The Comparative Value of Feline Virology Research: Can Findings from the Feline Lentiviral Vaccine Be Translated to Humans?
Abstract
:1. Introduction
2. A Commercial FIV Vaccine
3. Why Do Some Virus Strains Resist Vaccine-Induced Protection?
4. A Switch in Receptor Usage Phenotype Occurs with Time Post Infection
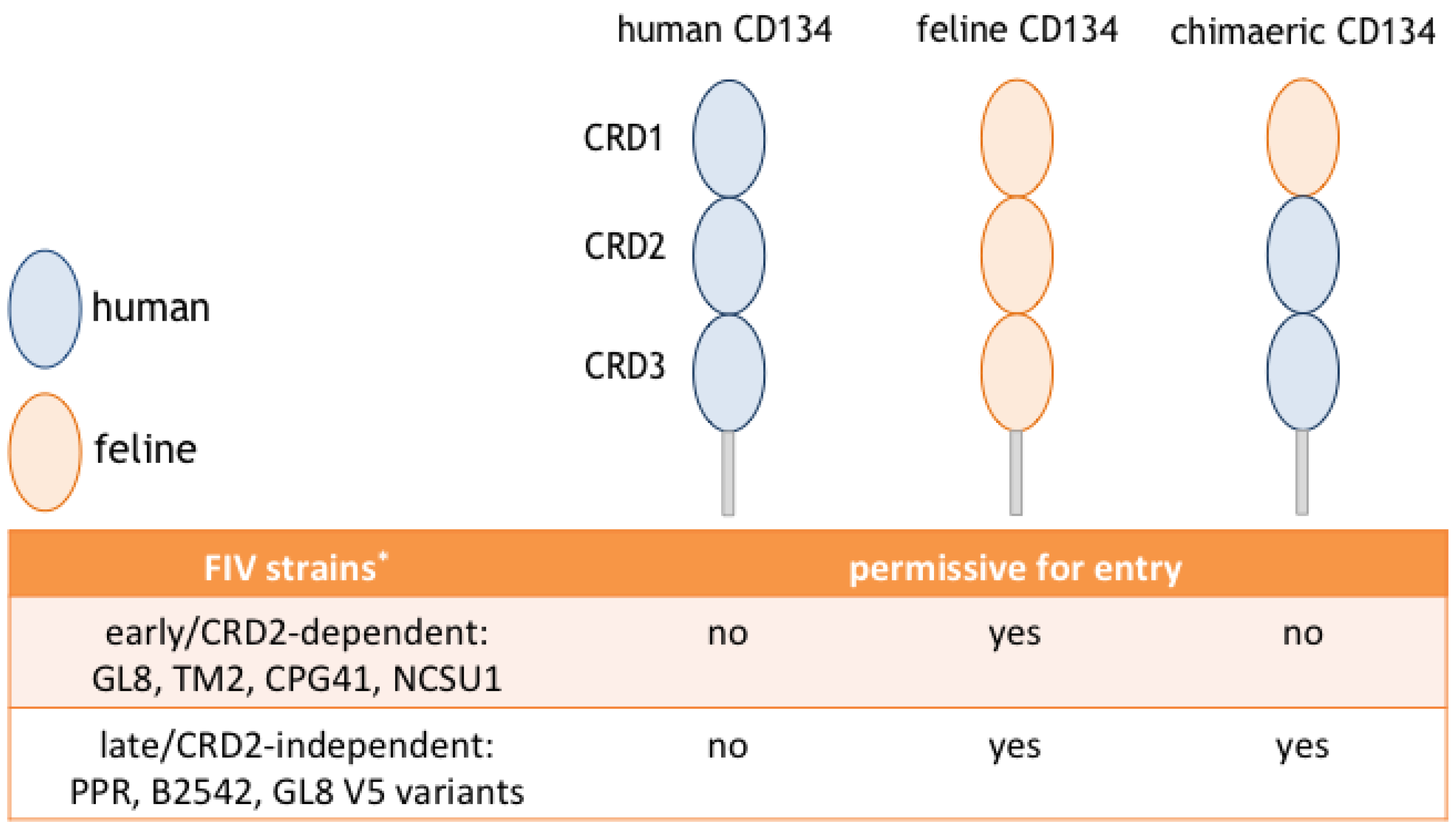
5. Different Modes of Interaction with CD134; CRD2-Dependent and -Independent Isolates
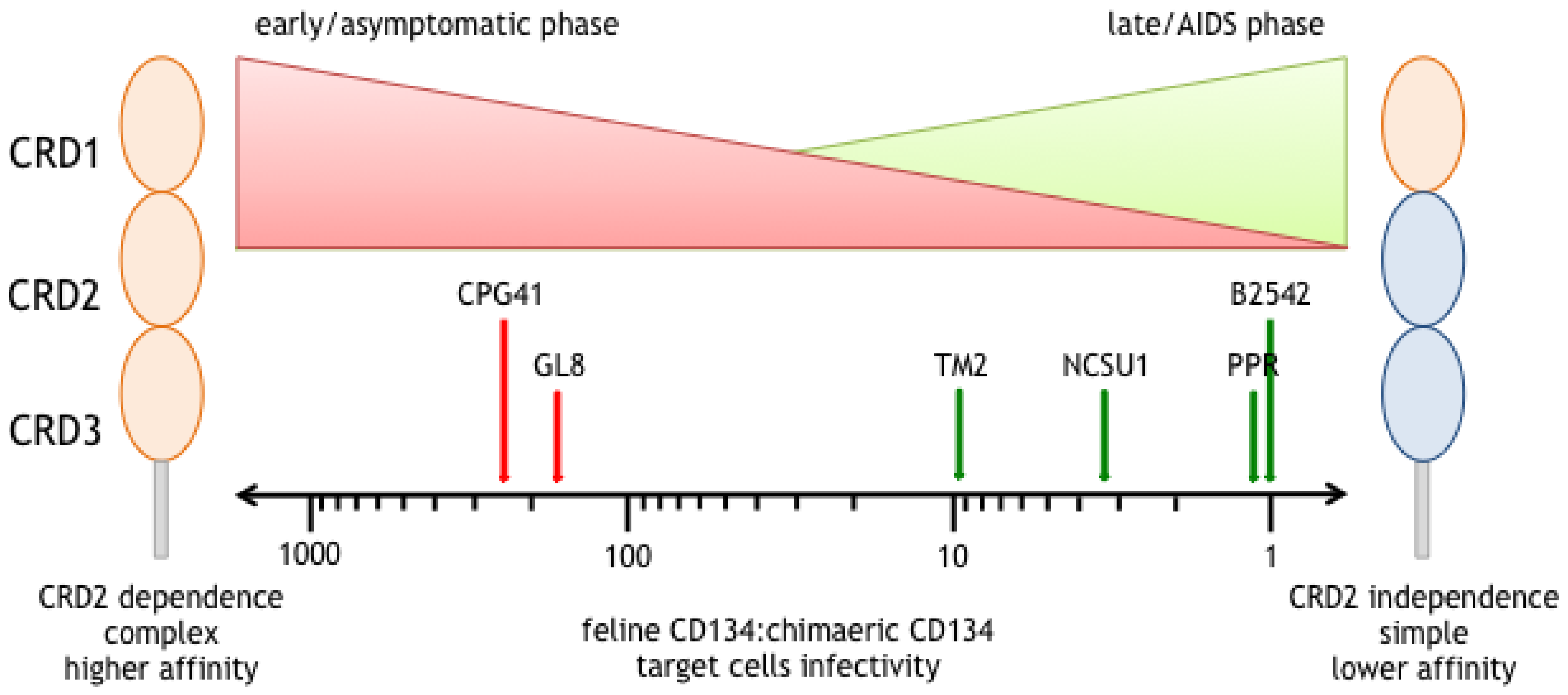
6. CRD2-Independent Variants Emerge in Cats Infected both Experimentally and Naturally with FIV
7. CRD2-Independent Variants Are a Consequence, Not the Cause of Immunodeficiency
8. CRD2-Dependent Variants Resist Vaccine Protection
9. Enhancement of Lentiviral Infection Following Vaccination
10. Conclusions
Acknowledgments
Conflicts of Interest
References
- Willett, B.J.; Flynn, J.N.; Hosie, M.J. FIV infection of the domestic cat: An animal model for AIDS. Immunol. Today 1997, 18, 182–189. [Google Scholar] [CrossRef]
- Bendinelli, M.; Pistello, M.; Lombardi, S.; Poli, A.; Garzelli, C.; Matteucci, D.; Ceccherini-Nelli, L.; Malvaldi, G.; Tozzini, F. Feline immunodeficiency virus: An interesting model for AIDS studies and an important cat pathogen. Clin. Microbiol. Rev. 1995, 8, 87–112. [Google Scholar] [PubMed]
- Bienzle, D. FIV in cats—A useful model of HIV in people? Vet. Immunol. Immunopathol. 2014, 159, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Hesselink, W.; Sondermeijer, P.; Pouwels, H.; Verblakt, E.; Dhore, C. Vaccination of cats against feline immunodeficiency virus (FIV): A matter of challenge. Vet. Microbiol. 1999, 69, 109–110. [Google Scholar] [CrossRef]
- Tellier, M.C.; Pu, R.; Pollock, D.; Vitsky, A.; Tartaglia, J.; Paoletti, E.; Yamamoto, J.K. Efficacy evaluation of prime-boost protocol: Canarypoxvirus-based feline immunodeficiency virus (FIV) vaccine and inactivated FIV-infected cell vaccine against heterologous FIV challenge in cats. AIDS 1998, 12, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Matteucci, D.; Poli, A.; Mazzetti, P.; Sozzi, S.; Bonci, F.; Isola, P.; Zaccaro, L.; Giannecchini, S.; Calandrella, M.; Pistello, M.; et al. Immunogenicity of an anti-clade B feline immunodeficiency fixed-cell virus vaccine in field cats. J. Virol. 2000, 74, 10911–10919. [Google Scholar] [CrossRef] [PubMed]
- Hohdatsu, T.; Okada, S.; Motokawa, K.; Aizawa, C.; Yamamoto, J.K.; Koyama, H. Effect of dual-subtype vaccine against feline immunodeficiency virus infection. Vet. Microbiol. 1997, 58, 155–165. [Google Scholar] [CrossRef]
