The Comparative Diagnostic Features of Canine and Human Lymphoma
Abstract
:1. Introduction
2. Classification of Canine and Human Lymphoma
2.1. Basic Histologic Approach
2.2. FNAC in the Diagnosis of Lymphoma
2.3. Flow Cytometry in the Diagnosis and Classification of Lymphoproliferative Disorders
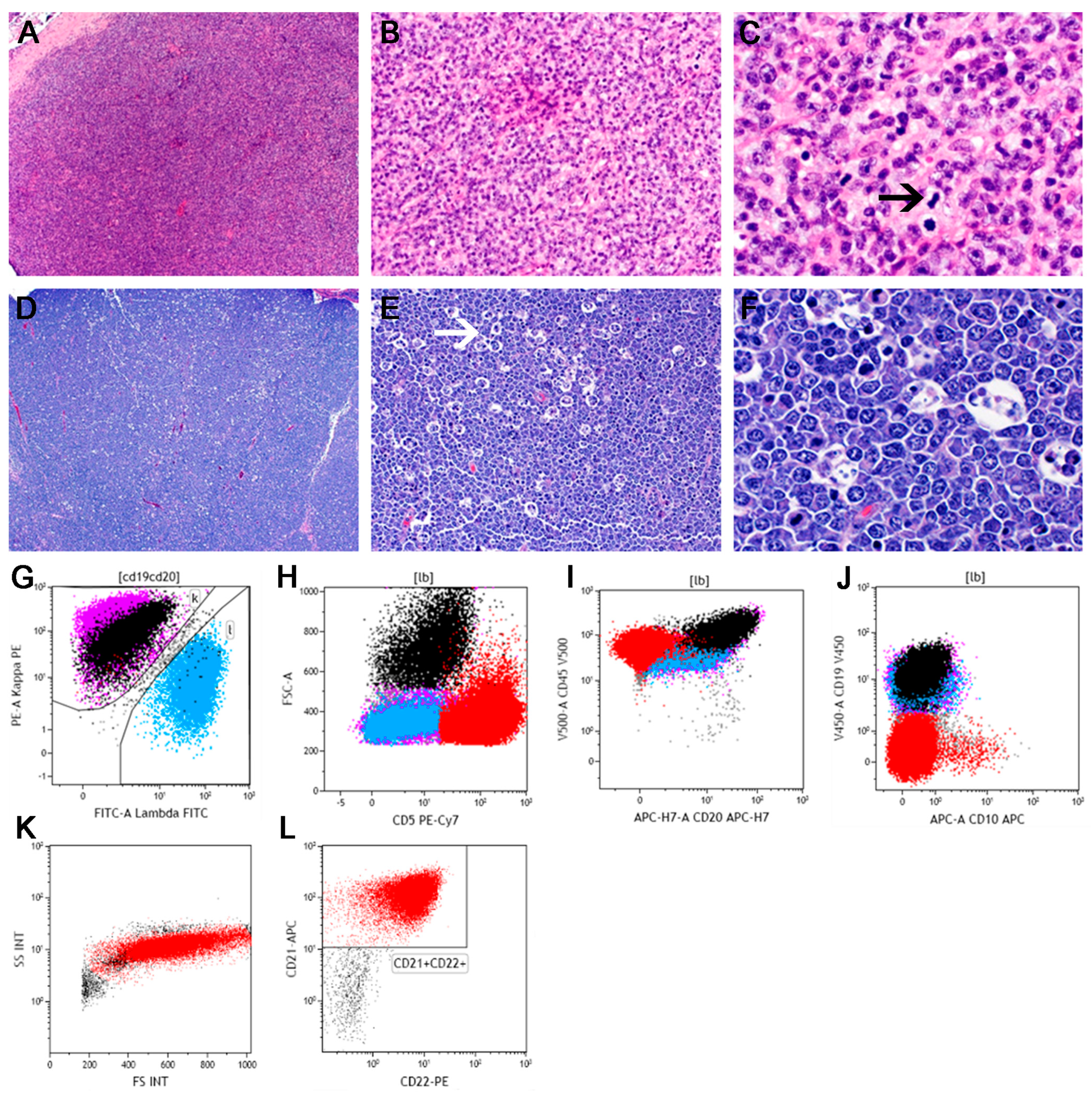
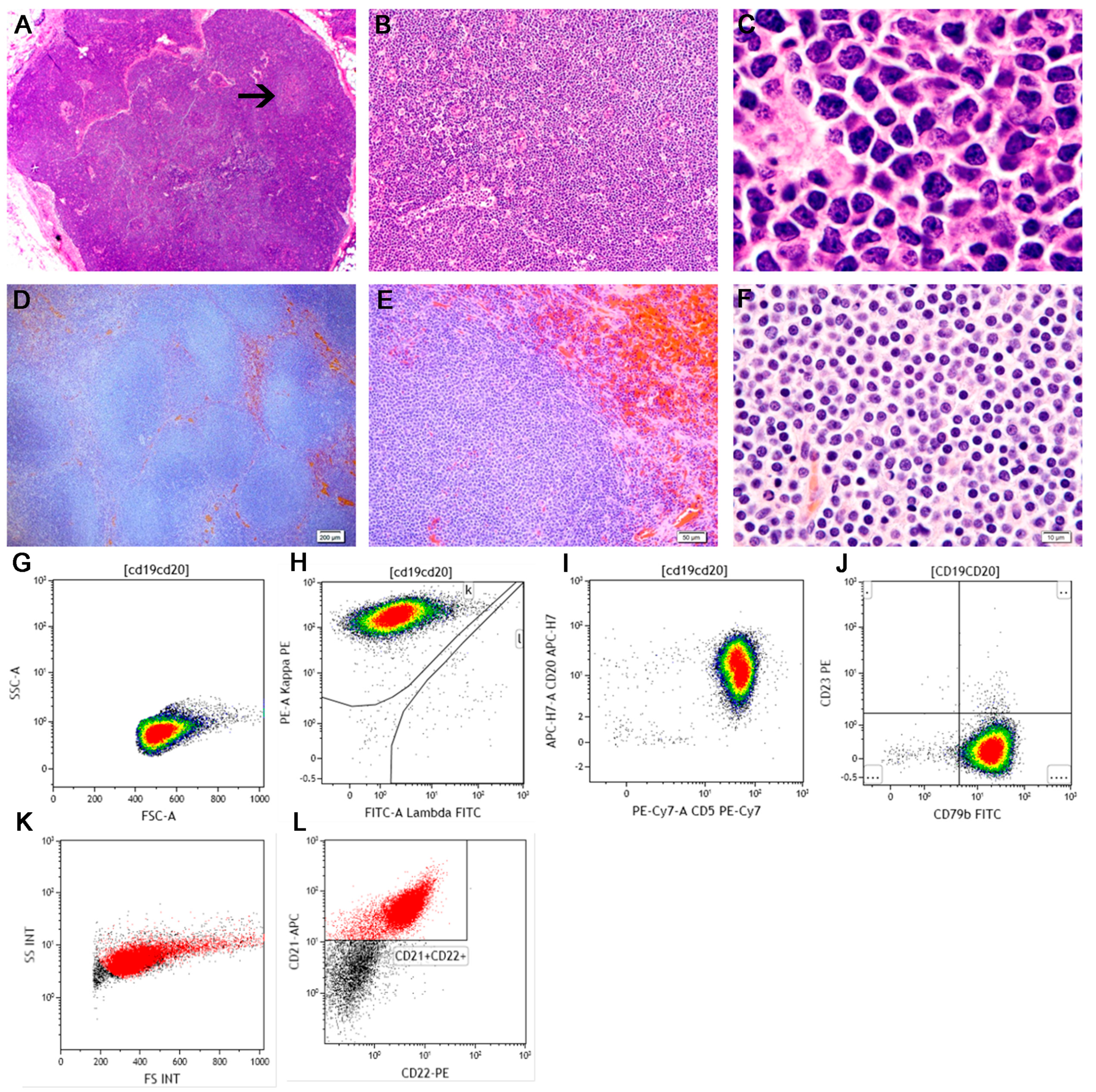
3. Precursor Neoplasms
4. T-Cell Neoplasms
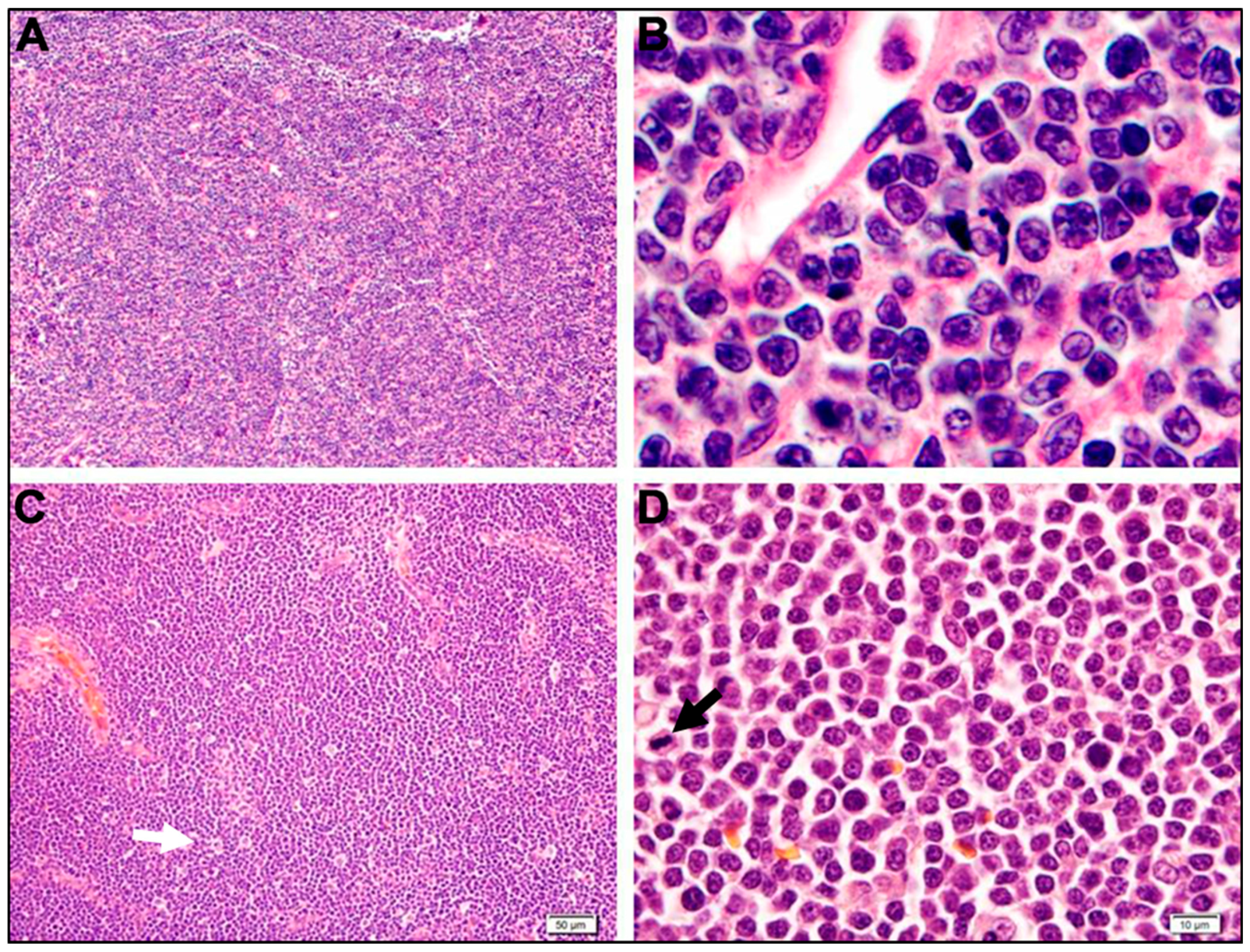
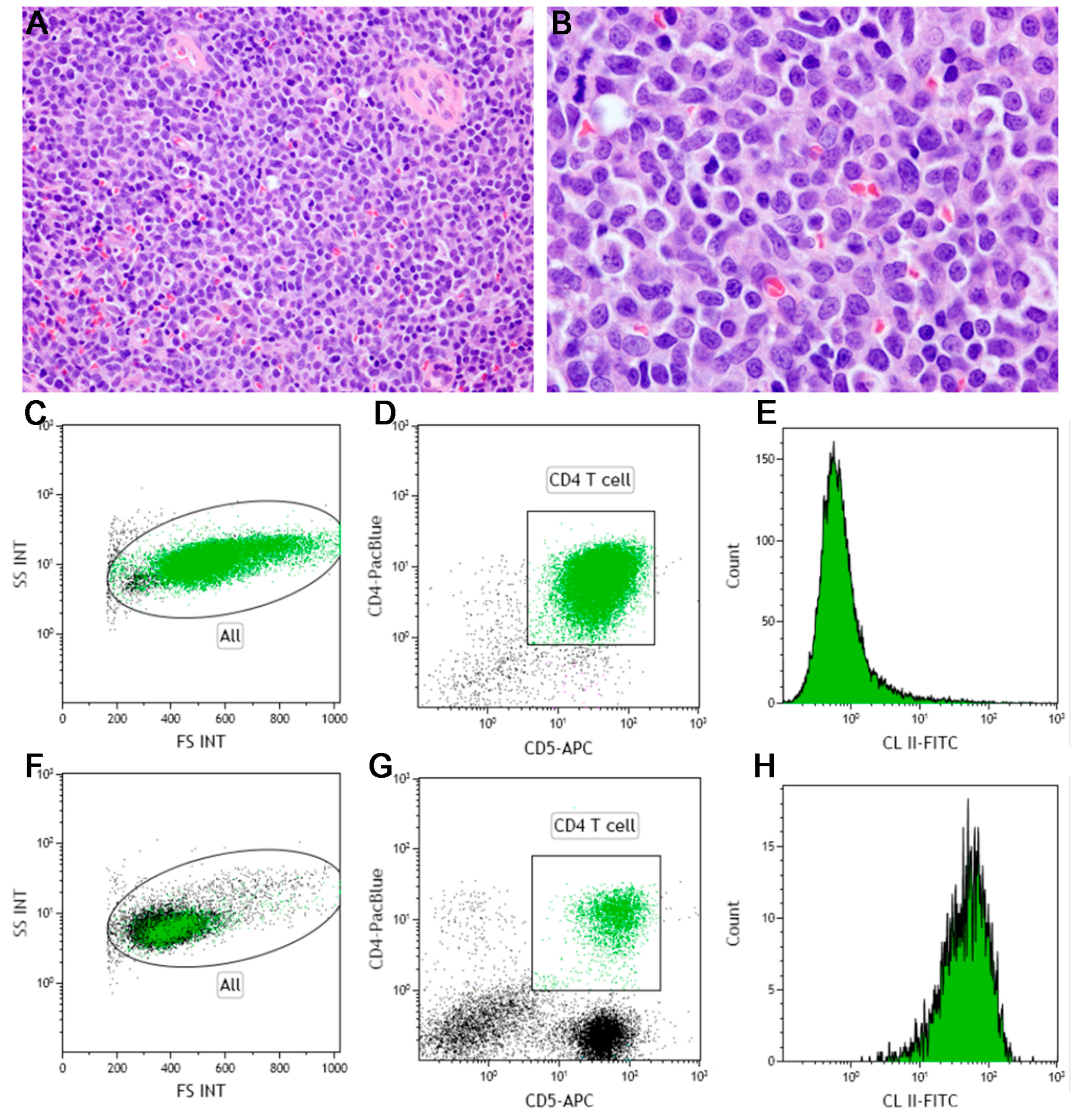
4.1. Peripheral T-Cell, Not Otherwise Specified (PTCL, NOS)
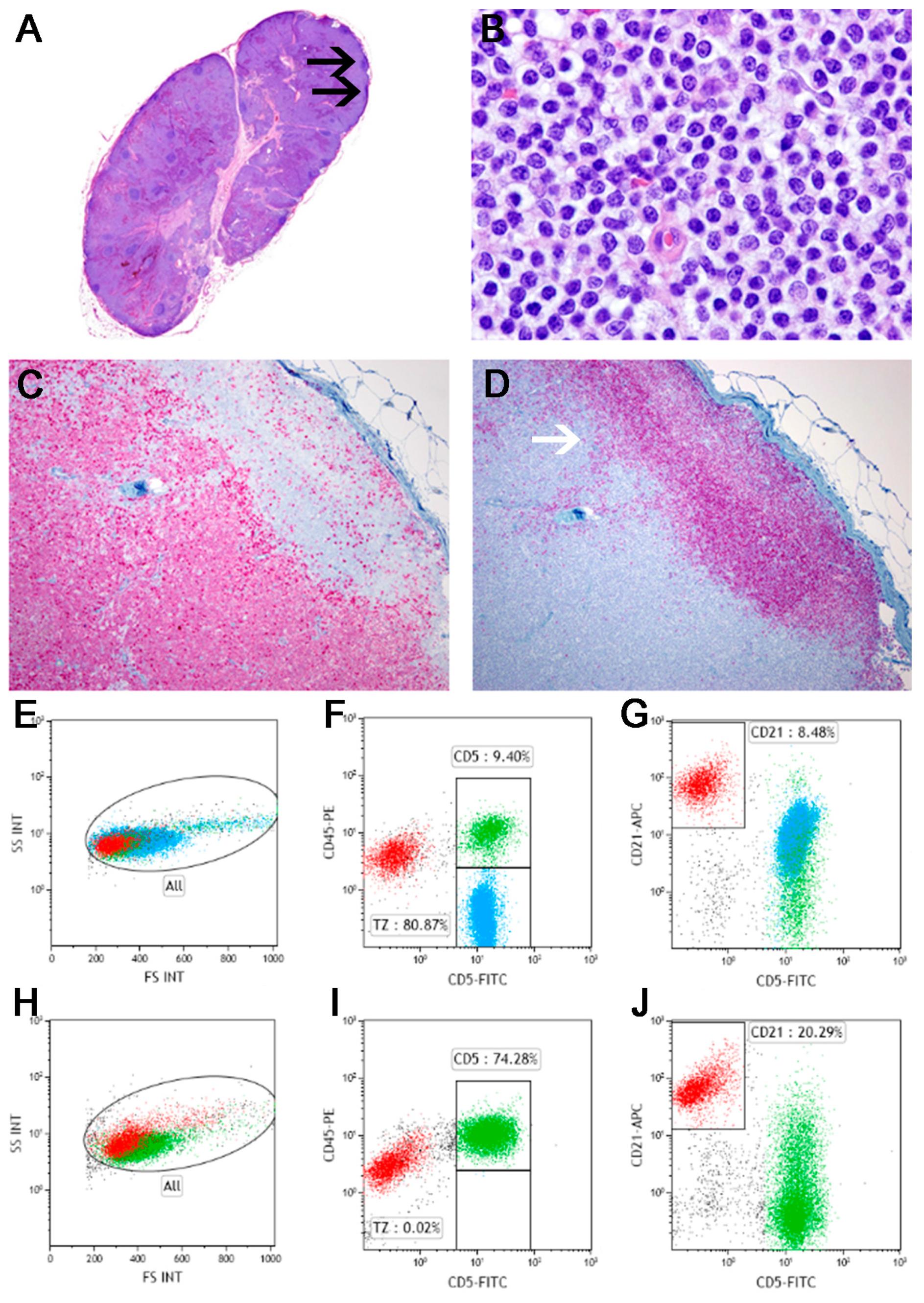
4.2. T-Zone Lymphoma
5. B-Cell Neoplasms
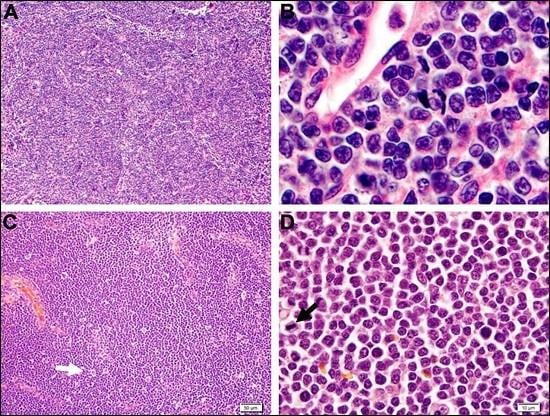
5.1. Diffuse Large B-Cell Lymphoma
5.2. Marginal Zone Lymphoma
5.2.1. Splenic Marginal Zone Lymphoma
5.2.2. Nodal Marginal Zone Lymphoma
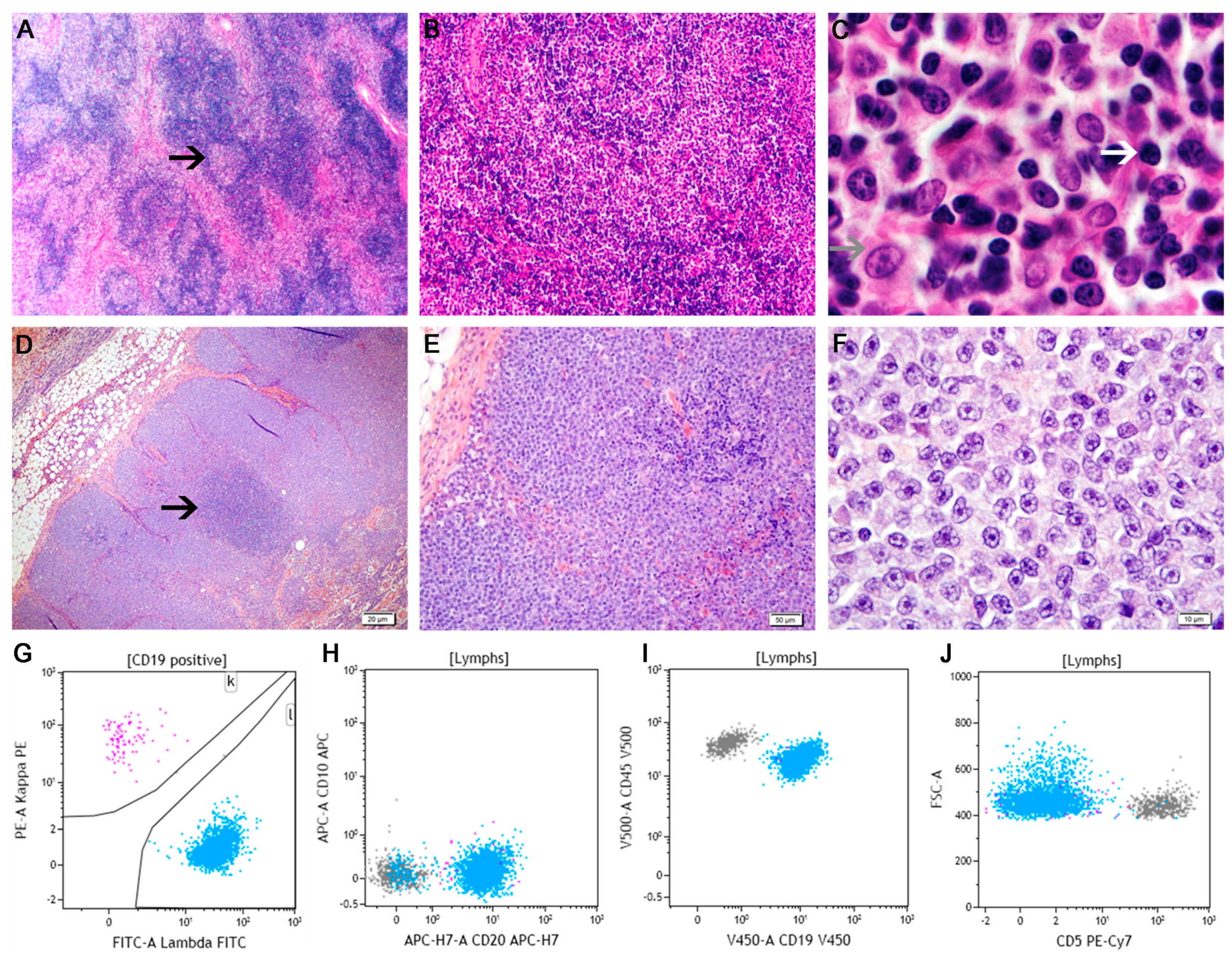
5.3. Mantle Cell Lymphoma
5.4. Follicular Lymphoma
6. Conclusions
Author Contributions
Conflicts of Interest
References
- SEER Cancer Statistics Review, 1975–2012. Howlader, N.; Krapcho, M.; Garshell, J.; Miller, D.; Altekruse, S.F.; Kosary, C.L.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; et al. (Eds.) National Cancer Institute: Bethesda, MD, USA, 2015.
- Merlo, D.F.; Rossi, L.; Pellegrino, C.; Ceppi, M.; Cardellino, U.; Capurro, C.; Ratto, A.; Sambucco, P.L.; Sestito, V.; Tanara, G.; et al. Cancer incidence in pet dogs: Findings of the animal tumor registry of Genoa, Italy. J. Vet. Intern. Med. 2008, 22, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Dorn, C.R.; Taylor, D.O.; Chaulk, L.E.; Hibbard, H.H. The prevalence of spontaneous neoplasms in a defined canine population. Am. J. Public Health Nations Health 1966, 56, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Dorn, C.R.; Taylor, D.O.; Schneider, R.; Hibbard, H.H.; Klauber, M.R. Survey of animal neoplasms in Alameda and Contra Costa Counties, California. II. Cancer morbidity in dogs and cats from Alameda County. J. Natl. Cancer Inst. 1968, 40, 307–318. [Google Scholar] [PubMed]
- Dorn, C.R.; Taylor, D.O.; Schneider, R. The epidemiology of canine leukemia and lymphoma. Bibl. Haematol 1970, 403–415. [Google Scholar]
- Teske, E. Malignant lymphomas in dogs: A review with reference to non-Hodgkin lymphoma in man. Tijdschr Diergeneeskd 1994, 119, 705–717. [Google Scholar] [PubMed]
- Dorn, C.R.; Taylor, D.O.; Hibbard, H.H. Epizootiologic characteristics of canine and feline leukemia and lymphoma. Am. J. Vet. Res. 1967, 28, 993–1001. [Google Scholar] [PubMed]
- Dobson, J.M.; Samuel, S.; Milstein, H.; Rogers, K.; Wood, J.L. Canine neoplasia in the UK: Estimates of incidence rates from a population of insured dogs. J. Small Anim. Pract. 2002, 43, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Chiu, B.C.; Hou, N. Epidemiology and etiology of non-hodgkin lymphoma. Cancer Treat. Res. 2015, 165, 1–25. [Google Scholar] [PubMed]
- Bassig, B.A.; Au, W.-Y.; Mang, O.; Ngan, R.; Morton, M.L.; Ip, D.K.M.; Hu, W.; Zheng, T.; Seow, W.J.; Xu, J.; et al. Subtype-specific incidence rates of lymphoid malignancies in Hong Kong compared to the United States, 2001–2010. Cancer Epidemiol. 2016, 42, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Ponce, F.; Marchal, T.; Magnol, J.P.; Turinelli, V.; Ledieu, D.; Bonnefont, C.; Pastor, M.; Delignette, M.L.; Fournel-Fleury, C. A morphological study of 608 cases of canine malignant lymphoma in France with a focus on comparative similarities between canine and human lymphoma morphology. Vet. Pathol. 2010, 47, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Vezzali, E.; Parodi, A.L.; Marcato, P.S.; Bettini, G. Histopathologic classification of 171 cases of canine and feline non-Hodgkin lymphoma according to the WHO. Vet. Comp. Oncol. 2010, 8, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Valli, V.E.; Kass, P.H.; San Myint, M.; Scott, F. Canine lymphomas: Association of classification type, disease stage, tumor subtype, mitotic rate, and treatment with survival. Vet. Pathol. 2013, 50, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Campo, E.; Swerdlow, S.H.; Harris, N.L.; Pileri, S.; Stein, H.; Jaffe, E.S. The 2008 WHO classification of lymphoid neoplasms and beyond: Evolving concepts and practical applications. Blood 2011, 117, 5019–5032. [Google Scholar] [CrossRef] [PubMed]
- Valli, V.E.; Myint, M.S.; Barthel, A.; Bienzle, D.; Caswell, J.; Colbatzky, F.; Durham, A.; Ehrhart, E.J.; Johnson, Y.; Jones, C.; et al. Classification of canine malignant lymphomas according to the World Health Organization criteria. Vet. Pathol. 2011, 48, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Flood-Knapik, K.E.; Durham, A.C.; Gregor, T.P.; Sanchez, M.D.; Durney, M.E.; Sorenmo, K.U. Clinical, histopathological and immunohistochemical characterization of canine indolent lymphoma. Vet. Comp. Oncol. 2013, 11, 272–286. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J.; Vardiman, J.W. WHO classification of tumours of the haematopoietic and lymphoid tissues, 4th ed.; WHO Classification of Tumours; IARC: Lyon, France, 2008. [Google Scholar]
