The Epidemiology of Q Fever in England and Wales 2000–2015
Abstract
:1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
- Establishing a period of enhanced surveillance, where data on the potential source of an individual’s infection is collected.
- Conducting a laboratory survey to determine the proportion of cases reported to the reference laboratories, and any geographical variation in policy.
- Analysing the geographical variation in the case dataset, to determine whether it is real or ascertainment bias, and to explore reasons for the variation.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cutler, S.; Bouzid, M.; Cutler, R. Q Fever. J. Infect. 2007, 54, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Raoult, D.; Marrie, T.J.; Mege, J.L. Natural history and pathophysiology of Q fever. Lancet Infect. Dis. 2005, 5, 219–226. [Google Scholar] [CrossRef]
- Public Health England. Health Protection Report: Latest Infection Reports. Available online: https://www.gov.uk/government/collections/health-protection-report-latest-infection-reports#zoonoses (accessed on 20 April 2017).
- Scottish Government. Q Fever. Available online: http://www.gov.scot/Topics/farmingrural/Agriculture/animal-welfare/News/PregLambFAQ/QFever (accessed on 20 April 2017).
- Maurin, M.; Raoult, D.Q. Fever. Clin. Microbiol. Rev. 1999, 12, 518–553. [Google Scholar] [PubMed]
- Georgiev, M.; Afonso, A.; Neubauer, G.; Needham, H.; Thiery, R.; Rodolakis, A.; Roest, H.J.; Stark, K.D.; Stegeman, J.A.; Vellema, P.; et al. Q fever in humans and farm animals in four European countries, 1982–2010. Euro Surveill. 2013, 18. [Google Scholar]
- Wilson, L.E.; Souper, S.; Prempeh, H.; Young, D.; Pollock, K.G.J.; Stewart, W.C.; Browning, L.M.; Donaghy, M. Investigation of a Q Fever outbreak in a Scottish co-located slaughterhouse and cutting plant. Zoonoses Public Health 2010, 57, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Health Protection Scotland. Coxiella burnetii (Q fever), Scotland, Annual Totals. Available online: http://www.hps.scot.nhs.uk/giz/resourcedetail.aspx?id=1458 (accessed on 21 April 2017).
- Schneeberger, P.M.; Wintenberger, C.; van der Hoek, W.; Stahl, J.P. Q fever in the Netherlands, 2007–2010: What we learned from the largest outbreak ever. Med. Mal. Infect. 2014, 44, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Defra. Zoonoses Report UK. Available online: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/447771/pb13987-zoonoses-report-2013.pdf (accessed on 20 April 2017).
- HM Government. Notifiable Diseases and Causative Organisms: How to Report. Available online: https://www.gov.uk/guidance/notifiable-diseases-and-causative-organisms-how-to-report#list-of-notifiable-organisms-causative-agents (accessed on 20 April 2017).
- Van Woerden, H.C.; Mason, B.W.; Nehaul, L.K.; Smith, R.; Salmon, R.L.; Healy, B.; Valappil, M.; Westmoreland, D.; de Martin, S.; Evans, M.R.; et al. Q fever outbreak in an industrial setting. Emerg. Infect. Dis. 2004, 10, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Wallensten, A.; Moore, P.; Webster, H.; Johnson, C.; van der Burgt, G.; Pritchard, G.; Ellis-Iversen, J.; Oliver, I. Q fever outbreak in Cheltenham, United Kingdon, in 2007 and the use of dispersion modelling to investigate the possibility of airborne spread. Euro Surveill. 2010, 15. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19521 (accessed on 20 April 2017).
- ONS. Population estimates for UK, England and Wales, Scotland and Northern Ireland. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/populationestimatesforukenglandandwalesscotlandandnorthernireland (accessed on 20 April 2017).
- Defra. The Cattle Book 2008. Available online: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/69220/pb13572-cattlebook-2008-090804.pdf (accessed on 20 April 2017).
- Defra. Distribution of Sheep in UK on 02 June 2005. Available online: http://webarchive.nationalarchives.gov.uk/20130402151656/http://archive.defra.gov.uk/foodfarm/farmanimal/diseases/vetsurveillance/reports/documents/rp6150.pdf (accessed on 20 April 2017).