- Uhl, E.W.; Heaton-Jones, T.G.; Pu, R.; Yamamoto, J.K. FIV vaccine development and its importance to veterinary and human medicine: A review FIV vaccine 2002 update and review. Vet. Immunol. Immunopathol. 2002, 90, 113–132. [Google Scholar] [CrossRef]
- Pu, R.; Coleman, J.; Omori, M.; Arai, M.; Hohdatsu, T.; Huang, C.; Tanabe, T.; Yamamoto, J.K. Dual-subtype FIV vaccine protects cats against in vivo swarms of both homologous and heterologous subtype FIV isolates. AIDS 2001, 15, 1225–1237. [Google Scholar] [CrossRef] [PubMed]
- Kusuhara, H.; Hohdatsu, T.; Okumura, M.; Sato, K.; Suzuki, Y.; Motokawa, K.; Gemma, T.; Watanabe, R.; Huang, C.; Arai, S.; et al. Dual-subtype vaccine (Fel-O-Vax FIV) protects cats against contact challenge with heterologous subtype B FIV infected cats. Vet. Microbiol. 2005, 108, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Doornenbal, E.; Ingwersen, W.; Cloutier, G. Feline leukemia and feline immunodeficiency virus in Canada—A comment. Can. Vet. J. 2012, 53, 9–10. [Google Scholar] [PubMed]
- Huang, C.; Conlee, D.; Gill, M.; Chu, H.J. Dual-subtype feline immunodeficiency virus vaccine provides 12 months of protective immunity against heterologous challenge. J. Feline Med. Surg. 2010, 12, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Girard, M.P.; Osmanov, S.; Assossou, O.M.; Kieny, M.P. Human immunodeficiency virus (HIV) immunopathogenesis and vaccine development: A review. Vaccine 2011, 29, 6191–6218. [Google Scholar] [CrossRef] [PubMed]
- Robinson, H.L. HIV/AIDS vaccines: 2007. Clin. Pharmacol. Ther. 2007, 82, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Wijesundara, D.K.; Jackson, R.J.; Ramshaw, I.A.; Ranasinghe, C. Human immunodeficiency virus-1 vaccine design: Where do we go now? Immunol. Cell Biol. 2011, 89, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Flynn, N.M.; Forthal, D.N.; Harro, C.D.; Judson, F.N.; Mayer, K.H.; Para, M.F.; rgp120 HIV Vaccine Study Group. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J. Infect. Dis. 2005, 191, 654–665. [Google Scholar] [PubMed]
- Gilbert, P.B.; Peterson, M.L.; Follmann, D.; Hudgens, M.G.; Francis, D.P.; Gurwith, M.; Heyward, W.L.; Jobes, D.V.; Popovic, V.; Self, S.G.; et al. Correlation between immunologic responses to a recombinant glycoprotein 120 vaccine and incidence of HIV-1 infection in a phase 3 HIV-1 preventive vaccine trial. J. Infect. Dis. 2005, 191, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Pitisuttithum, P.; Gilbert, P.; Gurwith, M.; Heyward, W.; Martin, M.; van Griensven, F.; Hu, D.; Tappero, J.W.; Choopanya, K.; Bangkok Vaccine Evaluation Group. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J. Infect. Dis. 2006, 194, 1661–1671. [Google Scholar] [CrossRef] [PubMed]