- Valli, V.E. Veterinary comparative hematopathology, 1st ed.; Blackwell Pub: Ames, IA, USA, 2007. [Google Scholar]
- Ghielmini, M. ESMO Guidelines consensus conference on malignant lymphoma 2011 part 1: Diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL) and chronic lymphocytic leukemia (CLL). Ann. Oncol. 2013, 24, 561–576. [Google Scholar] [CrossRef] [PubMed]
- Thalheim, L.; Williams, L.E.; Borst, L.B.; Fogle, J.E.; Suter, S.E. Lymphoma immunophenotype of dogs determined by immunohistochemistry, flow cytometry, and polymerase chain reaction for antigen receptor rearrangements. J. Vet. Intern. Med. 2013, 27, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.J.; Hong, S.W.; Jeong, H.J.; Yoon, S.O. The diagnostic approach to fine-needle aspiration of malignant lymphoma: using cytomorphology and immunocytochemistry with cell transfer method. Diagn Cytopathol. 2014, 42, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Lioe, T.F.; Elliott, H.; Allen, D.C.; Spence, R.A. The role of fine needle aspiration cytology (FNAC) in the investigation of superficial lymphadenopathy; uses and limitations of the technique. Cytopathology 1999, 10, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Meda, B.A.; Buss, D.H.; Woodruff, R.D.; Cappellari, J.O.; Rainer, R.O.; Powell, B.L.; Geisinger, K.R. Diagnosis and subclassification of primary and recurrent lymphoma. The usefulness and limitations of combined fine-needle aspiration cytomorphology and flow cytometry. Am. J. Clin. Pathol. 2000, 113, 688–699. [Google Scholar] [PubMed]
- Bienzle, D.; Vernau, W. The diagnostic assessment of canine lymphoma: Implications for treatment. Clin. Lab. Med. 2011, 31, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Demurtas, A.; Accinelli, G.; Pacchioni, D.; Godio, L.; Novero, D.; Bussolati, G.; Palestro, G.; Papotti, M.; Stacchini, A. Utility of flow cytometry immunophenotyping in fine-needle aspirate cytologic diagnosis of non-Hodgkin lymphoma: A series of 252 cases and review of the literature. Appl Immunohistochem Mol. Morphol. 2010, 18, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Sokolowska, J.; Micun, J.; Zabielska, K.; Malicka, E.; Lechowski, R. Immunohistochemical study of expression of immunoglobulins in canine B-cell lymphomas. Pol. J. Vet. Sci. 2010, 13, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Lana, S.; Eickhoff, J.; Marcus, E.; Avery, P.R.; Morley, P.S.; Avery, A.C. Class II major histocompatibility complex expression and cell size independently predict survival in canine B-cell lymphoma. J. Vet. Intern. Med. 2011, 25, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Barrena, S. Flow cytometry immunophenotyping of fine-needle aspiration specimens: Utility in the diagnosis and classification of non-Hodgkin lymphomas. Histopathology 2011, 58, 906–918. [Google Scholar] [CrossRef] [PubMed]
- Greig, B.; Oldaker, T.; Warzynski, M.; Wood, B. 2006 Bethesda International Consensus recommendations on the immunophenotypic analysis of hematolymphoid neoplasia by flow cytometry: recommendations for training and education to perform clinical flow cytometry. Cytometry. B Clin. Cytom. 2007, 72 Suppl 1, S23–S33. [Google Scholar] [CrossRef] [PubMed]
- Arun, S.S.; Breuer, W.; Hermanns, W. Immunohistochemical examination of light-chain expression (lambda/kappa ratio) in canine, feline, equine, bovine and porcine plasma cells. Zentralbl. Veterinarmed. A. 1996, 43, 573–576. [Google Scholar] [CrossRef] [PubMed]
- A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood 1997, 89, 3909–3918.
- Bassan, R.; Maino, E.; Cortelazzo, S. Lymphoblastic lymphoma: An updated review on biology, diagnosis and treatment. Eur. J. Haematol. 2016, 96, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Tasca, S.; Carli, E.; Caldin, M.; Menegazzo, L.; Furlanello, T.; Gallego, L.S. Hematologic abnormalities and flow cytometric immunophenotyping results in dogs with hematopoietic neoplasia: 210 Cases (2002–2006). Vet. Clin. Pathol. 2009, 38, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Racke, F.K.; Borowitz, M.J. Precursor B- and T-Cell Neoplasms. In Hematopathology; Jaffe, E.S., Harris, N.L., Vardiman, J.W., Campo, E., Arber, D.A., Eds.; Elsevier Saunders: St. Louis, MO, USA, 2011; pp. 629–639. [Google Scholar]
- You, M.J.; Medeiros, L.J.; Hsi, E.D. T-lymphoblastic leukemia/lymphoma. Am. J. Clin Pathol. 2015, 144, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Cortelazzo, S.; Ponzoni, M.; Ferreri, A.J.; Hoelzer, D. Lymphoblastic lymphoma. Crit Rev. Oncol. Hematol. 2011, 79, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.C. Proposed criteria for classification of acute myeloid leukemia in dogs and cats. Vet. Clin. Pathol. 1991, 20, 63–82. [Google Scholar] [CrossRef] [PubMed]
