A Longitudinal Observational Study on Lactation-Associated Changes in Procalcitonin, Protein Carbonyl Content, Asymmetric Dimethylarginine, and Symmetric Dimethylarginine in Dairy Cattle
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- Animals and Management
- Inclusion criteria
- Sampling
- Determination of plasma PCT, PCC, ADMA, and SDMA
- Statistical analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PCT | Procalcitonin |
| PCC | Protein carbonyl content |
| ADMA | Asymmetric dimethylarginine |
| SDMA | Symmetric dimethylarginine |
| BCS | Body condition score |
| SCC | Somatic cell count |
| CMT | California Mastitis Test |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| iNOS | Inducible nitric oxide synthase |
| DDAH | Dimethylarginine dimethylaminohydrolase |
| DIM | Days in milk |
| ROS | Reactive oxygen species |
References
- Tufarelli, V.; Colonna, M.A.; Losacco, C.; Puvaca, N. Biological health markers associated with oxidative stress in dairy cows during lactation period. Metabolites 2023, 13, 405. [Google Scholar] [CrossRef]
- Drackley, J.K. Biology of dairy cows during the transition period: The final frontier? J. Dairy Sci. 1999, 82, 2259–2273. [Google Scholar] [CrossRef]
- Caixeta, L.S.; Omontese, B.O. Monitoring and improving the metabolic health of dairy cows during the transition period. Animals 2021, 11, 352. [Google Scholar] [CrossRef]
- Sundrum, A. Metabolic disorders in the transition period indicate that the dairy cows’ ability to adapt is overstressed. Animals 2015, 5, 978–1020. [Google Scholar] [CrossRef]
- Sordillo, L.M.; O’Boyle, N.; Gandy, J.C.; Corl, C.M.; Hamilton, E. Shifts in thioredoxin reductase activity and oxidant status in mononuclear cells obtained from transition dairy cattle. J. Dairy Sci. 2007, 90, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Mezzetti, M.; Cattaneo, L.; Passamonti, M.M.; Lopreiato, V.; Minuti, A.; Trevisi, E. The transition period updated: A review of the new insights into the adaptation of dairy cows to the new lactation. Dairy 2021, 2, 617–636. [Google Scholar] [CrossRef]
- Perera, T.R.; Skerrett-Byrne, D.A.; Gibb, Z.; Nixon, B.; Swegen, A. The future of biomarkers in veterinary medicine: Emerging approaches and associated challenges. Animals 2022, 12, 2194. [Google Scholar] [CrossRef]
- Ercan, N.; Tuzcu, N.; Basbug, O.; Tuzcu, M.; Alim, A. Diagnostic value of serum procalcitonin, neopterin, and gamma interferon in neonatal calves with septicemic colibacillosis. J. Vet. Diagn. Investig. 2016, 28, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, F.; Meucci, V.; Divers, T.J.; Boccardo, A.; Pravettoni, D.; Meylan, M.; Belloli, A.G.; Sgorbini, M. Plasma procalcitonin concentration in healthy calves and those with septic systemic inflammatory response syndrome. Vet. J. 2018, 234, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Akyüz, E.; Gökce, G. Neopterin, procalcitonin, clinical biochemistry, and hematology in calves with neonatal sepsis. Trop. Anim. Health Prod. 2021, 53, 354. [Google Scholar] [CrossRef]