- Buchbinder, S.P.; Mehrotra, D.V.; Duerr, A.; Fitzgerald, D.W.; Mogg, R.; Li, D.; Gilbert, P.B.; Lama, J.R.; Marmor, M.; Del Rio, C.; et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): A double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 2008, 372, 1881–1893. [Google Scholar] [CrossRef]
- McElrath, M.J.; De Rosa, S.C.; Moodie, Z.; Dubey, S.; Kierstead, L.; Janes, H.; Defawe, O.D.; Carter, D.K.; Hural, J.; Akondy, R.; et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: A case-cohort analysis. Lancet 2008, 372, 1894–1905. [Google Scholar] [CrossRef]
- Rerks-Ngarm, S.; Pitisuttithum, P.; Nitayaphan, S.; Kaewkungwal, J.; Chiu, J.; Paris, R.; Premsri, N.; Namwat, C.; de Souza, M.; Adams, E.; et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 2009, 361, 2209–2220. [Google Scholar] [CrossRef] [PubMed]
- Duerr, A.; Huang, Y.; Buchbinder, S.; Coombs, R.W.; Sanchez, J.; del Rio, C.; Casapia, M.; Santiago, S.; Gilbert, P.; Corey, L.; et al. Extended follow-up confirms early vaccine-enhanced risk of HIV acquisition and demonstrates waning effect over time among participants in a randomized trial of recombinant adenovirus HIV vaccine (Step Study). J. Infect. Dis. 2012, 206, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Dunham, S.P.; Bruce, J.; MacKay, S.; Golder, M.; Jarrett, O.; Neil, J.C. Limited efficacy of an inactivated feline immunodeficiency virus vaccine. Vet. Rec. 2006, 158, 561–562. [Google Scholar] [CrossRef] [PubMed]
- Beczkowski, P.M.; Harris, M.; Techakriengkrai, N.; Beatty, J.A.; Willett, B.J.; Hosie, M.J. Neutralising antibody response in domestic cats immunised with a commercial feline immunodeficiency virus (FIV) vaccine. Vaccine 2015, 33, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Westman, M.E.; Malik, R.; Hall, E.; Harris, M.; Norris, J.M. The protective rate of the feline immunodeficiency virus vaccine: An Australian field study. Vaccine 2016, 34, 4752–4758. [Google Scholar] [CrossRef] [PubMed]
- Shimojima, M.; Miyazawa, T.; Ikeda, Y.; McMonagle, E.L.; Haining, H.; Akashi, H.; Takeuchi, Y.; Hosie, M.J.; Willett, B.J. Use of CD134 as a primary receptor by the feline immunodeficiency virus. Science 2004, 303, 1192–1195. [Google Scholar] [CrossRef] [PubMed]
- Willett, B.J.; Hosie, M.J.; Neil, J.C.; Turner, J.D.; Hoxie, J.A. Common mechanism of infection by lentiviruses. Nature 1997. [Google Scholar] [CrossRef] [PubMed]