- Stokol, T.; Schaefer, D.M.; Shuman, M.; Belcher, N.; Dong, L. Alkaline phosphatase is a useful cytochemical marker for the diagnosis of acute myelomonocytic and monocytic leukemia in the dog. Vet. Clin. Pathol. 2015, 44, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Fournel-Fleury, C. Canine T-cell lymphomas: A morphological, immunological, and clinical study of 46 new cases. Vet. Pathol. 2002, 39, 92–109. [Google Scholar] [CrossRef] [PubMed]
- Avery, P.R.; Burton, J.; Bromberek, J.L.; Seelig, D.M.; Elmslie, R.; Correa, S.; Ehrhart, E.J.; Morley, P.S.; Avery, A.C. Flow cytometric characterization and clinical outcome of CD4+ T-cell lymphoma in dogs: 67 cases. J. Vet. Intern. Med. 2014, 28, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Lurie, D.M.; Milner, R.J.; Suter, S.E.; Vernau, W. Immunophenotypic and cytomorphologic subclassification of T-cell lymphoma in the boxer breed. Vet. Immunol Immunopathol 2008, 125, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Gelain, M.E.; Mazzilli, M.; Riondato, F.; Marconato, L.; Comazzi, S. Aberrant phenotypes and quantitative antigen expression in different subtypes of canine lymphoma by flow cytometry. Veterinary Immunology & Immunopathology 2008, 121, 179–188. [Google Scholar]
- Williams, M.J.; Avery, A.C.; Lana, S.E.; Hillers, K.R.; Bachand, A.M.; Avery, P.R. Canine lymphoproliferative disease characterized by lymphocytosis: Immunophenotypic markers of prognosis. J. Vet. Intern. Med. 2008, 22, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Adam, F.; Villiers, E.; Watson, S.; Coyne, K.; Blackwood, L. Clinical pathological and epidemiological assessment of morphologically and immunologically confirmed canine leukaemia. Vet. Comp. Oncol. 2009, 7, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Vernau, W.; Moore, P.F. An immunophenotypic study of canine leukemias and preliminary assessment of clonality by polymerase chain reaction. Vet. Immunol Immunopathol 1999, 69, 145–164. [Google Scholar] [CrossRef]
- Ponce, F.; Magnol, J.P.; Blavier, A.; Bonnefont, C.; Ghernati, I.; Felman, P.; Buff, S.; Fournel-Fleury, C. Clinical, morphological, and immunological study of 13 cases of canine lymphoblastic lymphoma: Comparison with the human entity. Comp. Clin. Path. 2003, 12, 75–83. [Google Scholar] [CrossRef]
- Williams, M.J.; Avery, A.C.; Lana, S.E.; Hillers, K.R.; Bachand, A.M.; Avery, P.R. Canine lymphoproliferative disease characterized by lymphocytosis: Immunophenotypic markers of prognosis. J. Vet. Int. Med. 2008, 22, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Gorczyca, W. Immunophenotypic Markers: Differential Diagnosis. In Atlas of Differential Diagnosis in Neoplastic Hematopathology; CRC Press: Boca Raton, FL, USA, 2014; pp. 119–155. [Google Scholar]
- Bhargava, P.; Kallakury, B.V.; Ross, J.S.; Azumi, N.; Bagg, A. CD79a is heterogeneously expressed in neoplastic and normal myeloid precursors and megakaryocytes in an antibody clone-dependent manner. Am. J. Clin Pathol. 2007, 128, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Modiano, J.F. Distinct B-cell and T-cell lymphoproliferative disease prevalence among dog breeds indicates heritable risk. Cancer Res. 2005, 65, 5654–5661. [Google Scholar] [CrossRef] [PubMed]
- Piccaluga, P.P.; Tabanelli, V.; Pileri, S.A. Molecular genetics of peripheral T-cell lymphomas. Int. J. Hematol. 2014, 99, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Piccaluga, P.P. Gene expression analysis of peripheral T cell lymphoma, unspecified, reveals distinct profiles and new potential therapeutic targets. J. Clin. Invest. 2007, 117, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y. Peripheral T-cell lymphomas with a follicular growth pattern are derived from follicular helper T cells (TFH) and may show overlapping features with angioimmunoblastic T-cell lymphomas. Am. J. Surg. Pathol. 2009, 33, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J. Molecular signatures to improve diagnosis in peripheral T-cell lymphoma and prognostication in angioimmunoblastic T-cell lymphoma. Blood 2010, 115, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Gallamini, A. Peripheral T-cell lymphoma unspecified (PTCL-U): A new prognostic model from a retrospective multicentric clinical study. Blood 2004, 103, 2474–2479. [Google Scholar] [CrossRef] [PubMed]
- Savage, K.J.; Ferreri, A.J.; Zinzani, P.L.; Pileri, S.A. Peripheral T-cell lymphoma--Not otherwise specified. Crit Rev. Oncol. Hematol. 2011, 79, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Vose, J.; Armitage, J.; Weisenburger, D.; International, T.C.L.P. International peripheral T-cell and natural killer/T-cell lymphoma study: Pathology findings and clinical outcomes. J. Clin. Oncol. 2008, 26, 4124–4130. [Google Scholar] [PubMed]
