Comparative Aspects of Molecular Mechanisms of Drug Resistance through ABC Transporters and Other Related Molecules in Canine Lymphoma
Abstract
:1. Introduction
2. Transporters Associated with Drug Resistance
2.1. P-gp
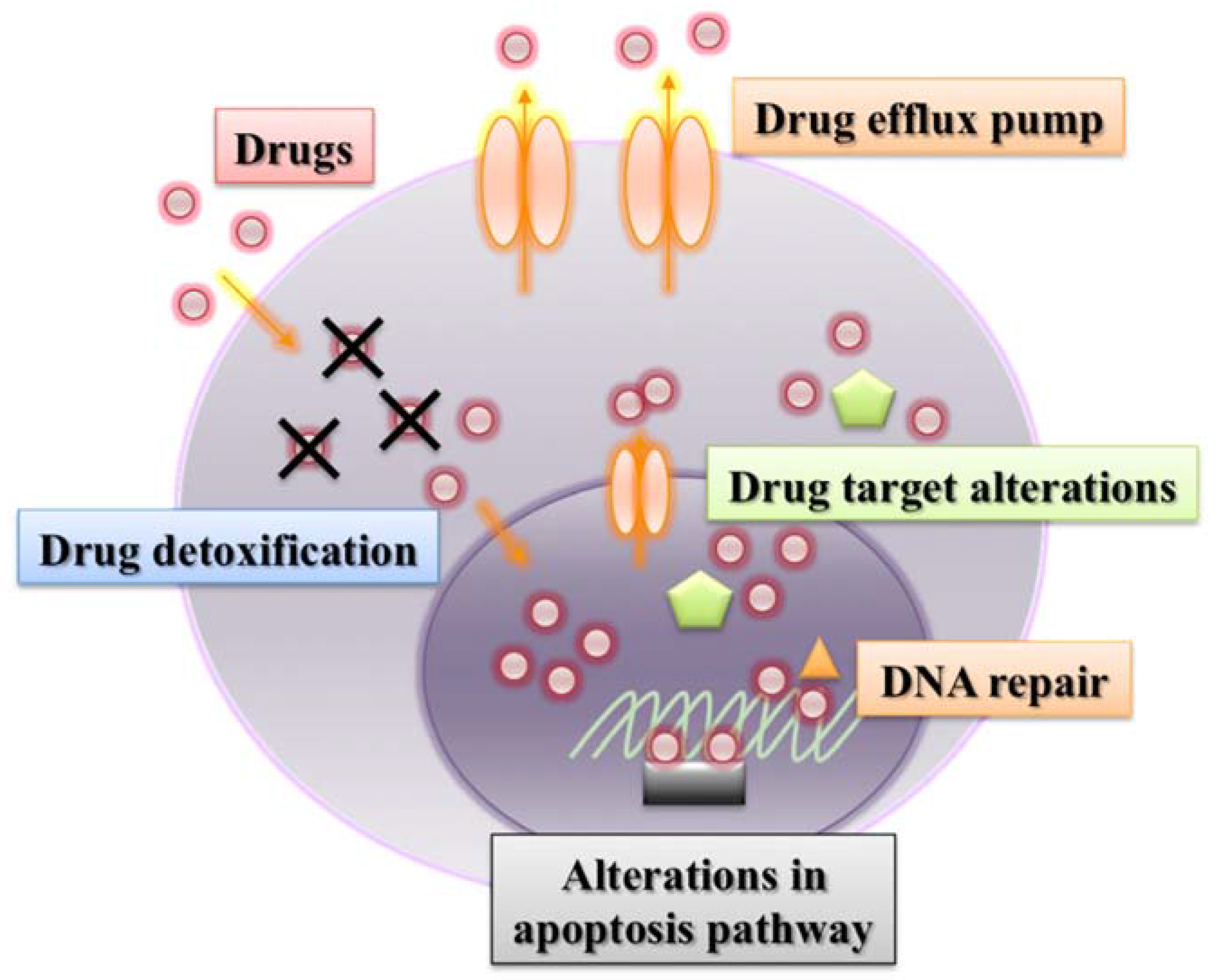
2.2. Other ABC Transporters
| P-gp | MRP1 | BCRP | LRP |
|---|---|---|---|
| Vinca alkaloids | Vinca alkaloids | Anthracyclines | Vinca alkaloids |
| Anthracyclines | Anthracyclines | Nucleoside analogs | Anthracyclines |
| Epipodophyllotoxins | Epipodophyllotoxins | Epipodophyllotoxins | Epipodophyllotoxins |
| Taxanes | Camptothecin | Camptothecin | Taxanes |
| Camptothecin | Methotrexate | Methotrexate | Platinum-containing drugs |
| Methotrexate | Nitrogen mustard | ||
| Other antibiotics (Actinomycin-D, Mitomycin-C) | |||
2.3. Lung Resistance Protein (LRP)
3. Molecular Mechanisms of Regulation of the Expression and Enhancement of P-gp Functions
3.1. Genetic Changes
3.2. Alternative Promoter

3.3. Epigenetic Regulations
3.4. Activation of Intracellular Signaling
4. Possible Strategies to Overcome Drug Resistance through Overexpression of Drug Transporters
4.1. Inhibition of the P-gp Functions
4.2. Reduction of P-gp Expression
5. Other Mechanisms Associated with Drug Resistance
5.1. Drug Target Alterations
5.2. Drug Detoxification
5.3. Increased DNA Damage Repair
5.4. Alterations in Apoptosis Pathways
6. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References and Notes
- Dobson, J.M.; Samuel, S.; Milstein, H.; Rogers, K.; Wood, J.L. Canine neoplasia in the UK: Estimates of incidence rates from a population of insured dogs. J. Small Anim. Pract. 2002, 43, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Garrett, L.D.; Thamm, D.H.; Chun, R.; Dudley, R.; Vail, D.M. Evaluation of a 6-month chemotherapy protocol with no maintenance therapy for dogs with lymphoma. J. Vet. Intern. Med. 2002, 16, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.; Nolte, I.; Eberle, N.; Abbrederis, N.; Killich, M.; Hirschberger, J. Treatment of dogs with lymphoma using a 12-week, maintenance-free combination chemotherapy protocol. J. Vet. Intern. Med. 2006, 20, 948–954. [Google Scholar] [CrossRef] [PubMed]
- MacEwen, E.G. Spontaneous tumors in dogs and cats: Models for the study of cancer biology and treatment. Cancer Metastasis Rev. 1990, 9, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Vail, D.M.; MacEwen, E.G. Spontaneously occurring tumors of companion animals as models for human cancer. Cancer Investig. 2000, 18, 781–792. [Google Scholar] [CrossRef]
- Czuczman, M.S.; Leonard, J.P.; Williams, M.E. Recent advances in the treatment of mantle cell lymphoma: A post-ash 2009 discussion. Clin. Adv. Hematol. Oncol. 2010, 8, A1–A15. [Google Scholar] [PubMed]
- Sonneveld, P.; de Ridder, M.; van der Lelie, H.; Nieuwenhuis, K.; Schouten, H.; Mulder, A.; van Reijswoud, I.; Hop, W.; Lowenberg, B. Comparison of doxorubicin and mitoxantrone in the treatment of elderly patients with advanced diffuse non-Hodgkin’s lymphoma using CHOP versus CNOP chemotherapy. J. Clin. Oncol. 1995, 13, 2530–2539. [Google Scholar] [PubMed]
- Kathawala, R.J.; Gupta, P.; Ashby, C.R., Jr.; Chen, Z.S. The modulation of ABC transporter-mediated multidrug resistance in cancer: A review of the past decade. Drug Resist. Updates 2015, 18C, 1–17. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, R. The pharmacology of cancer resistance. Anticancer Res. 2007, 27, 1267–1272. [Google Scholar] [PubMed]
- Bergman, P.J. Mechanisms of anticancer drug resistance. Vet. Clin. N. Am. Small Anim. Pract. 2003, 33, 651–667. [Google Scholar] [CrossRef]
- Moscow, J.A.; Fairchild, C.R.; Madden, M.J.; Ransom, D.T.; Wieand, H.S.; O’Brien, E.E.; Poplack, D.G.; Cossman, J.; Myers, C.E.; Cowan, K.H. Expression of anionic glutathione-s-transferase and P-glycoprotein genes in human tissues and tumors. Cancer Res. 1989, 49, 1422–1428. [Google Scholar] [PubMed]
- Miller, T.P.; Grogan, T.M.; Dalton, W.S.; Spier, C.M.; Scheper, R.J.; Salmon, S.E. P-glycoprotein expression in malignant lymphoma and reversal of clinical drug resistance with chemotherapy plus high-dose verapamil. J. Clin. Oncol. 1991, 9, 17–24. [Google Scholar] [PubMed]
- Wilson, W.H.; Bates, S.E.; Fojo, A.; Bryant, G.; Zhan, Z.; Regis, J.; Wittes, R.E.; Jaffe, E.S.; Steinberg, S.M.; Herdt, J.; et al. Controlled trial of dexverapamil, a modulator of multidrug resistance, in lymphomas refractory to EPOCH chemotherapy. J. Clin. Oncol. 1995, 13, 1995–2004. [Google Scholar] [PubMed]