- English, R.V.; Nelson, P.; Johnson, C.M.; Nasisse, M.; Tompkins, W.A.; Tompkins, M.B. Development of clinical disease in cats experimentally infected with feline immunodeficiency virus. J. Infect. Dis. 1994, 170, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Grossman, Z.; Grossman, Z.; Meier-Schellersheim, M.; Paul, W.E.; Picker, L.J. Pathogenesis of HIV infection: What the virus spares is as important as what it destroys. Nat. Med. 2006, 12, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Margolis, L.; Shattock, R. Selective transmission of CCR5-utilizing HIV-1: The 'gatekeeper' problem resolved? Nat. Rev. Microbiol. 2006, 4, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Regoes, R.R.; Bonhoeffer, S. The HIV coreceptor switch: A population dynamical perspective. Trends Microbiol. 2005, 13, 269–277. [Google Scholar] [CrossRef] [PubMed]
- De Parseval, A.; Chatterji, U.; Morris, G.; Sun, P.; Olson, A.J.; Elder, J.H. Structural mapping of CD134 residues critical for interaction with feline immunodeficiency virus. Nat. Struct. Mol. Biol. 2005, 12, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Willett, B.J.; McMonagle, E.L.; Bonci, F.; Pistello, M.; Hosie, M.J. Mapping the domains of CD134 as a functional receptor for feline immunodeficiency virus. J. Virol. 2006, 80, 7744–7747. [Google Scholar] [CrossRef] [PubMed]
- Willett, B.J.; McMonagle, E.L.; Ridha, S.; Hosie, M.J. Differential utilization of CD134 as a functional receptor by diverse strains of feline immunodeficiency virus. J. Virol. 2006, 80, 3386–3394. [Google Scholar] [CrossRef] [PubMed]
- Willett, B.J.; Kraase, M.; Logan, N.; McMonagle, E.L.; Samman, A.; Hosie, M.J. Modulation of the virus-receptor interaction by mutations in the V5 loop of feline immunodeficiency virus (FIV) following in vivo escape from neutralising antibody. Retrovirology 2010. [Google Scholar] [CrossRef] [PubMed]
- Willett, B.J.; McMonagle, E.L.; Logan, N.; Schneider, P.; Hosie, M.J. Enforced covalent trimerisation of soluble feline CD134 (OX40)-ligand generates a functional antagonist of feline immunodeficiency virus. Mol. Immunol. 2009, 46, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Willett, B.J.; McMonagle, E.L.; Logan, N.; Spiller, O.B.; Schneider, P.; Hosie, M.J. Probing the interaction between feline immunodeficiency virus and CD134 by using the novel monoclonal antibody 7D6 and the CD134 (Ox40) ligand. J. Virol. 2007, 81, 9665–9679. [Google Scholar] [CrossRef] [PubMed]
- Diehl, L.J.; Mathiason-Dubard, C.K.; O'Neil, L.L.; Obert, L.A.; Hoover, E.A. Induction of accelerated feline immunodeficiency virus disease by acute-phase virus passage. J. Virol. 1995, 69, 6149–6157. [Google Scholar] [PubMed]
- Hosie, M.J.; Jarrett, O. Serological responses of cats to feline immunodeficiency virus. AIDS 1990, 4, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Fukasawa, M.; Hasegawa, A.; Maki, N.; Ikuta, K.; Takahashi, E.; Hayami, M.; Mikami, T. Molecular cloning of a novel isolate of feline immunodeficiency virus biologically and genetically different from the original U.S. isolate. J. Virol. 1991, 65, 1572–1577. [Google Scholar] [PubMed]