- Thomas, D.R.; Salmon, R.L.; Smith, R.M.M.; Caul, E.O.; Treweek, L.; Kench, S.M.; Coleman, T.J.; Meadows, D.; Morgan-Capner, P.; Sillis, M. Epidemiology of Q fever in the UK. In Rickettsiae and Rickettsial Diseases; Kazar, J., Toman, R., Eds.; Slovak Academy of Sciences: Bratislava, Slovakia, 1996; pp. 512–517. [Google Scholar]
- Maltezou, H.C.; Raoult, D. Q fever in children. Lancet Infect. Dis. 2002, 2, 686–691. [Google Scholar] [CrossRef]
- McCaughey, C.; McKenna, J.; McKenna, C.; Coyle, P.V.; O´Neill, H.J.; Wyatt, D.E.; Smyth, B.; Murray, L.J. Human seroprevalence to Coxiella burnetii (Q fever) in Northern Ireland. Zoonoses Public Health 2008, 55, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Orr, H.J.; Christensen, H.; Smyth, B.; Dance, D.A.B.; Carrington, D.; Paul, I.; Stuart, J.M. Case-control study for risk factors for Q fever in southwest England and Northern Ireland. Euro. Surveill. 2006, 11, 260–262. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=655 (accessed on 20 April 2017). [PubMed]