- Yahanda, A.M.; Alder, K.M.; Fisher, G.A.; Brophy, N.A.; Halsey, J.; Hardy, R.I.; Gosland, M.P.; Lum, B.L.; Sikic, B.I. Phase I trial of etoposide with cyclosporine as a modulator of multidrug resistance. J. Clin. Oncol. 1992, 10, 1624–1634. [Google Scholar] [PubMed]
- Matsuura, S.; Koto, H.; Ide, K.; Fujino, Y.; Setoguchi-Mukai, A.; Ohno, K.; Tsujimoto, H. Induction of chemoresistance in a cultured canine cell line by retroviral transduction of the canine multidrug resistance 1 gene. Am. J. Vet. Res. 2007, 68, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Bergman, P.J.; Ogilvie, G.K.; Powers, B.E. Monoclonal antibody c219 immunohistochemistry against P-glycoprotein: Sequential analysis and predictive ability in dogs with lymphoma. J. Vet. Intern. Med. 1996, 10, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Hughes, C.S.; Fine, R.L.; Page, R.L. P-glycoprotein expression in canine lymphoma: A relevant, intermediate model of multidrug resistance. Cancer 1996, 77, 1892–1898. [Google Scholar] [CrossRef]
- Moore, A.S.; Leveille, C.R.; Reimann, K.A.; Shu, H.; Arias, I.M. The expression of P-glycoprotein in canine lymphoma and its association with multidrug resistance. Cancer Investig. 1995, 13, 475–479. [Google Scholar] [CrossRef]
- Tomiyasu, H.; Goto-Koshino, Y.; Takahashi, M.; Fujino, Y.; Ohno, K.; Tsujimoto, H. Quantitative analysis of mrna for 10 different drug resistance factors in dogs with lymphoma. J. Vet. Med. Sci. 2010, 72, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, M.; Ikura, Y.; Fukushima, H.; Shirai, N.; Sugama, Y.; Suekane, T.; Hirayama, M.; Hino, M.; Ueda, M. Immunohistochemical expression of multidrug resistance proteins as a predictor of poor response to chemotherapy and prognosis in patients with nodal diffuse large B-cell lymphoma. Oncology 2005, 68, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, K.M.; Mucha, J.; Majchrzak, K.; Motyl, T.; Krol, M. Expression and role of PGP, BCRP, MRP1 and MRP3 in multidrug resistance of canine mammary cancer cells. BMC Vet. Res. 2013, 9. [Google Scholar] [CrossRef] [PubMed]
- Honscha, K.U.; Schirmer, A.; Reischauer, A.; Schoon, H.A.; Einspanier, A.; Gabel, G. Expression of ABC-transport proteins in canine mammary cancer: Consequences for chemotherapy. Reprod. Domest. Anim. 2009, 44 (Suppl 2), 218–223. [Google Scholar] [CrossRef] [PubMed]
- Pozniak, B.; Pawlak, A.; Obminska-Mrukowicz, B. Flow cytometric assessment of P-glycoprotein and multidrug resistance-associated protein activity and expression in canine lymphoma. In Vivo 2015, 29, 149–153. [Google Scholar] [PubMed]
- Galimberti, S.; Nagy, B.; Benedetti, E.; Pacini, S.; Brizzi, S.; Caracciolo, F.; Papineschi, F.; Ciabatti, E.; Guerrini, F.; Fazzi, R.; et al. Evaluation of the MDR1, ABCG2, topoisomerases IIalpha and GSTpi gene expression in patients affected by aggressive mantle cell lymphoma treated by the R-Hyper-CVAD regimen. Leuk. Lymphoma 2007, 48, 1502–1509. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Singh, R.R.; Cho-Vega, J.H.; Drakos, E.; Davuluri, Y.; Khokhar, F.A.; Fayad, L.; Medeiros, L.J.; Vega, F. Sonic hedgehog signaling proteins and ATP-binding cassette G2 are aberrantly expressed in diffuse large B-cell lymphoma. Mod. Pathol. 2009, 22, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Zandvliet, M.; Teske, E.; Schrickx, J.A.; Mol, J.A. A longitudinal study of ABC transporter expression in canine multicentric lymphoma. Vet. J. 2015, 205, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Scheper, R.J.; Broxterman, H.J.; Scheffer, G.L.; Kaaijk, P.; Dalton, W.S.; van Heijningen, T.H.; van Kalken, C.K.; Slovak, M.L.; de Vries, E.G.; van der Valk, P.; et al. Overexpression of a M(r) 110,000 vesicular protein in non-P-glycoprotein-mediated multidrug resistance. Cancer Res. 1993, 53, 1475–1479. [Google Scholar] [PubMed]
- Gromicho, M.; Dinis, J.; Magalhaes, M.; Fernandes, A.R.; Tavares, P.; Laires, A.; Rueff, J.; Rodrigues, A.S. Development of imatinib and dasatinib resistance: Dynamics of expression of drug transporters ABCB1, ABCC1, ABCG2, MVP, and SLC22A1. Leuk. Lymphoma 2011, 52, 1980–1990. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, M.A.; Shoemaker, R.H.; Flens, M.J.; Scheffer, G.L.; Wu, L.; Prather, T.R.; Scheper, R.J. Overlapping phenotypes of multidrug resistance among panels of human cancer-cell lines. Int. J. Cancer 1996, 65, 230–237. [Google Scholar] [CrossRef]
