.G.; Ruiz, A.; Cervantes, R.; Torres, A.P.; Cabrera, N.; et al. Leishmania Lipophosphoglycan (LPG) activates NK cells through Toll-like receptor-2. Mol. Biochem. Parasitol. 2003, 130, 65–74. [Google Scholar] [CrossRef]
- Halliday, A.; Bates, P.A.; Chance, M.L.; Taylor, M.J. Toll-like receptor 2 (TLR2) plays a role in controlling cutaneous leishmaniasis in vivo, but does not require activation by parasite Lipophosphoglycan. Parasites Vectors 2016, 9, 532. [Google Scholar] [CrossRef] [PubMed]
- Paltrinieri, S.; Gradoni, L.; Roura, X.; Zatelli, A.; Zini, E. Laboratory tests for diagnosing and monitoring canine leishmaniasis. Vet. Clin. Pathol. 2016, 45, 552–578. [Google Scholar] [CrossRef] [PubMed]
- McMahon, L.A.; House, A.K.; Catchpole, B.; Elson-Riggins, J.; Riddle, A.; Smith, K.; Werling, D.; Burgener, I.A.; Allenspach, K. Expression of Toll-like receptor 2 in duodenal biopsies from dogs with inflammatory bowel disease is associated with severity of disease. Vet. Immunol. Immunopathol. 2010, 135, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Riggio, M.P.; Lappin, D.F.; Bennett, D. Bacteria and Toll-like receptor and cytokine mRNA expression profiles associated with canine arthritis. Vet. Immunol. Immunopathol. 2014, 160, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Chotimanukul, S.; Sirivaidyapong, S. The localization of Toll-like receptor 2 (TLR2) in the endometrium and the cervix of dogs at different stages of the oestrous cycle and with pyometra. Reprod. Domest. Anim. 2012, 47, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Mercier, E.; Peters, I.R.; Day, M.J.; Clercx, C.; Peeters, D. Toll- and Nod-like receptor mRNA expression in canine sino-nasal aspergillosis and idiopathic lymphoplasmacytic rhinitis. Vet. Immunol. Immunopathol. 2012, 145, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.W.; Kim, S.J.; Cho, H.I.; Lee, S.M. Damps activating innate immune responses in sepsis. Ageing Res. Rev. 2015, 24, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Fonseca, D.C.M.; Oliveira-Rovai, F.M.; Rodas, L.A.C.; Beloti, C.A.C.; Torrecilha, R.B.P.; Ito, P.; Avanco, S.V.; Cipriano, R.S.; Utsunomiya, Y.T.; Hiramoto, R.M.; et al. Dog skin parasite load, TLR-2, IL-10 and TNF-alpha expression and infectiousness. Parasites Immunol. 2017, 39, e12493. [Google Scholar] [CrossRef] [PubMed]
- Tuon, F.F.; Fernandes, E.R.; Duarte, M.I.; Amato, V.S. Expression of TLR2 and TLR4 in lesions of patients with tegumentary american leishmaniasis. Rev. Inst. Med. Trop. Sao Paulo 2012, 54, 159–163. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montserrat-Sangrà, S.; Ordeix, L.; Martínez-Orellana, P.; Solano-Gallego, L. Parasite Specific Antibody Levels, Interferon-γ and TLR2 and TLR4 Transcripts in Blood from Dogs with Different Clinical Stages of Leishmaniosis. Vet. Sci. 2018, 5, 31. https://doi.org/10.3390/vetsci5010031
Montserrat-Sangrà S, Ordeix L, Martínez-Orellana P, Solano-Gallego L. Parasite Specific Antibody Levels, Interferon-γ and TLR2 and TLR4 Transcripts in Blood from Dogs with Different Clinical Stages of Leishmaniosis. Veterinary Sciences. 2018; 5(1):31. https://doi.org/10.3390/vetsci5010031
Chicago/Turabian StyleMontserrat-Sangrà, Sara, Laura Ordeix, Pamela Martínez-Orellana, and Laia Solano-Gallego. 2018. "Parasite Specific Antibody Levels, Interferon-γ and TLR2 and TLR4 Transcripts in Blood from Dogs with Different Clinical Stages of Leishmaniosis" Veterinary Sciences 5, no. 1: 31. https://doi.org/10.3390/vetsci5010031
APA StyleMontserrat-Sangrà, S., Ordeix, L., Martínez-Orellana, P., & Solano-Gallego, L. (2018). Parasite Specific Antibody Levels, Interferon-γ and TLR2 and TLR4 Transcripts in Blood from Dogs with Different Clinical Stages of Leishmaniosis. Veterinary Sciences, 5(1), 31. https://doi.org/10.3390/vetsci5010031