- Yang, J.S.; English, R.V.; Ritchey, J.W.; Davidson, M.G.; Wasmoen, T.; Levy, J.K.; Gebhard, D.H.; Tompkins, M.B.; Tompkins, W.A. Molecularly cloned feline immunodeficiency virus NCSU1 JSY3 induces immunodeficiency in specific-pathogen-free cats. J. Virol. 1996, 70, 3011–3017. [Google Scholar] [PubMed]
- Kraase, M.; Sloan, R.; Klein, D.; Logan, N.; McMonagle, L.; Biek, R.; Willett, B.J.; Hosie, M.J. Feline immunodeficiency virus env gene evolution in experimentally infected cats. Vet. Immunol. Immunopathol. 2010, 134, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Beczkowski, P.M.; Techakriengkrai, N.; Logan, N.; McMonagle, E.; Litster, A.; Willett, B.J.; Hosie, M.J. Emergence of CD134 cysteine-rich domain 2 (CRD2)-independent strains of feline immunodeficiency virus (FIV) is associated with disease progression in naturally infected cats. Retrovirology 2014. [Google Scholar] [CrossRef] [PubMed]
- Willett, B.J.; Kraase, M.; Logan, N.; McMonagle, E.; Varela, M.; Hosie, M.J. Selective expansion of viral variants following experimental transmission of a reconstituted feline immunodeficiency virus quasispecies. PLoS ONE 2013, 8, e54871. [Google Scholar] [CrossRef] [PubMed]
- Techakriengkrai, N. Investigating the Role of Target Cell Availability in the Pathogenesis of Feline Immunodeficiency Virus Infection. Ph.D. Thesis, University of Glasgow, Glasgow, UK, 2016. [Google Scholar]
- Connor, R.I.; Sheridan, K.E.; Ceradini, D.; Choe, S.; Landau, N.R. Change in coreceptor use correlates with disease progression in HIV-1--infected individuals. J. Exp. Med. 1997, 185, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Hammond, S.A.; Raabe, M.L.; Issel, C.J.; Montelaro, R.C. Evaluation of antibody parameters as potential correlates of protection or enhancement by experimental vaccines to equine infectious anemia virus. Virology 1999, 262, 416–430. [Google Scholar] [CrossRef] [PubMed]
- Giannecchini, S.; Isola, P.; Sichi, O.; Matteucci, D.; Pistello, M.; Zaccaro, L.; Del Mauro, D.; Bendinelli, M. AIDS vaccination studies using an ex vivo feline immunodeficiency virus model: Failure to protect and possible enhancement of challenge infection by four cell-based vaccines prepared with autologous lymphoblasts. J. Virol. 2002, 76, 6882–6892. [Google Scholar] [CrossRef] [PubMed]
- Hosie, M.J.; Osborne, R.; Reid, G.; Neil, J.C.; Jarrett, O. Enhancement after feline immunodeficiency virus vaccination. Vet. Immunol. Immunopathol. 1992, 35, 191–197. [Google Scholar] [CrossRef]