- Kitazono, M.; Sumizawa, T.; Takebayashi, Y.; Chen, Z.S.; Furukawa, T.; Nagayama, S.; Tani, A.; Takao, S.; Aikou, T.; Akiyama, S. Multidrug resistance and the lung resistance-related protein in human colon carcinoma SW-620 cells. J. Natl. Cancer Inst. 1999, 91, 1647–1653. [Google Scholar] [CrossRef] [PubMed]
- Yasunami, T.; Wang, Y.H.; Tsuji, K.; Takanashi, M.; Yamada, Y.; Motoji, T. Multidrug resistance protein expression of adult T-cell leukemia/lymphoma. Leuk. Res. 2007, 31, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Ohno, N.; Tani, A.; Uozumi, K.; Hanada, S.; Furukawa, T.; Akiba, S.; Sumizawa, T.; Utsunomiya, A.; Arima, T.; Akiyama, S. Expression of functional lung resistance—Related protein predicts poor outcome in adult T-cell leukemia. Blood 2001, 98, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Filipits, M.; Jaeger, U.; Simonitsch, I.; Chizzali-Bonfadin, C.; Heinzl, H.; Pirker, R. Clinical relevance of the lung resistance protein in diffuse large B-cell lymphomas. Clin. Cancer Res. 2000, 6, 3417–3423. [Google Scholar] [PubMed]
- Hifumi, T.; Miyoshi, N.; Kawaguchi, H.; Nomura, K.; Yasuda, N. Immunohistochemical detection of proteins associated with multidrug resistance to anti-cancer drugs in canine and feline primary pulmonary carcinoma. J. Vet. Med. Sci. 2010, 72, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Assaraf, Y.G.; Molina, A.; Schimke, R.T. Sequential amplification of dihydrofolate reductase and multidrug resistance genes in Chinese Hamster Ovary cells selected for stepwise resistance to the lipid-soluble antifolate trimetrexate. J. Biol. Chem. 1989, 264, 18326–18334. [Google Scholar] [PubMed]
- Huff, L.M.; Wang, Z.; Iglesias, A.; Fojo, T.; Lee, J.S. Aberrant transcription from an unrelated promoter can result in MDR-1 expression following drug selection in vitro and in relapsed lymphoma samples. Cancer Res. 2005, 65, 11694–11703. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.H.; Chen, C.J.; Kriegler, M.; Roninson, I.B. An altered pattern of cross-resistance in multidrug-resistant human cells results from spontaneous mutations in the mdr1 (P-glycoprotein) gene. Cell 1988, 53, 519–529. [Google Scholar] [CrossRef]
- Devine, S.E.; Ling, V.; Melera, P.W. Amino acid substitutions in the sixth transmembrane domain of P-glycoprotein alter multidrug resistance. Proc. Natl. Acad. Sci. USA 1992, 89, 4564–4568. [Google Scholar] [CrossRef] [PubMed]
- Mickley, L.A.; Spengler, B.A.; Knutsen, T.A.; Biedler, J.L.; Fojo, T. Gene rearrangement: A novel mechanism for MDR-1 gene activation. J. Clin. Investig. 1997, 99, 1947–1957. [Google Scholar] [CrossRef] [PubMed]
- Ejendal, K.F.; Hrycyna, C.A. Multidrug resistance and cancer: The role of the human ABC transporter ABCG2. Curr. Protein Pept. Sci. 2002, 3, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Unadkat, J.D. Role of the breast cancer resistance protein (ABCG2) in drug transport. AAPS J. 2005, 7, E118–E133. [Google Scholar] [CrossRef] [PubMed]
- Abbott, B.L.; Colapietro, A.M.; Barnes, Y.; Marini, F.; Andreeff, M.; Sorrentino, B.P. Low levels of ABCG2 expression in adult AML blast samples. Blood 2002, 100, 4594–4601. [Google Scholar] [CrossRef] [PubMed]
- Suvannasankha, A.; Minderman, H.; O’Loughlin, K.L.; Nakanishi, T.; Greco, W.R.; Ross, D.D.; Baer, M.R. Breast cancer resistance protein (BCRP/MXR/ABCG2) in acute myeloid leukemia: Discordance between expression and function. Leukemia 2004, 18, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Labialle, S.; Gayet, L.; Marthinet, E.; Rigal, D.; Baggetto, L.G. Transcriptional regulators of the human multidrug resistance 1 gene: Recent views. Biochem. Pharmacol. 2002, 64, 943–948. [Google Scholar] [CrossRef]
- Raguz, S.; Randle, R.A.; Sharpe, E.R.; Foekens, J.A.; Sieuwerts, A.M.; Meijer-van Gelder, M.E.; Melo, J.V.; Higgins, C.F.; Yague, E. Production of P-glycoprotein from the MDR1 upstream promoter is insufficient to affect the response to first-line chemotherapy in advanced breast cancer. Int. J. Cancer 2008, 122, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Pastan, I.; Gottesman, M.M. Isolation and sequence of the promoter region of the human multidrug-resistance (P-glycoprotein) gene. J. Biol. Chem. 1987, 262, 17432–17436. [Google Scholar] [PubMed]
- van Groenigen, M.; Valentijn, L.J.; Baas, F. Identification of a functional initiator sequence in the human MDR1 promoter. Biochim. Biophys. Acta 1993, 1172, 138–146. [Google Scholar] [CrossRef]