- El-Deeb, W.; Elsohaby, I.; Fayez, M.; Mkrtchyan, H.V.; El-Etriby, D.; ElGioushy, M. Use of procalcitonin, neopterin, haptoglobin, serum amyloid A and proinflammatory cytokines in diagnosis and prognosis of bovine respiratory disease in feed-lot calves under field conditions. Acta Trop. 2020, 204, 105336. [Google Scholar] [CrossRef]
- Koshiishi, T.; Shibutani, S.; Chuma, T.; Iwata, H. Measurement of serum procalcitonin concentrations in calves with bovine respiratory disease. Jpn. J. Vet. Res. 2023, 71, 65–71. [Google Scholar]
- Sala, G.; Orsetti, C.; Meucci, V.; De Marchi, L.; Sgorbini, M.; Bonelli, F. Case–control study: Endogenous procalcitonin and protein carbonylated content as a potential biomarker of subclinical mastitis in dairy cows. Vet. Sci. 2023, 10, 670. [Google Scholar] [CrossRef] [PubMed]
- Neumann, S.; Siegert, S.; Fischer, A. Procalcitonin as an Endogenous Biomarker for Mastitis in Cows. Animals 2023, 13, 2204. [Google Scholar] [CrossRef]
- El-Deeb, W.; Fayez, M.; Alhumam, N.; Elsohaby, I.; Quadri, S.A.; Mkrtchyan, H. The effect of staphylococcal mastitis including resistant strains on serum procalcitonin, neopterin, acute phase response and stress biomarkers in Holstein dairy cows. PeerJ 2021, 9, e11511. [Google Scholar] [CrossRef]
- Bonelli, F.; Madrigali, A.; Sgorbini, M.; Meucci, V.; Battaglia, F.; Guélat-Brechbuehl, M.; Sala, G.; Meylan, M. Case-Control study: Evaluation of plasma procalcitonin concentration as an indicator of inflammation in healthy and sick cows. Res. Vet. Sci. 2023, 155, 56–61. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta 2003, 329, 23–38. [Google Scholar] [CrossRef]
- Andrei, S.; Matei, S.; Rugină, D.; Bogdan, L.; Stefanut, C. Interrelationships between the content of oxidative markers, antioxidative status, and somatic cell count in cow’s milk. Czech J. Anim. Sci. 2016, 61, 407–413. [Google Scholar] [CrossRef]
- Gabai, G.; De Luca, E.; Miotto, G.; Zin, G.; Stefani, A.; Da Dalt, L.; Barberio, A.; Celi, P. Relationship between protein oxidation biomarkers and uterine health in dairy cows during the postpartum period. Antioxidants 2019, 8, 21. [Google Scholar] [CrossRef]
- Molayi-Jabdaragi, N.; Esmaeilnejad, B.; Mohammadi, V. Evaluation of oxidative/nitrosative stress biomarkers and DNA damage in buffaloes naturally infected with Theileria annulata. Microb. Pathog. 2020, 138, 103821. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. Toxic dimethylarginines: Asymmetric dimethylarginine (ADMA) and symmetric dimethylarginine (SDMA). Toxins 2017, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Oliva-Damaso, E.; Oliva-Damaso, N.; Rodriguez-Esparragon, F.; Payan, J.; Baamonde-Laborda, E.; Gonzalez-Cabrera, F.; Santana-Estupiñan, R.; Rodriguez-Perez, J.C. Asymmetric (ADMA) and symmetric (SDMA) dimethylarginines in chronic kidney disease: A clinical approach. Int. J. Mol. Sci. 2019, 20, 3668. [Google Scholar] [CrossRef] [PubMed]