- De Leval, L.; Savilo, E.; Longtine, J.; Ferry, J.A.; Harris, N.L. Peripheral T-cell lymphoma with follicular involvement and a CD4+/bcl-6+ phenotype. Am. J. Surg. Pathol. 2001, 25, 395–400. [Google Scholar] [CrossRef] [PubMed]
- De Leval, L.; Ralfkiaer, E.; Jaffe, E.S. Peripheral T-cell Lymphoma, Not Otherwise Specified. In Hematopathology; Jaffe, E.S., Harris, N.L., Vardiman, J.W., Campo, E., Arber, D.A., Eds.; Elsevier Saunders: St. Louis, MO, USA, 2011; pp. 541–553. [Google Scholar]
- Piccaluga, P.P.; Agostinelli, C.; Tripodo, C.; Gazzola, A.; Bacci, F.; Sabattini, E.; Pileri, S.A.; European T-cell Lymphoma Study Group. Peripheral T-cell lymphoma classification: The matter of cellular derivation. Expert Rev. Hematol. 2011, 4, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Went, P. Marker expression in peripheral T-cell lymphoma: A proposed clinical-pathologic prognostic score. J. Clin. Oncol. 2006, 24, 2472–2479. [Google Scholar] [CrossRef] [PubMed]
- Onaindia, A. CD30 Expression by B and T Cells: A Frequent Finding in Angioimmunoblastic T-Cell Lymphoma and Peripheral T-Cell Lymphoma-Not Otherwise Specified. Am. J. Surg. Pathol. 2016, 40, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Geissinger, E.; Bonzheim, I.; Roth, S.; Rosenwald, A.; Muller-Hermelink, H.K.; Rudiger, T. CD52 expression in peripheral T-cell lymphomas determined by combined immunophenotyping using tumor cell specific T-cell receptor antibodies. Leuk Lymphoma 2009, 50, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yuan, C.M.; Hubacheck, J.; Janik, J.E.; Wilson, W.; Morris, J.C.; Jasper, G.A.; Stetler-Stevenson, M. Variable CD52 expression in mature T cell and NK cell malignancies: Implications for alemtuzumab therapy. Br. J. Haematol 2009, 145, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Weisenburger, D.D. Peripheral T-cell lymphoma, not otherwise specified: A report of 340 cases from the International Peripheral T-cell Lymphoma Project. Blood 2011, 117, 3402–3408. [Google Scholar] [CrossRef] [PubMed]
- Seelig, D.M.; Avery, P.; Webb, T.; Yoshimoto, J.; Bromberek, J.; Ehrhart, E.J.; Avery, A.C. Canine T-Zone Lymphoma: Unique Immunophenotypic Features, Outcome, and Population Characteristics. J. Vet. Intern. Med. 2014, 28, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Godde-Salz, E.; Schwarze, E.W.; Stein, H.; Lennert, K.; Grote, W. Cytogenetic findings in T-zone lymphoma. J. Cancer Res. Clin. Oncol. 1981, 101, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Valli, V.E.; Vernau, W.; de Lorimier, L.P.; Graham, P.S.; Moore, P.F. Canine indolent nodular lymphoma. Vet. Pathol. 2006, 43, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Martini, V.; Marconato, L.; Poggi, A.; Riondato, F.; Aresu, L.; Cozzi, M.; Comazzi, S. Canine small clear cell/T-zone lymphoma: Clinical presentation and outcome in a retrospective case series. Vet. Comp. Oncol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Teske, E. Cytology of the lymphoid tissues. In Manual of Diagnostic Cytology of the Dog and Cat; Dunn, J., Ed.; Wiley Blackwell: West Sussex, UK, 2014; pp. 330–356. [Google Scholar]
- Helbron, D.; Brittinger, G.; Lennert, K. T-zone lymphoma--clinical symptoms, therapy, and prognosis (author’s transl). Blut 1979, 39, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Lennert, K.; Feller, A.C. Histopathology of Non-Hodgkin’s Lymphoma (Based on the Updated Kiel Classification); Springer: Berlin, Germany, 1992. [Google Scholar]
- Martini, V.; Poggi, A.; Riondato, F.; Gelain, M.E.; Aresu, L.; Comazzi, S. Flow-cytometric detection of phenotypic aberrancies in canine small clear cell lymphoma. Vet. Comp. Oncol. 2015, 13, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Nogai, H.; Dorken, B.; Lenz, G. Pathogenesis of non-Hodgkin’s lymphoma. J. Clin. Oncol. 2011, 29, 1803–1811. [Google Scholar] [CrossRef] [PubMed]
- Richards, K.L.; Motsinger-Reif, A.A.; Chen, H.W.; Fedoriw, Y.; Fan, C.; Nielsen, D.M.; Small1, G.W.; Thomas, R.; Smith, C.; Dave, S.S. Gene profiling of canine B-cell lymphoma reveals germinal center and postgerminal center subtypes with different survival times, modeling human DLBCL. Cancer Res. 2013, 73, 5029–5039. [Google Scholar] [CrossRef] [PubMed]
- Mudaliar, M.A.; Haggart, R.D.; Miele, G.; Sellar, G.; Tan, K.A.L.; Goodlad, J.R.; Milne, E.; Vail, D.M.; Kurzman, I.; Crowther, D.; et al. Comparative gene expression profiling identifies common molecular signatures of NF-kappaB activation in canine and human diffuse large B cell lymphoma (DLBCL). PLoS ONE 2013, 8, e72591. [Google Scholar] [CrossRef] [PubMed]