- Chen, K.G.; Wang, Y.C.; Schaner, M.E.; Francisco, B.; Duran, G.E.; Juric, D.; Huff, L.M.; Padilla-Nash, H.; Ried, T.; Fojo, T.; et al. Genetic and epigenetic modeling of the origins of multidrug-resistant cells in a human sarcoma cell line. Cancer Res. 2005, 65, 9388–9397. [Google Scholar] [CrossRef] [PubMed]
- Mealey, K.L.; Bentjen, S.A. Sequence and structural analysis of the presumed downstream promoter of the canine mdr1 gene. Vet. Comp. Oncol. 2003, 1, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.M.; Bird, A. DNA methylation landscapes: Provocative insights from epigenomics. Nat. Rev. Genet. 2008, 9, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Kantharidis, P.; El-Osta, A.; deSilva, M.; Wall, D.M.; Hu, X.F.; Slater, A.; Nadalin, G.; Parkin, J.D.; Zalcberg, J.R. Altered methylation of the human MDR1 promoter is associated with acquired multidrug resistance. Clin. Cancer Res. 1997, 3, 2025–2032. [Google Scholar] [PubMed]
- El-Osta, A.; Kantharidis, P.; Zalcberg, J.R.; Wolffe, A.P. Precipitous release of methyl-CpG binding protein 2 and histone deacetylase 1 from the methylated human multidrug resistance gene (MDR1) on activation. Mol. Cell. Biol. 2002, 22, 1844–1857. [Google Scholar] [CrossRef] [PubMed]
- Chekhun, V.F.; Kulik, G.I.; Yurchenko, O.V.; Tryndyak, V.P.; Todor, I.N.; Luniv, L.S.; Tregubova, N.A.; Pryzimirska, T.V.; Montgomery, B.; Rusetskaya, N.V.; et al. Role of DNA hypomethylation in the development of the resistance to doxorubicin in human MCF-7 breast adenocarcinoma cells. Cancer Lett. 2006, 231, 87–93. [Google Scholar] [CrossRef] [PubMed]
- David, G.L.; Yegnasubramanian, S.; Kumar, A.; Marchi, V.L.; De Marzo, A.M.; Lin, X.; Nelson, W.G. MDR1 promoter hypermethylation in MCF-7 human breast cancer cells: Changes in chromatin structure induced by treatment with 5-Aza-cytidine. Cancer Biol. Ther. 2004, 3, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Desiderato, L.; Davey, M.W.; Piper, A.A. Demethylation of the human MDR1 5′ region accompanies activation of P-glycoprotein expression in a HL60 multidrug resistant subline. Somat. Cell Mol. Genet. 1997, 23, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.B.; Park, J.H.; Min, Y.D.; Kim, K.J.; Choi, C.H. Epigenetic mechanisms involved in differential MDR1 mRNA expression between gastric and colon cancer cell lines and rationales for clinical chemotherapy. BMC Gastroenterol. 2008, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Wada, M.; Harada, T.; Nagayama, J.; Kusaba, H.; Ohshima, K.; Kozuru, M.; Komatsu, H.; Ueda, R.; Kuwano, M. Hypomethylation status of CpG sites at the promoter region and overexpression of the human MDR1 gene in acute myeloid leukemias. Blood 1998, 92, 4296–4307. [Google Scholar] [PubMed]
- Tada, Y.; Wada, M.; Kuroiwa, K.; Kinugawa, N.; Harada, T.; Nagayama, J.; Nakagawa, M.; Naito, S.; Kuwano, M. MDR1 gene overexpression and altered degree of methylation at the promoter region in bladder cancer during chemotherapeutic treatment. Clin. Cancer Res. 2000, 6, 4618–4627. [Google Scholar] [PubMed]
- Shi, C.J.; Wang, F.; Ren, M.F.; Mi, Y.J.; Yan, Y.Y.; To, K.K.; Dai, C.L.; Wang, Y.S.; Chen, L.M.; Tong, X.Z.; et al. Up-regulation of ABCB1/P-glycoprotein by escaping promoter hypermethylation indicates poor prognosis in hematologic malignancy patients with and without bone marrow transplantation. Leuk. Res. 2011, 35, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Kouzarides, T. Chromatin modifications and their function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.K.; Johnstone, R.W.; Zalcberg, J.R.; El-Osta, A. Epigenetic changes to the MDR1 locus in response to chemotherapeutic drugs. Oncogene 2005, 24, 8061–8075. [Google Scholar] [CrossRef] [PubMed]
- Tomiyasu, H.; Goto-Koshino, Y.; Fujino, Y.; Ohno, K.; Tsujimoto, H. Epigenetic regulation of the ABCB1 gene in drug-sensitive and drug-resistant lymphoid tumour cell lines obtained from canine patients. Vet. J. 2014, 199, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Tomiyasu, H.; Fujiwara-Igarashi, A.; Goto-Koshino, Y.; Fujino, Y.; Ohno, K.; Tsujimoto, H. Evaluation of DNA methylation profiles of the CpG island of the ABCB1 gene in dogs with lymphoma. Am. J. Vet. Res. 2014, 75, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Orom, U.A.; Nielsen, F.C.; Lund, A.H. MicroRNA-10a binds the 5'UTR of ribosomal protein mRNAs and enhances their translation. Mol. Cell 2008, 30, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Filipowicz, W.; Bhattacharyya, S.N.; Sonenberg, N. Mechanisms of post-transcriptional regulation by microRNAs: Are the answers in sight? Nat. Rev. Genet. 