- Bode-Böger, S.M.; Scalera, F.; Kielstein, J.T.; Martens-Lobenhoffer, J.; Breithardt, G.; Fobker, M.; Reinecke, H. Symmetrical dimethylarginine: A new combined parameter for renal function and extent of coronary artery disease. J. Am. Soc. Nephrol. 2006, 17, 1128–1134. [Google Scholar] [PubMed]
- Gori, E.; Pierini, A.; Lippi, I.; Meucci, V.; Perondi, F.; Marchetti, V. Evaluation of asymmetric dimethylarginine as an inflammatory and prognostic marker in dogs with acute pancreatitis. J. Vet. Intern. Med. 2020, 34, 1144–1149. [Google Scholar] [CrossRef]
- van Wijk, X.M.; Yun, C.; Lynch, K.L. Evaluation of biomarkers in sepsis: High dimethylarginine (ADMA and SDMA) concentrations are associated with mortality. J. Appl. Lab. Med. 2021, 6, 592–605. [Google Scholar] [CrossRef]
- Ertelt, A.; Stumpff, F.; Merle, R.; Kuban, S.; Bollinger, L.; Liertz, S.; Gehlen, H. Asymmetric dimethylarginine—A potential cardiac biomarker in horses. J. Vet. Cardiol. 2021, 33, 43–51. [Google Scholar] [CrossRef]
- Valente, C.; Guglielmini, C.; Baron Toaldo, M.; Romito, G.; Artusi, C.; Brugnolo, L.; Contiero, B.; Poser, H. Plasmatic dimethylarginines in dogs with myxomatous mitral valve disease. Front. Vet. Sci. 2021, 8, 738898. [Google Scholar]
- Ider, M.; Naseri, A.; Ok, M.; Uney, K.; Erturk, A.; Durgut, M.K.; Parlak, T.M.; Ismailoglu, N.; Kapar, M.M. Biomarkers in premature calves with and without respiratory distress syndrome. J. Vet. Intern. Med. 2021, 35, 2524–2533. [Google Scholar] [CrossRef]
- Mizuno, S.; Ishizaki, T.; Toga, H.; Sakai, A.; Isakova, J.; Taalaibekova, E.; Baiserkeev, Z.; Kojonazarov, B.; Aldashev, A. Endogenous asymmetric dimethylarginine pathway in high altitude adapted yaks. Biomed. Res. Int. 2015, 2015, 196904. [Google Scholar] [CrossRef]
- Bronzo, V.; Sala, G.; Ciabattini, I.; Orsetti, C.; Armenia, G.; Meucci, V.; De Marchi, L.; Bertelloni, F.; Sgorbini, M.; Bonelli, F. Endogenous symmetric dimethylarginine (SDMA) and asymmetrical dimethylarginine (ADMA) levels in healthy cows and cows with subclinical and clinical mastitis—A comparative study. Animals 2025, 15, 527. [Google Scholar] [CrossRef] [PubMed]
- Schepers, E.; Speer, T.; Bode-Böger, S.M.; Fliser, D.; Kielstein, J.T. Dimethylarginines ADMA and SDMA: The real water-soluble small toxins? Semin. Nephrol. 2014, 34, 97–105. [Google Scholar] [CrossRef]
- Schlesinger, S.; Sonntag, S.R.; Lieb, W.; Maas, R. Asymmetric and symmetric dimethylarginine as risk markers for total mortality and cardiovascular outcomes: A systematic review and meta-analysis of prospective studies. PLoS ONE 2016, 11, e0165811. [Google Scholar] [CrossRef] [PubMed]
- Dahlem, D.P.; Neiger, R.; Schweighauser, A.; Francey, T.; Yerramilli, M.; Obare, E.; Steinbach, S.M.L. Plasma symmetric dimethylarginine concentration in dogs with acute kidney injury and chronic kidney disease. J. Vet. Intern. Med. 2017, 31, 799–804. [Google Scholar] [CrossRef]
- Sargent, H.J.; Elliott, J.; Jepson, R.E. The new age of renal biomarkers: Does SDMA solve all of our problems? J. Small Anim. Pract. 2021, 62, 71–81. [Google Scholar] [CrossRef]