- Frantz, A.M.; Sarver, A.L.; Ito, D.; Phang, T.L.; Karimpour-Fard, A.; Scott, M.C.; Valli, V.E.O.; Lindblad-Toh, K.; Burgess, K.E.; Husbands, B.D.; et al. Molecular profiling reveals prognostically significant subtypes of canine lymphoma. Vet. Pathol. 2013, 50, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Martelli, M.; Ferreri, A.J.; Agostinelli, C.; Di Rocco, A.; Pfreundschuh, M.; Pileri, S.A. Diffuse large B-cell lymphoma. Crit Rev. Oncol. Hematol. 2013, 87, 146–171. [Google Scholar] [CrossRef] [PubMed]
- Comazzi, S.; Aresu, L.; Marconato, L. Transformation of Canine Lymphoma/Leukemia to More Aggressive Diseases: Anecdotes or Reality? Front. Vet. Sci. 2015, 2, 42. [Google Scholar] [CrossRef] [PubMed]
- Mey, U.; Hitz, F.; Lohri, A.; Pederiva, S.; Taverna, C.; Tzankov, A.; Meier, O.; Yeow, K.; Renner, C. Diagnosis and treatment of diffuse large B-cell lymphoma. Swiss. Med. Wkly. 2012, 142, w13511. [Google Scholar] [CrossRef] [PubMed]
- Aresu, L.; Aricò, A.; Ferraresso, F.; Martini, V.; Comazzi, C.S.; Riondato, F.; Giantin, G.; Dacasto, M.; Guadagnin, E.; Frayssinet, P.; et al. Minimal residual disease detection by flow cytometry and PARR in lymph node, peripheral blood and bone marrow, following treatment of dogs with diffuse large B-cell lymphoma. Vet. J. 2014, 200, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Sehn, L.H.; Scott, D.W.; Chhanabhai, M.; Berry, B.; Ruskova, A.; Berkahn, L.; Connors, J.M.; Gascoyne, R.D. Impact of concordant and discordant bone marrow involvement on outcome in diffuse large B-cell lymphoma treated with R-CHOP. J. Clin. Oncol. 2011, 29, 1452–1457. [Google Scholar] [CrossRef] [PubMed]
- Raskin, R.E.; Krehbiel, J.D. Prevalence of leukemic blood and bone marrow in dogs with multicentric lymphoma. J. Am. Vet. Med. Assoc. 1989, 194, 1427–1429. [Google Scholar] [PubMed]
- Chan, A.C.L.; Chan, J.K.C. Diffuse Large B-cell Lymphoma. In Hematopathology; Jaffe, E.S., Harris, N.L., Vardiman, J.W., Campo, E., Arber, D.A., Eds.; Elsevier Saunders: St. Louis, MO, USA, 2011; pp. 349–381. [Google Scholar]
- Engelhard, M.; Brittinger, G.; Huhn, D.; Gerhartz, H.G.; Meusers, P.; Siegert, W.; Thiel, T.; Wilmanns, W.; Aydemir, U.; Bierwolf, S.; et al. Subclassification of diffuse large B-cell lymphomas according to the Kiel classification: distinction of centroblastic and immunoblastic lymphomas is a significant prognostic risk factor. Blood 1997, 89, 2291–2297. [Google Scholar] [PubMed]
- Baars, J.W.; de Jong, D.; Willemse, E.M.; Gras, L.; Dalesio, O.; v Heerde, P.; Huygens, P.C.; vd Lelie, H.; Kr vd Borne, A.E. Diffuse large B-cell non-Hodgkin lymphomas: the clinical relevance of histological subclassification. Br. J. Cancer 1999, 79, 1770–1776. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.A.; Eisen, M.B.; Davis, R.E.; Ma, C.; Lossos, I.S.; Rosenwald, A.; Boldrick, J.C.; Sabet, H.; Tran, T.; Yu, X.; et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000, 403, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Rosenwald, A.; Wright, G.; Chan, W.C.; Connors, J.M.; Campo, E.; Fisher, R.I.; Gascoyne, R.D.; Muller-Hermelink, H.K.; Smeland, E.B.; Giltnane, J.M.; et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N. Engl. J. Med. 2002, 346, 1937–1947. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.H.; Dunleavy, K.; Pittaluga, S.; Hegde, U.; Grant, N.; Steinberg, S.M.; Raffeld, M.; Gutierrez, M.; Chabner, B.A.; Staudt, L.; et al. Phase II study of dose-adjusted EPOCH and rituximab in untreated diffuse large B-cell lymphoma with analysis of germinal center and post-germinal center biomarkers. J. Clin. Oncol. 2008, 26, 2717–2724. [Google Scholar] [CrossRef] [PubMed]
- Hans, C.P.; Weisenburger, D.D.; Greiner, T.C.; Gascoyne, R.D.; Delabie, J.; Ott, G.; Müller-Hermelink, H.K.; Campo, E.; Braziel, R.M.; Jaffe, E.S.; et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004, 103, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, M.K.; Kalpadakis, C.; Pangalis, G.A.; Kyrtsonis, M.C.; Vassilakopoulos, T.P. Nodal marginal zone lymphoma. Leuk Lymphoma 2014, 55, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- Oscier, D.; Owen, R.; Johnson, S. Splenic marginal zone lymphoma. Blood Rev. 2005, 19, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, S.J. Immunoglobulin gene rearrangement and cell surface antigen expression in acute lymphocytic leukemias of T cell and B cell precursor origins. J. Clin. Invest. 1983, 71, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Badine, E.; Richa, E.; El-Sayah, D.; Terjanian, T. A case report of splenic rupture due to marginal zone lymphoma. Ann. Hematol. 2007, 86, 845–846. [Google Scholar] [CrossRef] [PubMed]