2008, 9, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Sanchez, M.A.; Liu, J.; Hannon, G.J.; Parker, R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006, 20, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Control of protein synthesis and mRNA degradation by microRNAs. Curr. Opin. Cell Biol. 2008, 20, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Kovalchuk, O.; Filkowski, J.; Meservy, J.; Ilnytskyy, Y.; Tryndyak, V.P.; Chekhun, V.F.; Pogribny, I.P. Involvement of microRNA-451 in resistance of the MCF-7 breast cancer cells to chemotherapeutic drug doxorubicin. Mol. Cancer Ther. 2008, 7, 2152–2159. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.D.; Zhang, H.; Zhang, P.; Zheng, Y.S.; Zhang, X.J.; Han, B.W.; Luo, X.Q.; Xu, L.; Zhou, H.; Qu, L.H.; et al. Down-regulated miR-331-5p and miR-27a are associated with chemotherapy resistance and relapse in leukaemia. J. Cell. Mol. Med. 2011, 15, 2164–2175. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Hazari, S.; Mehra, S.; Kaushal, D.; Moroz, K.; Dash, S. Increased expression of P-glycoprotein and doxorubicin chemoresistance of metastatic breast cancer is regulated by miR-298. Am. J. Pathol. 2012, 180, 2490–2503. [Google Scholar] [CrossRef] [PubMed]
- Ikemura, K.; Yamamoto, M.; Miyazaki, S.; Mizutani, H.; Iwamoto, T.; Okuda, M. MicroRNA-145 post-transcriptionally regulates the expression and function of P-glycoprotein in intestinal epithelial cells. Mol. Pharmacol. 2013, 83, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, L.Y.; Spevak, C.C.; Wong, G.; Xia, W.; Gilad, E. Hyaluronan-CD44 interaction with protein kinase C(epsilon) promotes oncogenic signaling by the stem cell marker Nanog and the production of microRNA-21, leading to down-regulation of the tumor suppressor protein PDCD4, anti-apoptosis, and chemotherapy resistance in breast tumor cells. J. Biol. Chem. 2009, 284, 26533–26546. [Google Scholar] [PubMed]
- Boyerinas, B.; Park, S.M.; Murmann, A.E.; Gwin, K.; Montag, A.G.; Zillhardt, M.; Hua, Y.J.; Lengyel, E.; Peter, M.E. Let-7 modulates acquired resistance of ovarian cancer to Taxanes via IMP-1-mediated stabilization of multidrug resistance 1. Int. J. Cancer 2012, 130, 1787–1797. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tian, W.; Cai, H.; He, H.; Deng, Y. Down-regulation of microRNA-200c is associated with drug resistance in human breast cancer. Med. Oncol. 2012, 29, 2527–2534. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xia, F.; Ma, L.; Shan, J.; Shen, J.; Yang, Z.; Liu, J.; Cui, Y.; Bian, X.; Bie, P.; et al. MicroRNA-122 sensitizes HCC cancer cells to adriamycin and vincristine through modulating expression of MDR and inducing cell cycle arrest. Cancer Lett. 2011, 310, 160–169. [Google Scholar] [PubMed]
- Yang, L.; Li, N.; Wang, H.; Jia, X.; Wang, X.; Luo, J. Altered microRNA expression in cisplatin-resistant ovarian cancer cells and upregulation of miR-130a associated with MDR1/P-glycoprotein-mediated drug resistance. Oncol. Rep. 2012, 28, 592–600. [Google Scholar] [PubMed]
- Zhao, X.; Yang, L.; Hu, J.; Ruan, J. MiR-138 might reverse multidrug resistance of leukemia cells. Leuk. Res. 2010, 34, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wu, H.; Liu, X.; Evans, B.R.; Medina, D.J.; Liu, C.G.; Yang, J.M. Role of microRNA miR-27a and miR-451 in the regulation of MDR1/P-glycoprotein expression in human cancer cells. Biochem. Pharmacol. 2008, 76, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, Y.; Shen, H.; Li, H.; Long, L.; Hui, L.; Xu, W. MiR-137 restoration sensitizes multidrug-resistant MCF-7/ADM cells to anticancer agents by targeting YB-1. Acta Biochim. Biophys. Sin. (Shanghai) 2013, 45, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, R.; Luk, F.; Gong, J.; Mathys, J.M.; Grau, G.E.; Bebawy, M. Microparticle conferred microRNA profiles—Implications in the transfer and dominance of cancer traits. Mol. Cancer 2012, 11. [Google Scholar] [CrossRef] [PubMed]
- Katayama, K.; Yoshioka, S.; Tsukahara, S.; Mitsuhashi, J.; Sugimoto, Y. Inhibition of the mitogen-activated protein kinase pathway results in the down-regulation of P-glycoprotein. Mol. Cancer Ther. 2007, 6, 2092–2102. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Xu, W.; Luo, W.; Zhou, L.; Yong, W.; Chen, F.; Wu, C.; Chen, Q.; Han, X. Upregulation of mdr1 gene is related to activation of the MAPK/ERK signal transduction pathway and YB-1 nuclear translocation in B-cell lymphoma. Exp. Hematol. 2011, 39, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.M.; Vassil, A.D.; Hait, W.N. Activation of phospholipase C induces the expression of the multidrug resistance (MDR1) gene through the Raf-MAPK pathway. Mol. Pharmacol. 2001, 60, 674–680. [Google Scholar] [PubMed]