- Leone, M.; Honstettre, A.; Lepidi, H.; Capo, C.; Bayard, F.; Raoult, D.; Mege, J.L. Effect of sex on Coxiella burnetii infection: Protective role of 17β–Estradiol. J. Infect. Dis. 2004, 189, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Mehraj, V.; Textoris, J.; Capo, C.; Raoult, D.; Leone, M.; Mege, J.L. Overexpression of the Per2 gene in male patients with acute Q fever. J. Infect. Dis. 2012, 206, 1768–1770. [Google Scholar] [CrossRef] [PubMed]
- Hawker, J.I.; Ayres, J.G.; Blair, I.; Evans, M.R.; Smith, D.L.; Smith, E.G.; Burge, P.S.; Carpenter, M.J.; Caul, E.O.; Coupland, B.; et al. A large outbreak of Q fever in the West Midlands: windborne spread into a metropolitan area? Commun. Dis. Public Health 1998, 1, 180–187. [Google Scholar] [PubMed]
- CDC. Diagnosis and Management of Q fever–United States, 2013: Recommendations from CDC and the Q Fever Working Group. MMWR 2013, 62, 1–23. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6203a1.htm (accessed on 20 April 2017).
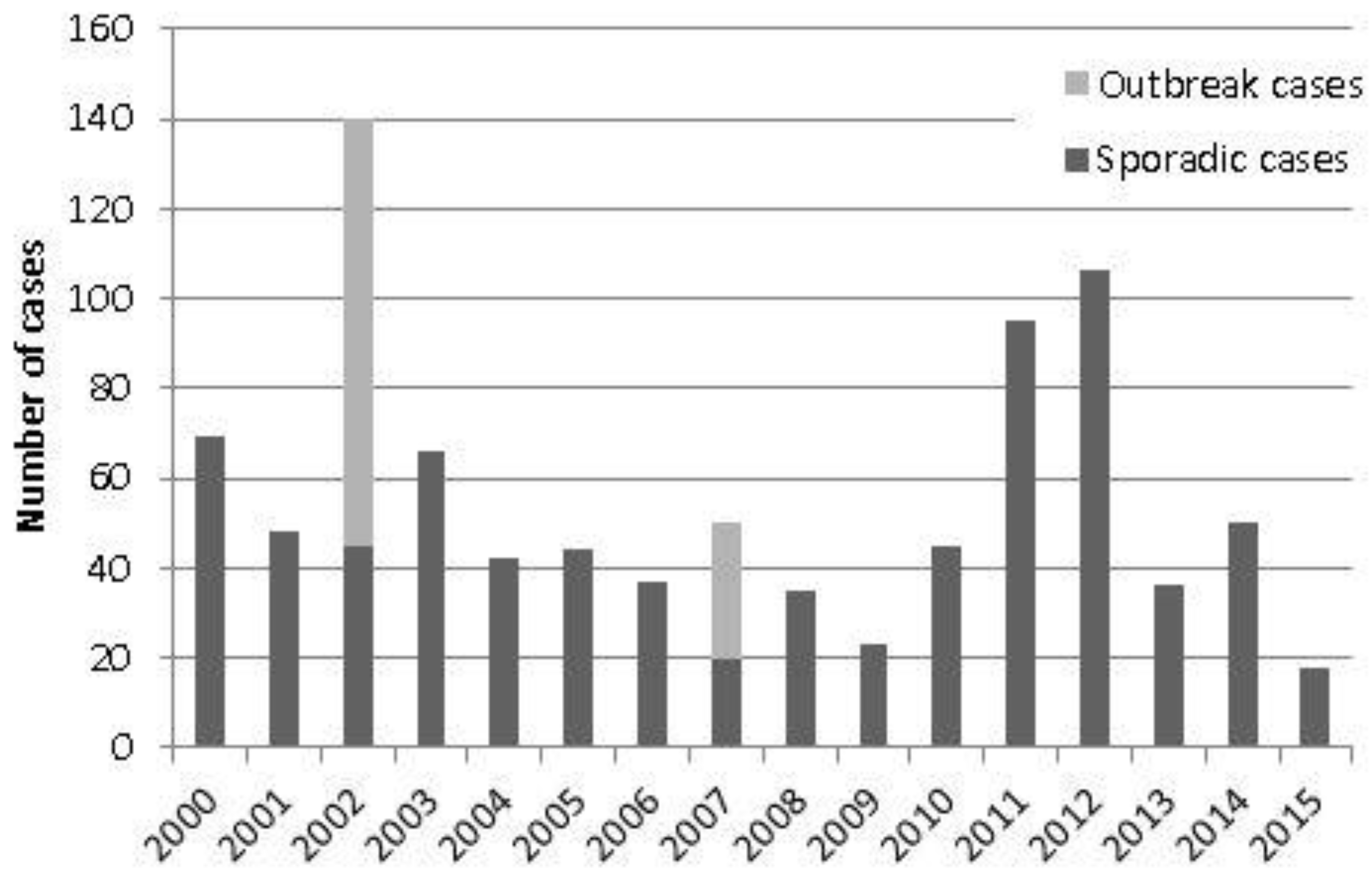
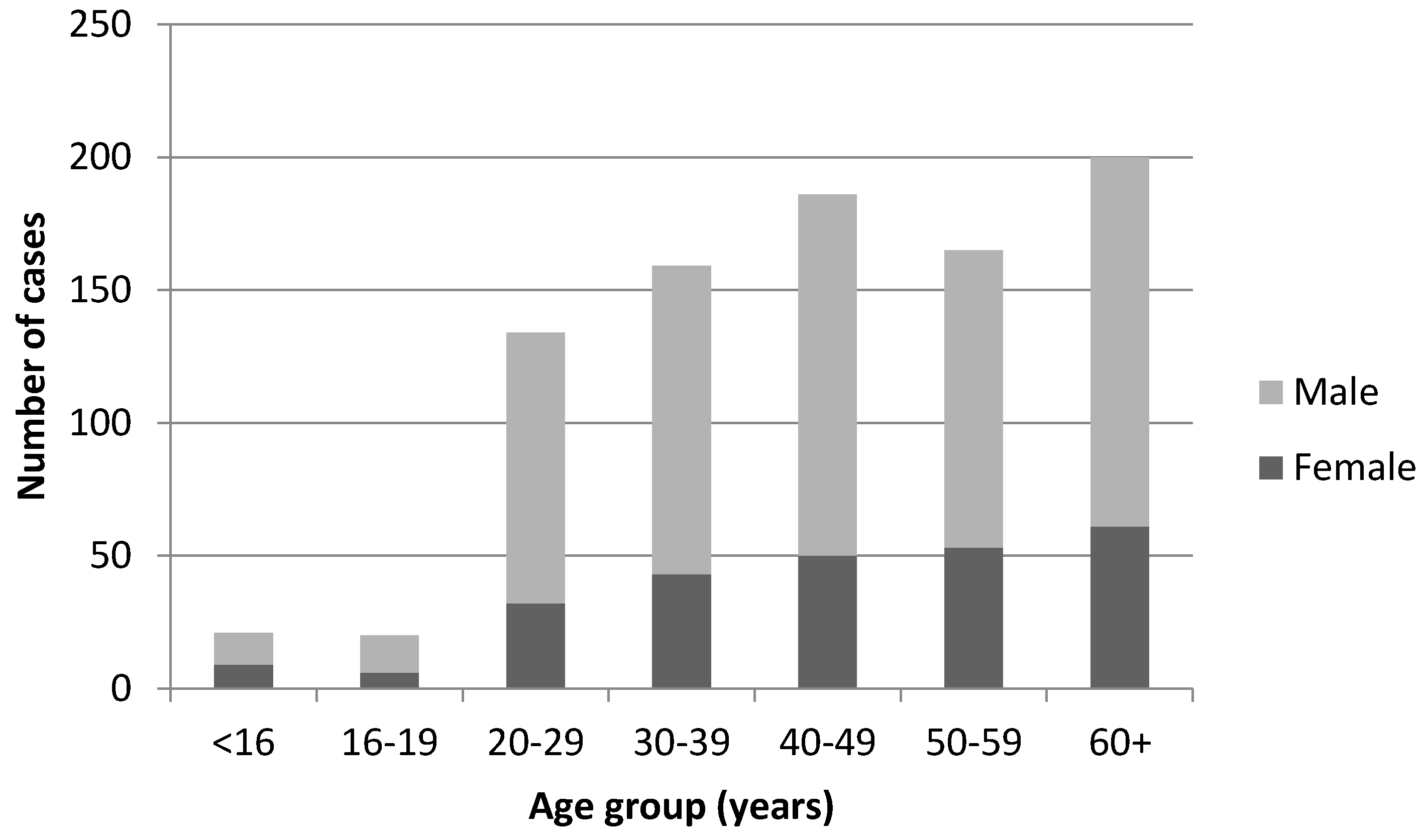
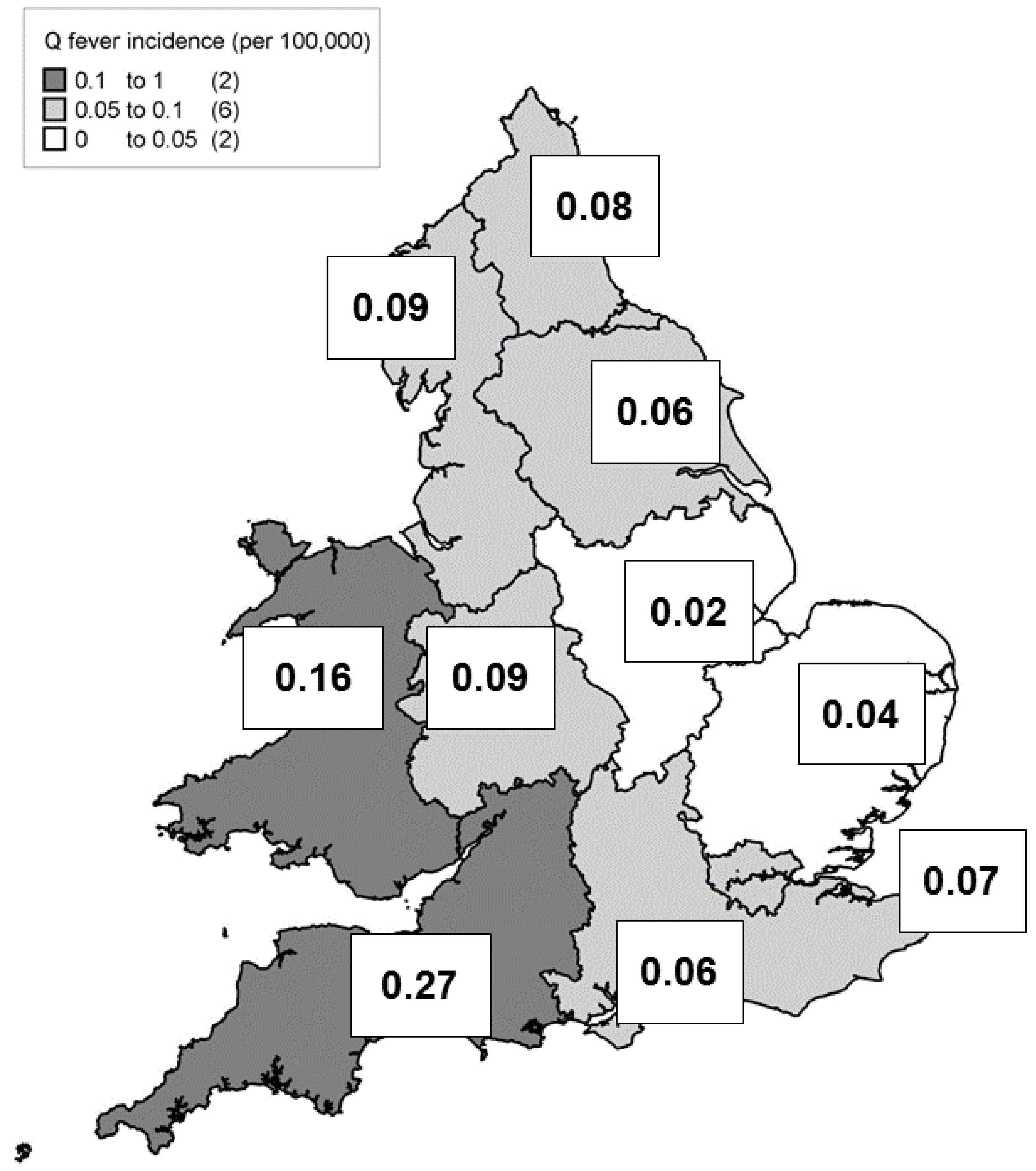
| Year | Total Cases | Sporadic Cases (Excl. Recognised Outbreak Cases) | Incidence Rates (Excl. Recognised Outbreak Cases) |
|---|---|---|---|
| 2015 | 18 | 18 | 0.03 |
| 2014 | 50 | 50 | 0.09 |
| 2013 | 36 | 36 | 0.07 |
| 2012 | 106 | 106 | 0.20 |
| 2011 | 95 | 95 | 0.18 |
| 2010 | 45 | 45 | 0.08 |
| 2009 | 23 | 23 | 0.04 |
| 2008 | 35 | 35 | 0.07 |
| 2007 | 50 | 20 | 0.04 |
| 2006 | 37 | 37 | 0.07 |
| 2005 | 44 | 44 | 0.08 |
| 2004 | 42 | 42 | 0.08 |
| 2003 | 66 | 66 | 0.12 |
| 2002 | 140 | 45 | 0.08 |
| 2001 | 48 | 48 | 0.09 |
| 2000 | 69 | 69 | 0.13 |
| Total | 904 | 779 | |