- Stefanello, D.; Valenti, P.; Zini, E.; Comazzi, S.; Gelain, M.E.; Roccabianca, P.; Avallone, G.; Caniatti, M.; Marconato, L. Splenic marginal zone lymphoma in 5 dogs (2001-2008). J. Vet. Intern. Med. 2011, 25, 90–93. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, D.; Moore, P.F.; Vernau, W.; Peauroi, J.R.; Rebhun, R.B.; Rodriguez, C.O., Jr.; Skorupski, K.A. Clinical characteristics and outcome in dogs with splenic marginal zone lymphoma. J. Vet. Intern. Med. 2013, 27, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Van den Brand, M.; van der Velden, W.J.; Diets, I.J.; Ector, G.I.; de Haan, A.F.; Stevens, W.B.; Hebeda, K.M.; Groenen, P.J.; van Krieken, H.J. Clinical features of patients with nodal marginal zone lymphoma compared to follicular lymphoma: similar presentation, but differences in prognostic factors and rate of transformation. Leuk Lymphoma 2015, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, E.S. Nodal Marginal Zone Lymphoma. In Hematopathology; Jaffe, E.S., Harris, N.L., Vardiman, J.W., Campo, E., Arber, D.A., Eds.; Elsevier Saunders: St. Louis, MO, USA, 2011; pp. 319–332. [Google Scholar]
- Nathwani, B.N. Marginal zone B-cell lymphoma: A clinical comparison of nodal and mucosa-associated lymphoid tissue types. Non-Hodgkin’s Lymphoma Classification Project. J. Clin. Oncol. 1999, 17, 2486–2492. [Google Scholar] [PubMed]
- Molina, T.J.; Lin, P.; Swerdlow, S.H.; Cook, J.R. Marginal zone lymphomas with plasmacytic differentiation and related disorders. Am. J. Clin. Pathol. 2011, 136, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Berger, F.; Felman, P.; Thieblemont, C.; Pradier, T.; Baseggio, L.; Bryon, P.A.; Salles, G.; Callet-Bauchu, E.; Coiffier, B. Non-MALT marginal zone B-cell lymphomas: A description of clinical presentation and outcome in 124 patients. Blood 2000, 95, 1950–1956. [Google Scholar] [PubMed]
- Kojima, M.; Inagaki, H.; Motoori, T.; Itoh, H.; Shimizu, K.; Tamaki, Y.; Murase, T.; Nakamura, S. Clinical implications of nodal marginal zone B-cell lymphoma among Japanese: Study of 65 cases. Cancer Sci. 2007, 98, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Mollejo, M.; Camacho, F.I.; Algara, P.; Ruiz-Ballesteros, E.; Garcia, J.F.; Piris, M.A. Nodal and splenic marginal zone B cell lymphomas. Hematol Oncol. 2005, 23, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Traverse-Glehen, A.; Felman, P.; Callet-Bauchu, E.; Gazzo, S.; Baseggio, L.; Bryon, P.A.; Thieblemont, C.; Coiffier, B.; Salles, G.; Berger, F. A clinicopathological study of nodal marginal zone B-cell lymphoma. A report on 21 cases. Histopathology 2006, 48, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Andersen, N.S.; Jensen, M.K.; de Nully Brown, P.; Geisler, C.H. A Danish population-based analysis of 105 mantle cell lymphoma patients: Incidences, clinical features, response, survival and prognostic factors. Eur. J. Cancer 2002, 38, 401–408. [Google Scholar] [CrossRef]
- Velders, G.A.; Kluin-Nelemans, J.C.; de Boer, C.J.; Hermans, J.; Noordijk, E.M.; Schuuring, E.; Kramer, M.H.; van Deijk, W.A.; Rahder, J.B.; Kluin, P.M.; et al. Mantle-cell lymphoma: A population-based clinical study. J. Clin. Oncol. 1996, 14, 1269–1274. [Google Scholar] [PubMed]
- Hitz, F.; Bargetzi, M.; Cogliatti, S.; Lohri, A.; Taverna, C.; Renner, C.; Mey, U. Diagnosis and treatment of mantle cell lymphoma. Swiss. Med. Wkly. 2013, 143, w13868. [Google Scholar] [CrossRef] [PubMed]
- Campo, E.; Jares, P.; Jaffe, E.S. Mantle Cell Lymphoma. In Hematopathology; Jaffe, E.S., Harris, N.L., Vardiman, J.W., Campo, E., Arber, D.A., Eds.; Elsevier Saunders: St. Louis, MO, USA, 2011; pp. 331–348. [Google Scholar]
- Ferrer, A.; Salaverria, L.; Bosch, F.; Villamor, N.; Rozman, M.; Beà, S.; Giné, E.; López-Guillerm, A.; Campo, E.; Montserrat, E. Leukemic involvement is a common feature in mantle cell lymphoma. Cancer 2007, 109, 2473–2480. [Google Scholar] [CrossRef] [PubMed]
- Vose, J.M. Mantle cell lymphoma: 2015 Update on diagnosis, risk-stratification, and clinical management. Am. J. Hematol. 2015, 90, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Wasman, J.; Rosenthal, N.S.; Farhi, D.C. Mantle cell lymphoma. Morphologic findings in bone marrow involvement. Am. J. Clin. Pathol. 1996, 106, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Vasef, M.A.; Medeiros, L.J.; Koo, C.; McCourty, A.; Brynes, R.K. Cyclin D1 immunohistochemical staining is useful in distinguishing mantle cell lymphoma from other low-grade B-cell neoplasms in bone marrow. Am. J. Clin. Pathol. 1997, 108, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Van Stee, L.L.; Boston, S.E.; Singh, A.; Romanelli, G.; Rubio-Guzman, A.; Scase, T.J. Outcome and Prognostic Factors for Canine Splenic Lymphoma Treated by Splenectomy (1995-2011). Vet. Surg. 2015, 44, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, F.; Ponzoni, M. The cellular origin of mantle cell lymphoma. Int J. Biochem. Cell. Biol. 2007, 39, 1747–1753. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute sponsored study of classifications of non-Hodgkin’s lymphomas: summary and description of a working formulation for clinical usage. The Non-Hodgkin’s Lymphoma Pathologic Classification Project. Cancer 1982, 49, 2112–2135.