- Zhao, B.X.; Sun, Y.B.; Wang, S.Q.; Duan, L.; Huo, Q.L.; Ren, F.; Li, G.F. Grape seed procyanidin reversal of p-glycoprotein associated multi-drug resistance via down-regulation of NF-kappaB and MAPK/ERK mediated YB-1 activity in A2780/T cells. PLoS ONE 2013, 8, e71071. [Google Scholar] [CrossRef] [PubMed]
- Tomiyasu, H.; Watanabe, M.; Sugita, K.; Goto-Koshino, Y.; Fujino, Y.; Ohno, K.; Sugano, S.; Tsujimoto, H. Regulations of ABCB1 and ABCG2 expression through MAPK pathways in acute lymphoblastic leukemia cell lines. Anticancer Res. 2013, 33, 5317–5323. [Google Scholar] [PubMed]
- Lu, F.; Hou, Y.Q.; Song, Y.; Yuan, Z.J. TFPI-2 downregulates multidrug resistance protein in 5-FU-resistant human hepatocellular carcinoma BEL-7402/5-FU cells. Anat. Rec. (Hoboken) 2013, 296, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ma, N.; Wang, J.; Song, J.; Bu, X.; Cheng, Y.; Sun, K.; Xiong, H.; Jiang, G.; Zhang, B.; et al. Increased p38-MAPK is responsible for chemotherapy resistance in human gastric cancer cells. BMC Cancer 2008, 8, 375. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Aneja, R.; Wang, H.; Sun, L.; Dong, X.; Huo, L.; Joshi, H.; Zhou, J. Modulation of multidrug resistance in cancer cells by the E3 ubiquitin ligase seven-in-absentia homologue 1. J. Pathol. 2008, 214, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Bark, H.; Choi, C.H. PSC833, cyclosporine analogue, downregulates MDR1 expression by activating JNK/c-jun/AP-1 and suppressing NF-kappaB. Cancer Chemother. Pharmacol. 2010, 65, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Bian, Y.; Zeng, S. Involvement of AP-1 and NF-kappaB in the up-regulation of P-gp in vinblastine resistant Caco-2 cells. Drug Metab. Pharmacokinet. 2014, 29, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.T.; Liu, Z.; Wei, Y.; Lin-Lee, Y.C.; Tatebe, S.; Mills, G.B.; Unate, H. Induction of human MDR1 gene expression by 2-acetylaminofluorene is mediated by effectors of the phosphoinositide 3-kinase pathway that activate NF-kappaB signaling. Oncogene 2002, 21, 1945–1954. [Google Scholar] [CrossRef] [PubMed]
- Han, C.Y.; Cho, K.B.; Choi, H.S.; Han, H.K.; Kang, K.W. Role of FoxO1 activation in MDR1 expression in adriamycin-resistant breast cancer cells. Carcinogenesis 2008, 29, 1837–1844. [Google Scholar] [CrossRef] [PubMed]
- Hui, R.C.; Francis, R.E.; Guest, S.K.; Costa, J.R.; Gomes, A.R.; Myatt, S.S.; Brosens, J.J.; Lam, E.W. Doxorubicin activates FOXO3a to induce the expression of multidrug resistance gene ABCB1 (MDR1) in K562 leukemic cells. Mol. Cancer Ther. 2008, 7, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Thevenod, F.; Chakraborty, P.K. The role of Wnt/beta-catenin signaling in renal carcinogenesis: Lessons from cadmium toxicity studies. Curr. Mol. Med. 2010, 10, 387–404. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.C.; Kania, K.D.; Wijesuriya, H.; Chawla, S.; Sethi, J.K.; Pulaski, L.; Romero, I.A.; Couraud, P.O.; Weksler, B.B.; Hladky, S.B.; et al. Activation of beta-catenin signalling by GSK-3 inhibition increases P-glycoprotein expression in brain endothelial cells. J. Neurochem. 2008, 106, 1855–1865. [Google Scholar] [PubMed]
- Correa, S.; Binato, R.; Du Rocher, B.; Castelo-Branco, M.T.; Pizzatti, L.; Abdelhay, E. Wnt/beta-catenin pathway regulates ABCB1 transcription in chronic myeloid leukemia. BMC Cancer 2012, 12, 303. [Google Scholar] [CrossRef] [PubMed]
- Tomiyasu, H.; Watanabe, M.; Goto-Koshino, Y.; Fujino, Y.; Ohno, K.; Sugano, S.; Tsujimoto, H. Regulation of expression of ABCB1 and LRP genes by mitogen-activated protein kinase/extracellular signal-regulated kinase pathway and its role in generation of side population cells in canine lymphoma cell lines. Leuk. Lymphoma 2013, 54, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Tomiyasu, H.; Goto-Koshino, Y.; Fujino, Y.; Ohno, K.; Tsujimoto, H. Antitumour effect and modulation of expression of the ABCB1 gene by perifosine in canine lymphoid tumour cell lines. Vet. J. 2014, 201, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Tomiyasu, H.; Goto-Koshino, Y.; Fujino, Y.; Ohno, K.; Tsujimoto, H. The regulation of the expression of ABCG2 gene through mitogen-activated protein kinase pathways in canine lymphoid tumor cell lines. J. Vet. Med. Sci. 2014, 76, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Krishna, R.; Mayer, L.D. Multidrug resistance (MDR) in cancer. Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur. J. Pharm. Sci. 2000, 11, 265–283. [Google Scholar] [CrossRef]