| Mean | 56.5 | 48.7 | 0.09 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halsby, K.D.; Kirkbride, H.; Walsh, A.L.; Okereke, E.; Brooks, T.; Donati, M.; Morgan, D. The Epidemiology of Q Fever in England and Wales 2000–2015. Vet. Sci. 2017, 4, 28. https://doi.org/10.3390/vetsci4020028
Halsby KD, Kirkbride H, Walsh AL, Okereke E, Brooks T, Donati M, Morgan D. The Epidemiology of Q Fever in England and Wales 2000–2015. Veterinary Sciences. 2017; 4(2):28. https://doi.org/10.3390/vetsci4020028
Chicago/Turabian StyleHalsby, Kate D., Hilary Kirkbride, Amanda L. Walsh, Ebere Okereke, Timothy Brooks, Matthew Donati, and Dilys Morgan. 2017. "The Epidemiology of Q Fever in England and Wales 2000–2015" Veterinary Sciences 4, no. 2: 28. https://doi.org/10.3390/vetsci4020028
APA StyleHalsby, K. D., Kirkbride, H., Walsh, A. L., Okereke, E., Brooks, T., Donati, M., & Morgan, D. (2017). The Epidemiology of Q Fever in England and Wales 2000–2015. Veterinary Sciences, 4(2), 28. https://doi.org/10.3390/vetsci4020028