- Harris, N.; de Leval, F.; Ferry, J.A. Follicular Lymphoma. In Hematopathology; Jaffe, E.S., Harris, N.L., Vardiman, J.W., Campo, E., Arber, D.A., Eds.; Elsevier Saunders: St. Louis, MO, USA, 2011; pp. 267–290. [Google Scholar]
- Hitz, F.; Ketterer, N.; Lohri, A.; Mey, U.; Pederiva, S.; Renner, C.; Taverna, C.; Hartmann, A.; Yeow, K.; Bodis, S.; et al. Diagnosis and treatment of follicular lymphoma. Swiss. Med. Wkly. 2011, 141, w13247. [Google Scholar] [CrossRef] [PubMed]
- Klapper, W. Pathobiology and diagnosis of follicular lymphoma. Semin. Diagn. Pathol. 2011, 28, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Mann, R.B.; Berard, C.W. Criteria for the cytologic subclassification of follicular lymphomas: A proposed alternative method. Hematol. Oncol. 1983, 1, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Raible, M.D.; Hsi, E.D.; Alkan, S. Bcl-6 protein expression by follicle center lymphomas. A marker for differentiating follicle center lymphomas from other low-grade lymphoproliferative disorders. Am. J. Clin. Pathol. 1999, 112, 101–107. [Google Scholar] [CrossRef] [PubMed]
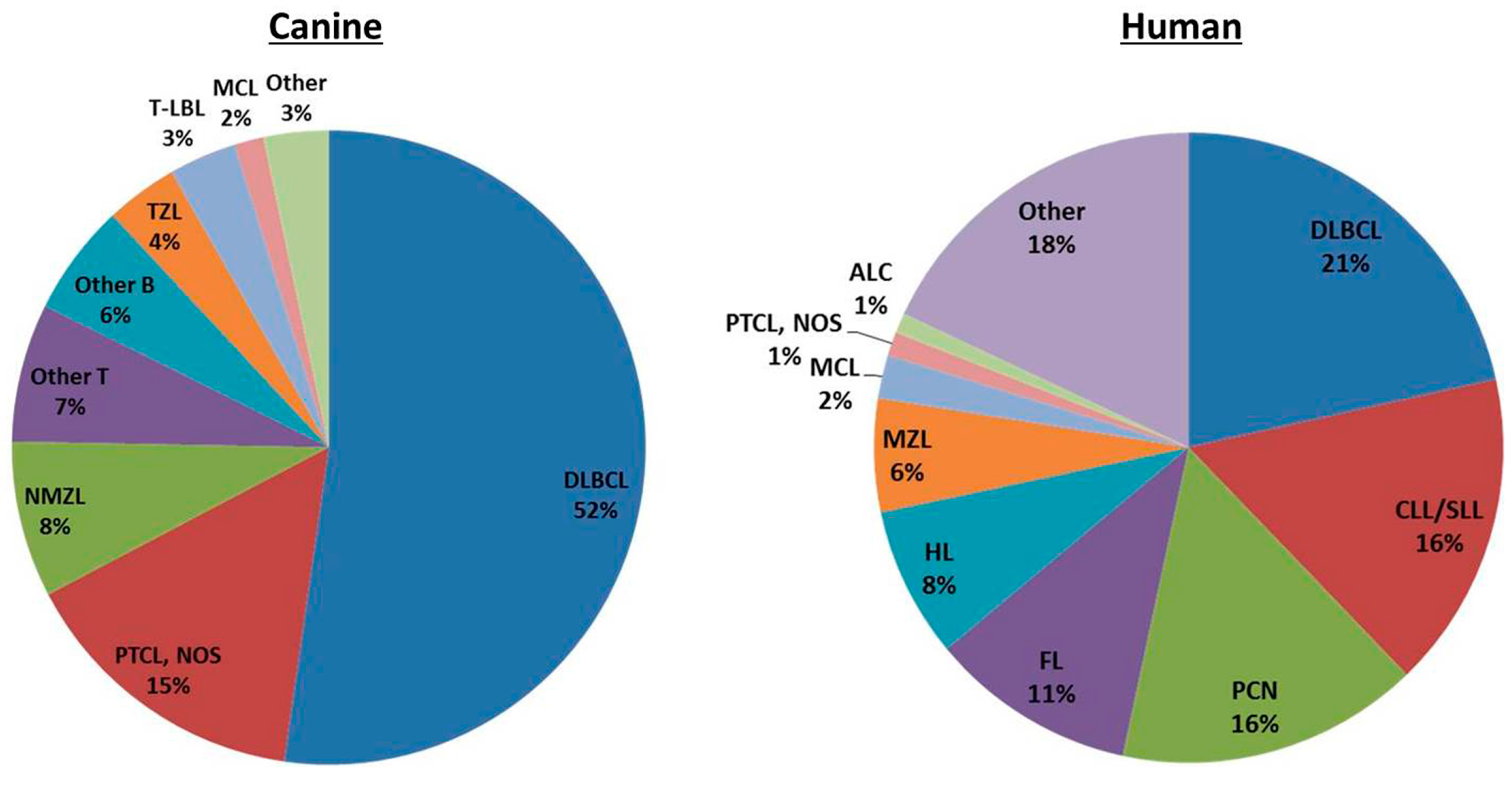
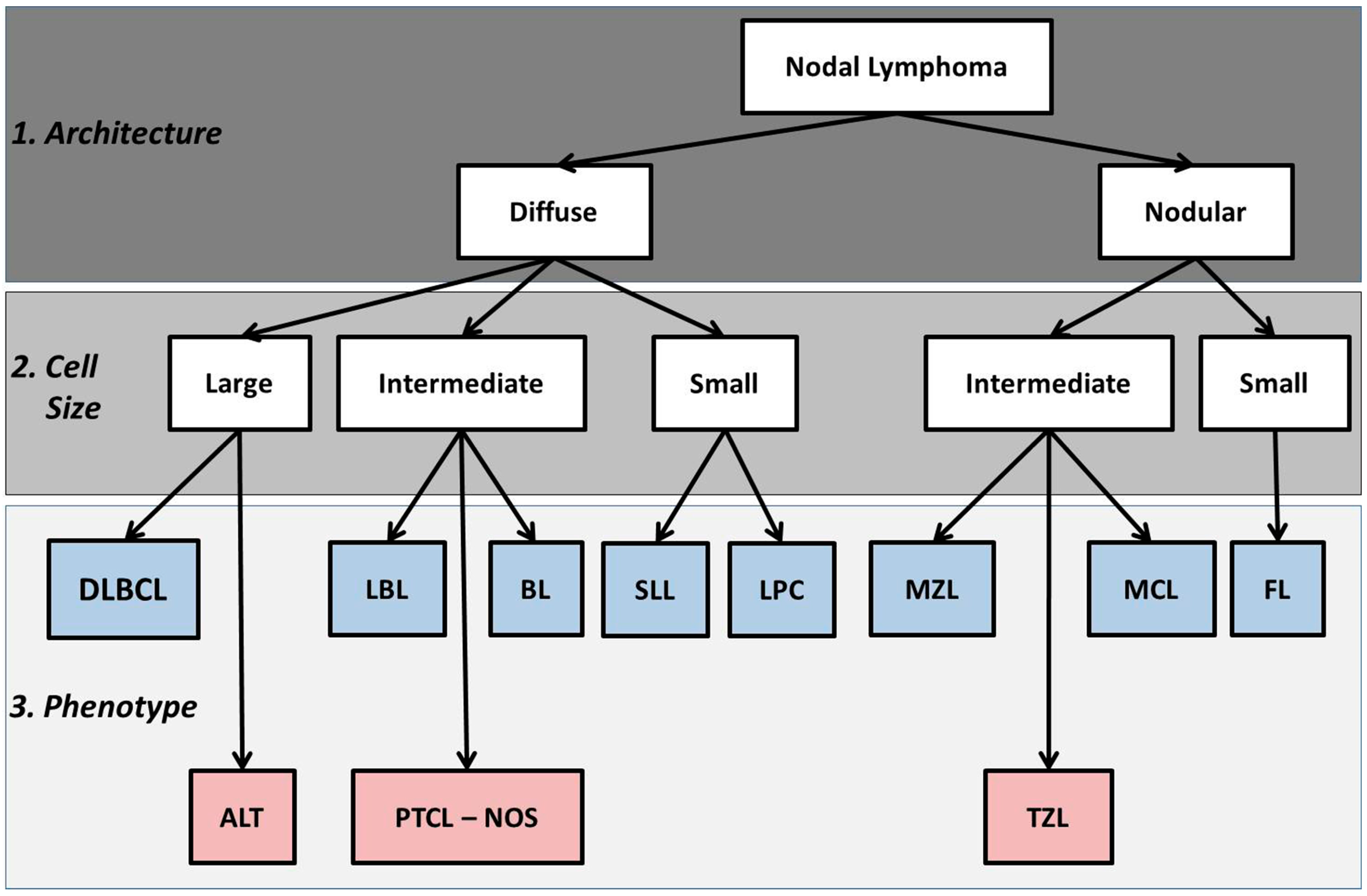

| B-Cell Neoplasms | T-Cell Neoplasms |
|---|---|
| B-lymphoblastic leukemia/lymphoma | T-lymphoblastic leukemia/lymphoma |
| Chronic lymphocytic leukemia/small lymphocytic lymphoma | T-cell prolymphocytic leukemia |
| B-cell prolymphocytic leukemia | T-cell large granular lymphocytic leukemia |
| Splenic marginal zone lymphoma | Chronic lymphoproliferative disorder of NK cells |
| Hairy cell leukemia | Aggressive NK-cell leukemia |
| Splenic lymphoma/leukemia, unclassifiable | EBV-positive T-cell disease of childhood |
| Lymphoplasmacytic lymphoma | Hydroa vacciniforme-like lymphoma |
| Heavy chain diseases | Adult T-cell leukemia/lymphoma |
| Plasma cell myeloma | Extranodal NK/T-cell lymphoma, nasal type |
| Solitary plasmacytoma of bone | Enteropathy-associated T-cell lymphoma * |
| Extraosseous plasmacytoma | Hepatosplenic T-cell lymphoma |
| MALT lymphoma | Subcutaneous panniculitis-like T-cell lymphoma |
| Nodal marginal zone lymphoma | Mycosis fungoides |
| Follicular lymphoma | Sézary syndrome |
| Primary cutaneous follicle centre lymphoma | Cutaneous CD30+ T-cell disorders |
| Mantle cell lymphoma | Peripheral T-cell lymphoma, NOS |
| Diffuse large B-cell lymphoma | T-Zone lymphoma ** |
| Lymphomatoid granulomatosis | Angioimmunoblastic T-cell lymphoma |
| Primary mediastinal large B-cell lymphoma | Anaplastic large cell lymphoma, ALK-positive |
| Intravascular large B-cell lymphoma | Anaplastic large cell lymphoma, ALK-negative |
| ALK-positive large B-cell lymphoma | |
| Plasmablastic lymphoma | |
| Large B-cell lymphoma in Castleman disease | |
| Primary effusion lymphoma | |
| Burkitt lymphoma | |
| B-cell lymphoma, unclassifiable |
| Disease | Canine | Human | ||
|---|---|---|---|---|
| Histology | Phenotype | Histology | Phenotype | |
| Precursor Neoplasms | Architecture: Diffuse | T-cell: CD45+, CD34+/−, CD5+/−, CD3+/−, CD4+/−, CD8− | Architecture: Diffuse | T-cell: CD34+, TdT+, cytoplasmic CD3+, CD5+ |
| Cytology: Intermediate sized cells, round nuclei, scant cytoplasm, high mitotic rate. | B-cell: CD45+, CD18+, CD34+/−, CD79a+, CD21+/− | Cytology: Small to large cells, varied cytoplasmic volume, high mitotic rate. | B-cell: CD34+, TdT+, CD19+, CD79a+, sIg− | |
| DLBCL | Architecture: Diffuse | CD20+, CD21+, CD45+, CD79a+, Pax5+, MHC II+ | Architecture: Diffuse | sIg+, CD45+, CD20+, CD22+, CD79a+, Pax5+, CD10+/− |
| Cytology: Large cells with round nuclei. One (central, immunoblast) or multiple (peripheral, centroblasts) nucleoli. High mitotic rate and “starry sky” appearance. | Cytology: Large cells with round nuclei. Both immunoblasts and centroblasts. High mitotic rate and “starry sky” appearance in a subset. | |||