- Szakacs, G.; Paterson, J.K.; Ludwig, J.A.; Booth-Genthe, C.; Gottesman, M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006, 5, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Binkhathlan, Z.; Lavasanifar, A. P-glycoprotein inhibition as a therapeutic approach for overcoming multidrug resistance in cancer: Current status and future perspectives. Curr. Cancer Drug Targets 2013, 13, 326–346. [Google Scholar] [CrossRef] [PubMed]
- Zandvliet, M.; Teske, E.; Schrickx, J.A. Multi-drug resistance in a canine lymphoid cell line due to increased P-glycoprotein expression, a potential model for drug-resistant canine lymphoma. Toxicol. In Vitro 2014, 28, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Chen, Z.S.; Ambudkar, S.V. Tyrosine kinase inhibitors as modulators of ABC transporter-mediated drug resistance. Drug Resist. Updates 2012, 15, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Zandvliet, M.; Teske, E.; Chapuis, T.; Fink-Gremmels, J.; Schrickx, J.A. Masitinib reverses doxorubicin resistance in canine lymphoid cells by inhibiting the function of P-glycoprotein. J. Vet. Pharmacol. Ther. 2013, 36, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Kang, H.; Fisher, M.; Juliano, R.L. Strategies for inhibition of MDR1 gene expression. Mol. Pharmacol. 2004, 66, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Pichler, A.; Zelcer, N.; Prior, J.L.; Kuil, A.J.; Piwnica-Worms, D. In vivo RNA interference-mediated ablation of MDR1 P-glycoprotein. Clin. Cancer Res. 2005, 11, 4487–4494. [Google Scholar] [CrossRef] [PubMed]
- Sen, R.; Natarajan, K.; Bhullar, J.; Shukla, S.; Fang, H.B.; Cai, L.; Chen, Z.S.; Ambudkar, S.V.; Baer, M.R. The novel BCR-ABL and FLT3 inhibitor ponatinib is a potent inhibitor of the MDR-associated ATP-binding cassette transporter ABCG2. Mol. Cancer Ther. 2012, 11, 2033–2044. [Google Scholar] [CrossRef] [PubMed]
- Chiarini, F.; Del Sole, M.; Mongiorgi, S.; Gaboardi, G.C.; Cappellini, A.; Mantovani, I.; Follo, M.Y.; McCubrey, J.A.; Martelli, A.M. The novel Akt inhibitor, perifosine, induces caspase-dependent apoptosis and downregulates P-glycoprotein expression in multidrug-resistant human T-acute leukemia cells by a JNK-dependent mechanism. Leukemia 2008, 22, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.M.; Morley, N. Rituximab in the treatment of non-Hodgkin's lymphoma—A critical evaluation of randomized controlled trials. Expert Opin. Biol. Ther. 2013, 13, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Beum, P.V.; Kennedy, A.D.; Williams, M.E.; Lindorfer, M.A.; Taylor, R.P. The shaving reaction: Rituximab/CD20 complexes are removed from mantle cell lymphoma and chronic lymphocytic leukemia cells by THP-1 monocytes. J. Immunol. 2006, 176, 2600–2609. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.A.; Grillo-Lopez, A.J.; White, C.A.; McLaughlin, P.; Czuczman, M.S.; Link, B.K.; Maloney, D.G.; Weaver, R.L.; Rosenberg, J.; Levy, R. Rituximab anti-CD20 monoclonal antibody therapy in non-Hodgkin's lymphoma: Safety and efficacy of re-treatment. J. Clin. Oncol. 2000, 18, 3135–3143. [Google Scholar] [PubMed]
- Kennedy, G.A.; Tey, S.K.; Cobcroft, R.; Marlton, P.; Cull, G.; Grimmett, K.; Thomson, D.; Gill, D. Incidence and nature of CD20-negative relapses following rituximab therapy in aggressive B-cell non-Hodgkin’s lymphoma: A retrospective review. Br. J. Haematol. 2002, 119, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Terui, Y.; Mishima, Y.; Sugimura, N.; Kojima, K.; Sakurai, T.; Mishima, Y.; Kuniyoshi, R.; Taniyama, A.; Yokoyama, M.; Sakajiri, S.; et al. Identification of CD20 C-terminal deletion mutations associated with loss of CD20 expression in non-Hodgkin's lymphoma. Clin. Cancer Res. 2009, 15, 2523–2530. [Google Scholar] [CrossRef] [PubMed]
- Halsey, C.H.; Gustafson, D.L.; Rose, B.J.; Wolf-Ringwall, A.; Burnett, R.C.; Duval, D.L.; Avery, A.C.; Thamm, D.H. Development of an in vitro model of acquired resistance to toceranib phosphate (Palladia(R)) in canine mast cell tumor. BMC Vet. Res. 2014, 10. [Google Scholar] [CrossRef] [PubMed]
- Tew, K.D. Glutathione-associated enzymes in anticancer drug resistance. Cancer Res. 1994, 54, 4313–4320. [Google Scholar] [PubMed]