- Richardson, J.; Moraillon, A.; Baud, S.; Cuisinier, A.M.; Sonigo, P.; Pancino, G. Enhancement of feline immunodeficiency virus (FIV) infection after DNA vaccination with the FIV envelope. J. Virol. 1997, 71, 9640–9649. [Google Scholar] [PubMed]
- Siebelink, K.H.; Tijhaar, E.; Huisman, R.C.; Huisman, W.; de Ronde, A.; Darby, I.H.; Francis, M.J.; Rimmelzwaan, G.F.; Osterhaus, A.D. Enhancement of feline immunodeficiency virus infection after immunization with envelope glycoprotein subunit vaccines. J. Virol. 1995, 69, 3704–3711. [Google Scholar] [PubMed]
- Karlas, J.A.; Siebelink, K.H.; Peer, M.A.; Huisman, W.; Cuisinier, A.M.; Rimmelzwaan, G.F.; Osterhaus, A.D. Vaccination with experimental feline immunodeficiency virus vaccines, based on autologous infected cells, elicits enhancement of homologous challenge infection. J. Gen. Virol. 1999, 80, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Vitu, C.; Fontaine, J.J.; Vignoni, M. Caprine arthritis-encephalitis: Trial of an adjuvant vaccine preparation. I. Clinical and virological study. Comp. Immunol. Microbiol. Infect. Dis. 1993, 16, 131–136. [Google Scholar] [CrossRef]
- Raabe, M.L.; Issel, C.J.; Montelaro, R.C. In vitro antibody-dependent enhancement assays are insensitive indicators of in vivo vaccine enhancement of equine infectious anemia virus. Virology 1999, 259, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Staprans, S.I.; Barry, A.P.; Silvestri, G.; Safrit, J.T.; Kozyr, N.; Sumpter, B.; Nguyen, H.; McClure, H.; Montefiori, D.; Cohen, J.I.; et al. Enhanced SIV replication and accelerated progression to AIDS in macaques primed to mount a CD4 T cell response to the SIV envelope protein. Proc. Natl. Acad. Sci. USA 2004, 101, 13026–13031. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Z.; Rushlow, K.E.; Issel, C.J.; Cook, R.F.; Cook, S.J.; Raabe, M.L.; Chong, Y.H.; Costa, L.; Montelaro, R.C. Enhancement of EIAV replication and disease by immunization with a baculovirus-expressed recombinant envelope surface glycoprotein. Virology 1994, 199, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Karlas, J.A.; Siebelink, K.H.; v Peer, M.A.; Huisman, W.; Rimmelzwaan, G.F.; Osterhaus, A.D. Accelerated viraemia in cats vaccinated with fixed autologous FIV-infected cells. Vet. Immunol. Immunopathol. 1998, 65, 353–365. [Google Scholar] [CrossRef]