- Ruegg, P.L.; Erskine, R.J. Mammary gland health. In Large Animal Internal Medicine: Diseases of Horses, Cattle, Sheep and Goats, 6th ed.; Smith, B.P., Ed.; Elsevier Mosby: St. Louis, MO, USA, 2019; pp. 1118–1150. [Google Scholar]
- Schukken, Y.; Wilson, D.; Welcome, F.; Garrison-Tikofsky, L.; Gonzalez, R. Monitoring udder health and milk quality using somatic cell counts. Vet. Res. 2003, 34, 579–596. [Google Scholar] [CrossRef]
- Ciaramella, P. Semeiologia Clinica Veterinaria, 1st ed.; Poletto Editore: Milano, Italy, 2014; pp. 1–630. [Google Scholar]
- Mee, J.F.; Buckley, F.; Ryan, D.; Dillon, P. Pre-breeding ovaro-uterine ultrasonography and its relationship with first service pregnancy rate in seasonal-calving dairy herds. Reprod. Domest. Anim. 2009, 44, 331–337. [Google Scholar] [CrossRef]
- Mee, J.F. The role of the veterinarian in bovine fertility management on modern dairy farms. Theriogenology 2007, 68, S257–S265. [Google Scholar] [CrossRef]
- Meucci, V.; Orsetti, C.; Sgorbini, M.; Battaglia, F.; Cresci, M.; Bonelli, F. Can procalcitonin be dosed in bovine milk using a commercial ELISA kit? Animals 2022, 12, 289. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L. & Randall, R.J. Protein measurement with the Folin phenol reagents. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Teerlink, T. HPLC analysis of ADMA and other methylated L-arginine analogs in biological fluids. J. Chromatogr. B 2007, 851, 21–29. [Google Scholar] [CrossRef]
- Sala, G.; Boccardo, A.; Ferrulli, V.; Meucci, V.; De Marchi, L.; Sgorbini, M.; Castelli, M.; Pravettoni, D.; Bonelli, F. Cross-sectional study: Can endogenous procalcitonin differentiate between healthy and bovine respiratory disease-affected preweaned dairy calves? Vet. Q. 2024, 44, 1–10. [Google Scholar] [CrossRef]
- Vijayan, A.L.; Ravindran, S.; Saikant, R.; Lakshmi, S.; Kartik, R. Procalcitonin: A promising diagnostic marker for sepsis and antibiotic therapy. J. Intensive Care 2017, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Sabzikar, Z.N.; Mohri, M.; Seifi, H.A. Variations of some adipokines, pro-inflammatory cytokines, oxidative stress biomarkers, and energy characteristics during the transition period in dairy cows. Vet. Res. Forum 2023, 14, 87. [Google Scholar]
- Müller, B.; White, J.C.; Nylén, E.S.; Snider, R.H.; Becker, K.L.; Habener, J.F. Ubiquitous expression of the calcitonin-i gene in multiple tissues in response to sepsis. J. Clin. Endocrinol. Metab. 2001, 86, 396–404. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kuhn, M.J.; Mavangira, V.; Gandy, J.C.; Sordillo, L.M. Production of 15-F2t-isoprostane as an assessment of oxidative stress in dairy cows at different stages of lactation. J. Dairy Sci. 2018, 101, 9287–9295. [Google Scholar] [CrossRef]
- Hasselgren, P.O. Pathways of muscle protein breakdown in injury and sepsis. Curr. Opin. Clin. Nutr. Metab. Care 1999, 2, 155–160. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Zhang, G.; Tobolski, D.; Zwierzchowski, G.; Mandal, R.; Wishart, D.S.; Ametaj, B.N. Identification of serum-predictive biomarkers for subclinical mastitis in dairy cows and new insights into the pathobiology of the disease. J. Agric. Food Chem. 2022, 70, 1724–1746. [Google Scholar] [CrossRef]