- Schisselbauer, J.C.; Silber, R.; Papadopoulos, E.; Abrams, K.; LaCreta, F.P.; Tew, K.D. Characterization of glutathione S-transferase expression in lymphocytes from chronic lymphocytic leukemia patients. Cancer Res. 1990, 50, 3562–3568. [Google Scholar] [PubMed]
- Ribrag, V.; Koscielny, S.; Carpiuc, I.; Cebotaru, C.; Vande Walle, H.; Talbot, M.; Fenaux, P.; Bosq, J. Prognostic value of GST-pi expression in diffuse large B-cell lymphomas. Leukemia 2003, 17, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Christmann, M.; Verbeek, B.; Roos, W.P.; Kaina, B. O(6)-Methylguanine-DNA methyltransferase (MGMT) in normal tissues and tumors: Enzyme activity, promoter methylation and immunohistochemistry. Biochim. Biophys. Acta 2011, 1816, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Silber, J.R.; Bobola, M.S.; Blank, A.; Chamberlain, M.C. O(6)-methylguanine-DNA methyltransferase in glioma therapy: Promise and problems. Biochim. Biophys. Acta 2012, 1826, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Reardon, D.A.; Desjardins, A.; Vredenburgh, J.J.; Quinn, J.A.; Austin, A.D.; Herndon, J.E., 2nd; McLendon, R.E.; Friedman, H.S. O6-methylguanine-DNA methyltransferase (MGMT) immunohistochemistry as a predictor of resistance to temozolomide in primary CNS lymphoma. J. Neurooncol. 2013, 114, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Orrenius, S.; Pervaiz, S.; Fadeel, B. Plasma membrane sequestration of apoptotic protease-activating factor-1 in human B-lymphoma cells: A novel mechanism of chemoresistance. Blood 2005, 105, 4070–4077. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.H.; Teruya-Feldstein, J.; Fest, T.; Harris, C.; Steinberg, S.M.; Jaffe, E.S.; Raffeld, M. Relationship of p53, bcl-2, and tumor proliferation to clinical drug resistance in non-Hodgkin’s lymphomas. Blood 1997, 89, 601–609. [Google Scholar] [PubMed]
- Gascoyne, R.D.; Adomat, S.A.; Krajewski, S.; Krajewska, M.; Horsman, D.E.; Tolcher, A.W.; O’Reilly, S.E.; Hoskins, P.; Coldman, A.J.; Reed, J.C.; et al. Prognostic significance of Bcl-2 protein expression and Bcl-2 gene rearrangement in diffuse aggressive non-Hodgkin’s lymphoma. Blood 1997, 90, 244–251. [Google Scholar] [PubMed]
- Hermine, O.; Haioun, C.; Lepage, E.; D’Agay, M.F.; Briere, J.; Lavignac, C.; Fillet, G.; Salles, G.; Marolleau, J.P.; Diebold, J.; et al. Prognostic significance of bcl-2 protein expression in aggressive non-Hodgkin’s lymphoma. Groupe d’Etude des lymphomes de l’Adulte (GELA). Blood 1996, 87, 265–272. [Google Scholar] [PubMed]
- Hill, M.E.; MacLennan, K.A.; Cunningham, D.C.; Vaughan Hudson, B.; Burke, M.; Clarke, P.; Di Stefano, F.; Anderson, L.; Vaughan Hudson, G.; Mason, D.; et al. Prognostic significance of BCL-2 expression and bcl-2 major breakpoint region rearrangement in diffuse large cell non-Hodgkin’s lymphoma: A British National Lymphoma Investigation Study. Blood 1996, 88, 1046–1051. [Google Scholar] [PubMed]
- Sanchez, E.; Chacon, I.; Plaza, M.M.; Munoz, E.; Cruz, M.A.; Martinez, B.; Lopez, L.; Martinez-Montero, J.C.; Orradre, J.L.; Saez, A.I.; et al. Clinical outcome in diffuse large B-cell lymphoma is dependent on the relationship between different cell-cycle regulator proteins. J. Clin. Oncol. 1998, 16, 1931–1939. [Google Scholar] [PubMed]
- Dhaliwal, R.S.; Kitchell, B.E.; Ehrhart, E.; Valli, V.E.; Dervisis, N.G. Clinicopathologic significance of histologic grade, pgp, and p53 expression in canine lymphoma. J. Am. Anim. Hosp. Assoc. 2013, 49, 175–184. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomiyasu, H.; Tsujimoto, H. Comparative Aspects of Molecular Mechanisms of Drug Resistance through ABC Transporters and Other Related Molecules in Canine Lymphoma. Vet. Sci. 2015, 2, 185-205. https://doi.org/10.3390/vetsci2030185
Tomiyasu H, Tsujimoto H. Comparative Aspects of Molecular Mechanisms of Drug Resistance through ABC Transporters and Other Related Molecules in Canine Lymphoma. Veterinary Sciences. 2015; 2(3):185-205. https://doi.org/10.3390/vetsci2030185
Chicago/Turabian StyleTomiyasu, Hirotaka, and Hajime Tsujimoto. 2015. "Comparative Aspects of Molecular Mechanisms of Drug Resistance through ABC Transporters and Other Related Molecules in Canine Lymphoma" Veterinary Sciences 2, no. 3: 185-205. https://doi.org/10.3390/vetsci2030185
APA StyleTomiyasu, H., & Tsujimoto, H. (2015). Comparative Aspects of Molecular Mechanisms of Drug Resistance through ABC Transporters and Other Related Molecules in Canine Lymphoma. Veterinary Sciences, 2(3), 185-205. https://doi.org/10.3390/vetsci2030185