- Osterhaus, A.D.; Tijhaar, E.; Huisman, R.C.; Huisman, W.; Darby, I.H.; Francis, M.J.; Rimmelzwaan, G.F.; Siebelink, K.H. Accelerated viremia in cats vaccinated with recombinant vaccinia virus expressing envelope glycoprotein of feline immunodeficiency virus. AIDS Res. Hum. Retrovirus. 1996, 12, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Kostrikis, L.G.; Cao, Y.; Ngai, H.; Moore, J.P.; Ho, D.D. Quantitative analysis of serum neutralization of human immunodeficiency virus type 1 from subtypes A, B, C, D, E, F, and I: Lack of direct correlation between neutralization serotypes and genetic subtypes and evidence for prevalent serum-dependent infectivity enhancement. J. Virol. 1996, 70, 445–458. [Google Scholar] [PubMed]
- Guillon, C.; Schutten, M.; Boers, P.H.; Gruters, R.A.; Osterhaus, A.D. Antibody-mediated enhancement of human immunodeficiency virus type 1 infectivity is determined by the structure of gp120 and depends on modulation of the gp120-CCR5 interaction. J. Virol. 2002, 76, 2827–2834. [Google Scholar] [CrossRef] [PubMed]
- Schutten, M.; Andeweg, A.C.; Bosch, M.L.; Osterhaus, A.D. Enhancement of infectivity of a non-syncytium inducing HIV-1 by sCD4 and by human antibodies that neutralize syncytium inducing HIV-1. Scand. J. Immunol. 1995, 41, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Schutten, M.; Andeweg, A.C.; Rimmelzwaan, G.F.; Osterhaus, A.D. Modulation of primary human immunodeficiency virus type 1 envelope glycoprotein-mediated entry by human antibodies. J. Gen. Virol. 1997, 78, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Samman, A. The Role of Virus Neutralisation in Immunity to Feline Immunodeficiency Virus Infection. Ph.D. Thesis, University of Glasgow, Glasgow, UK, 2010. [Google Scholar]
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosie, M.J.; Techakriengkrai, N.; Bęczkowski, P.M.; Harris, M.; Logan, N.; Willett, B.J. The Comparative Value of Feline Virology Research: Can Findings from the Feline Lentiviral Vaccine Be Translated to Humans? Vet. Sci. 2017, 4, 7. https://doi.org/10.3390/vetsci4010007
Hosie MJ, Techakriengkrai N, Bęczkowski PM, Harris M, Logan N, Willett BJ. The Comparative Value of Feline Virology Research: Can Findings from the Feline Lentiviral Vaccine Be Translated to Humans? Veterinary Sciences. 2017; 4(1):7. https://doi.org/10.3390/vetsci4010007
Chicago/Turabian StyleHosie, Margaret J., Navapon Techakriengkrai, Paweł M. Bęczkowski, Matthew Harris, Nicola Logan, and Brian J. Willett. 2017. "The Comparative Value of Feline Virology Research: Can Findings from the Feline Lentiviral Vaccine Be Translated to Humans?" Veterinary Sciences 4, no. 1: 7. https://doi.org/10.3390/vetsci4010007
APA StyleHosie, M. J., Techakriengkrai, N., Bęczkowski, P. M., Harris, M., Logan, N., & Willett, B. J. (2017). The Comparative Value of Feline Virology Research: Can Findings from the Feline Lentiviral Vaccine Be Translated to Humans? Veterinary Sciences, 4(1), 7. https://doi.org/10.3390/vetsci4010007