- Ametaj, B.; Zhang, G.; Dervishi, E.; Wishart, D. Urinary metabotyping around parturition indicates consistent metabolite signatures that can be used for monitoring and diagnosing of subclinical mastitis in dairy cows. J. Anim. Sci. 2018, 96, 19–20. [Google Scholar] [CrossRef]
- Haxhiaj, K. Blood and Urinary Metabotyping Reveals Potential Predictive Biomarkers for Identifying Dairy Cows at Risk of Subclinical Mastitis During the Dry-Off Period. Master’s Thesis, University of Alberta, Edmonton, AB, Canada, 2021. [Google Scholar]
- Böger, R.H. The emerging role of asymmetric dimethylarginine as a novel cardiovascular risk factor. Cardiovasc. Res. 2003, 59, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Teerlink, T.; Luo, Z.; Palm, F.; Wilcox, C.S. Cellular ADMA: Regulation and action. Pharmacol. Res. 2009, 60, 448–460. [Google Scholar] [CrossRef] [PubMed]
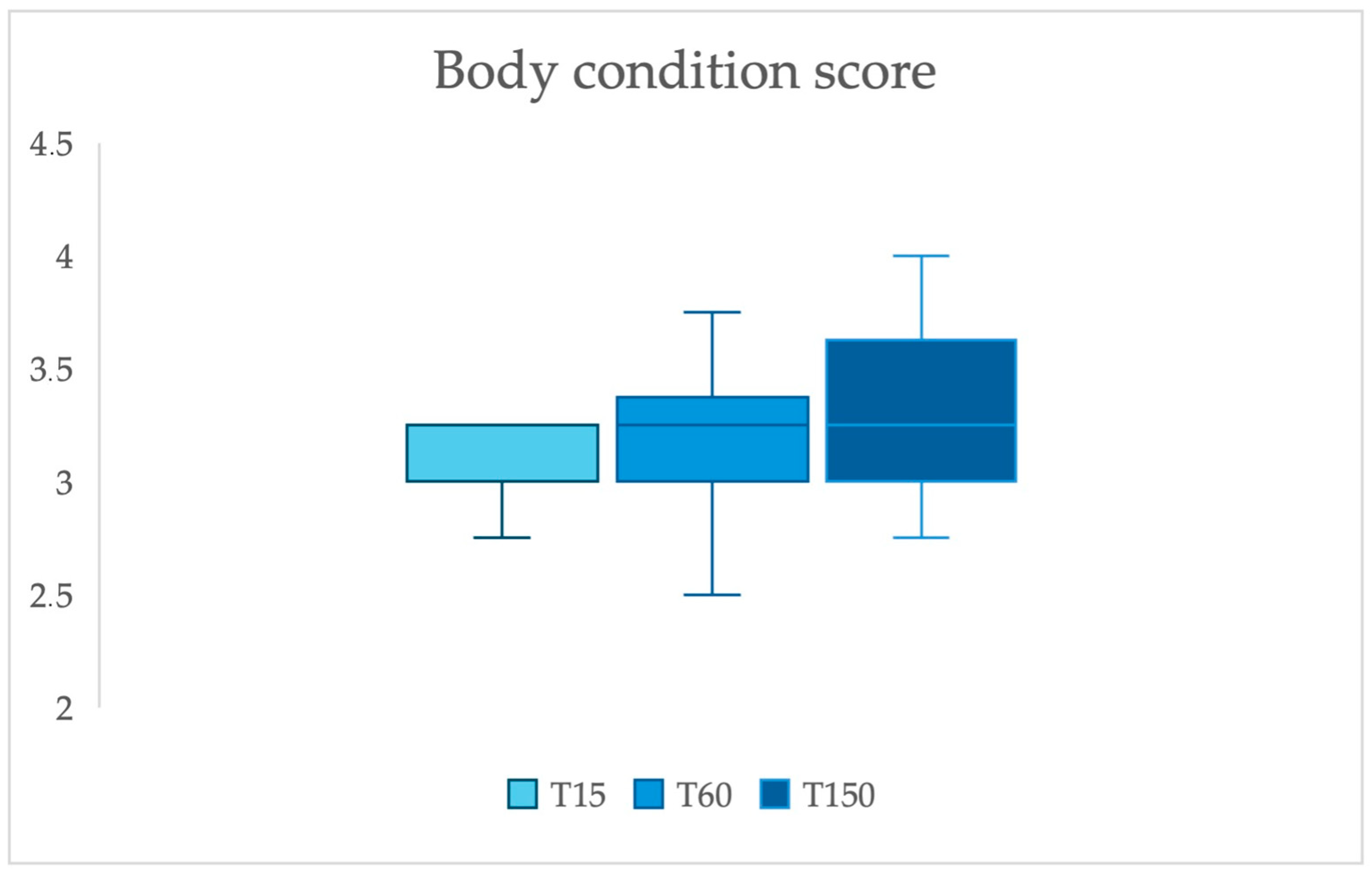
| 15 DIM | 60 DIM | 150 DIM | p Value | |
|---|---|---|---|---|
| PCT (pg/mL) | 64.29 (40.00–143.23) | 75.36 (40.00–161.46) | 77.50 (40.00–120.18) | 0.225 |
| PCC (nmol/mL/mg) | 0.17 (0.10–0.27) | 0.14 (0.08–0.22) | 0.19 (0.08–0.22) | 0.432 |
| ADMA (µmol/L) | 0.111 (0.085–0.155) | 0.104 (0.091–0.134) | 0.104 (0.095–0.141) | 0.867 |
| SDMA (µmol/L) | 0.110 (0.086–0.137) | 0.127 (0.091–0.157) | 0.100 (0.087–0.164) | 0.953 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sala, G.; Castelli, M.; Orsetti, C.; Armenia, G.; De Marchi, L.; Meucci, V.; Sgorbini, M.; Bonelli, F. A Longitudinal Observational Study on Lactation-Associated Changes in Procalcitonin, Protein Carbonyl Content, Asymmetric Dimethylarginine, and Symmetric Dimethylarginine in Dairy Cattle. Vet. Sci. 2025, 12, 895. https://doi.org/10.3390/vetsci12090895
Sala G, Castelli M, Orsetti C, Armenia G, De Marchi L, Meucci V, Sgorbini M, Bonelli F. A Longitudinal Observational Study on Lactation-Associated Changes in Procalcitonin, Protein Carbonyl Content, Asymmetric Dimethylarginine, and Symmetric Dimethylarginine in Dairy Cattle. Veterinary Sciences. 2025; 12(9):895. https://doi.org/10.3390/vetsci12090895
Chicago/Turabian StyleSala, Giulia, Matteo Castelli, Chiara Orsetti, Giovanni Armenia, Lucia De Marchi, Valentina Meucci, Micaela Sgorbini, and Francesca Bonelli. 2025. "A Longitudinal Observational Study on Lactation-Associated Changes in Procalcitonin, Protein Carbonyl Content, Asymmetric Dimethylarginine, and Symmetric Dimethylarginine in Dairy Cattle" Veterinary Sciences 12, no. 9: 895. https://doi.org/10.3390/vetsci12090895
APA StyleSala, G., Castelli, M., Orsetti, C., Armenia, G., De Marchi, L., Meucci, V., Sgorbini, M., & Bonelli, F. (2025). A Longitudinal Observational Study on Lactation-Associated Changes in Procalcitonin, Protein Carbonyl Content, Asymmetric Dimethylarginine, and Symmetric Dimethylarginine in Dairy Cattle. Veterinary Sciences, 12(9), 895. https://doi.org/10.3390/vetsci12090895