| Mantle Cell Lymphoma | Architecture: Nodular | CD20+, CD21+, CD45+, CD79a+, MHC II+ | Architecture: Nodular or diffuse | sIg+, CD19+, CD20+, CD22+, CD79a+, CD79b+, CD5+, cyclin D1+, CD23−, CD10− |
| Cytology: Small to intermediate-sized cells; scant cytoplasm, round nuclei with dense chromatin, and inconspicuous nucleoli. Varied mitotic rate. | Cytology: Very heterogeneous; small to large cells; round to irregular nuclei; varied nucleoli and mitotic rate. | |||
| Splenic Marginal Zone Lymphoma | Architecture: Nodular | CD20+, CD21+, CD45+, CD79a+, MHC II+ | Architecture: Nodular | sIg+, CD20+, IgD+, BCL2+, BCL6−, CD5−, CD10− |
| Cytology: Biphasic-Small cells with scant cytoplasm and round nuclei; intermediate-sized cells with abundant cytoplasm and irregular nuclei; rare mitotic figures. | ||||
| Cytology: Intermediate-sized cells; abundant pale cytoplasm; irregular nuclei with peripheralized chromatin and, a single nucleolus; rare mitotic figures. | ||||
| Nodal Marginal Zone Lymphoma | Architecture: Nodular | CD20+, CD21+, CD45+, CD79a+, MHC II+ | Architecture: Nodular to diffuse. | sIg+, CD19+, CD20+, CD22+, CD79a+, CD5−, CD10−, BCL6−, and Cyclin D1− |
| Cytology: Intermediate-sized cells with pale cytoplasm and irregular nuclei (centrocyte-like) and large cells with abundant pale cytoplasm and irregular nuclei (monocytoid). | ||||
| Cytology: Mixed. Mostly intermediate-sized cells with pale cytoplasm, irregular nuclei, peripheral chromatin, and one nucleolus. | ||||
| Follicular Lymphoma | Architecture: Nodular | CD20+, CD21+, CD45+, CD79a+, MHC II+ | Architecture: Nodular | sIg+, CD45+, CD19+, CD20+, CD22+, CD79a+, Pax5+, CD10+, BCL6+, BCL2+ |
| Cytology: Similar to the dog. | ||||
| Cytology: Mixed. Mostly small cells with clear cytoplasm, pale chromatin, and inconspicuous nucleoli (centrocytes) with fewer large cells with dark blue cytoplasm, vesicular nuclei, and 1–3 nucleoli (centroblasts). | ||||
| T-Cell: | ||||
| PTCL-NOS | Architecture: Diffuse | CD3+, CD79a−, CD21−, CD45+, CD5+, CD4+/−, CD8+/− | Architecture: Diffuse | CD3+, CD4+/> CD8+/−, CD7+/−, CD5+/−, CD2+, CD30+/− |
| Cytology: Small to large (heterogeneous) with irregular nuclei, variable chromatin, prominent nucleoli, and varied mitotic activity. | Cytology: Small to large (heterogeneous) with irregular nuclei, variable chromatin, prominent nucleoli, and varied mitotic activity. | |||
| TZL | Architecture: Nodular | CD45−, CD3+, CD5+, CD21+, CD4+/−, CD8+/− | Histologic Variant of PTCL, NOS | CD2+, CD3+, CD5+, CD4+ |
| Cytology: Small to intermediate-sized cells; moderate amount of pale cytoplasm; oval to elliptical nuclei with sharp, shallow indentations; nucleoli and mitotic figures are sparse. | Architecture: Nodular | |||
| Cytology: Small to intermediate-sized cells; moderate amount of pale cytoplasm; oval to elliptical nuclei with sharp, shallow indentations; nucleoli and mitotic figures are sparse. | ||||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seelig, D.M.; Avery, A.C.; Ehrhart, E.J.; Linden, M.A. The Comparative Diagnostic Features of Canine and Human Lymphoma. Vet. Sci. 2016, 3, 11. https://doi.org/10.3390/vetsci3020011
Seelig DM, Avery AC, Ehrhart EJ, Linden MA. The Comparative Diagnostic Features of Canine and Human Lymphoma. Veterinary Sciences. 2016; 3(2):11. https://doi.org/10.3390/vetsci3020011
Chicago/Turabian StyleSeelig, Davis M., Anne C. Avery, E. J. Ehrhart, and Michael A. Linden. 2016. "The Comparative Diagnostic Features of Canine and Human Lymphoma" Veterinary Sciences 3, no. 2: 11. https://doi.org/10.3390/vetsci3020011
APA StyleSeelig, D. M., Avery, A. C., Ehrhart, E. J., & Linden, M. A. (2016). The Comparative Diagnostic Features of Canine and Human Lymphoma. Veterinary Sciences, 3(2), 11. https://doi.org/10.3390/vetsci3020011