Preliminary Findings on Low-Dose 1cp-LSD for Canine Anxiety: Exploring the Role of Owner Neuroticism and Psychopathology
Simple Summary
Abstract
1. Introduction
2. Material and Methods
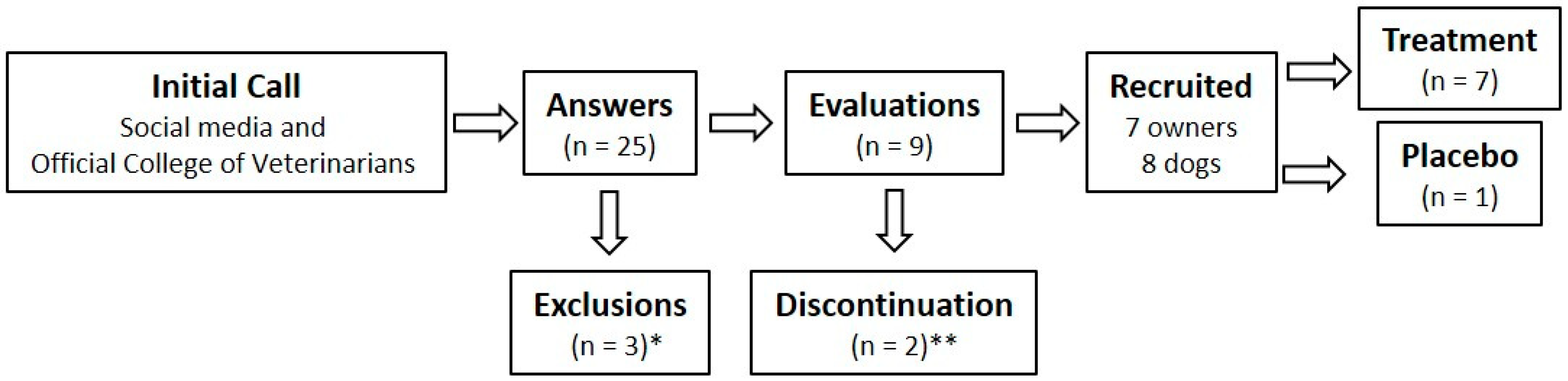
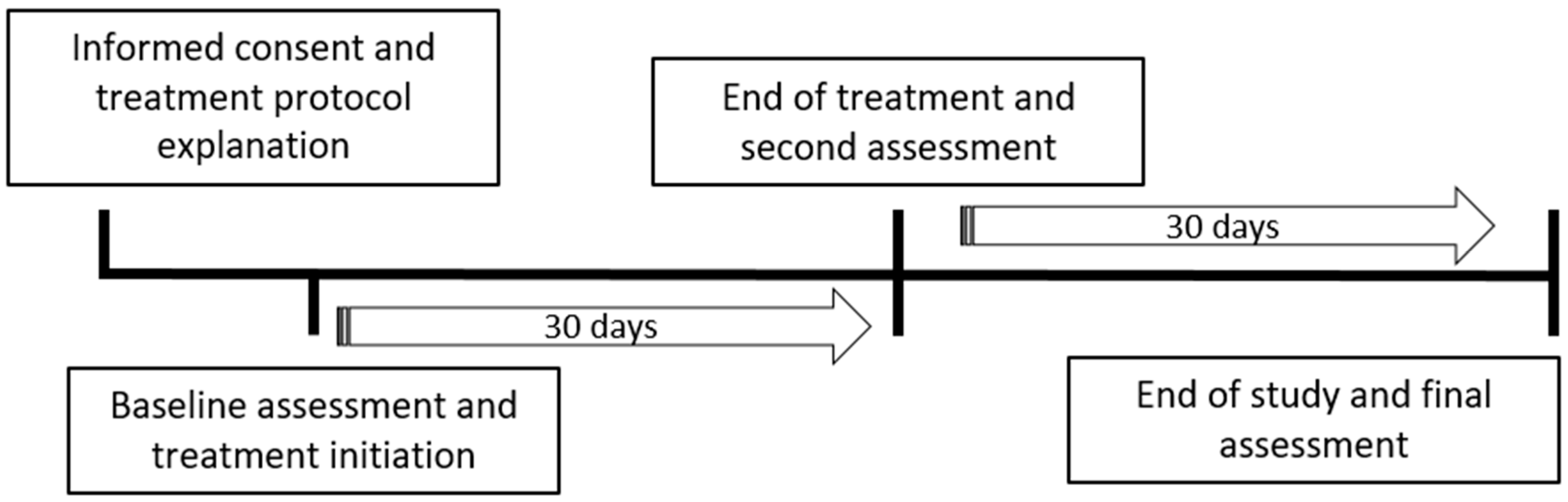
2.1. Study Design and Patient Recruitment
2.2. Rationale for the Sample Size
2.3. Instruments for Measuring Canine Anxiety and Human Neuroticism and Psychopathology
2.4. Dosage of 1cp-LSD
2.5. Statistical Analysis
3. Results
3.1. Descriptive Analysis of the Series
3.2. Role of 1cp-LSD and Owner Psychological Profile in Canine Anxiety: Baseline to End of Treatment
3.3. Role of Owner Psychological Profile in Canine Anxiety: End of Treatment to End of Study
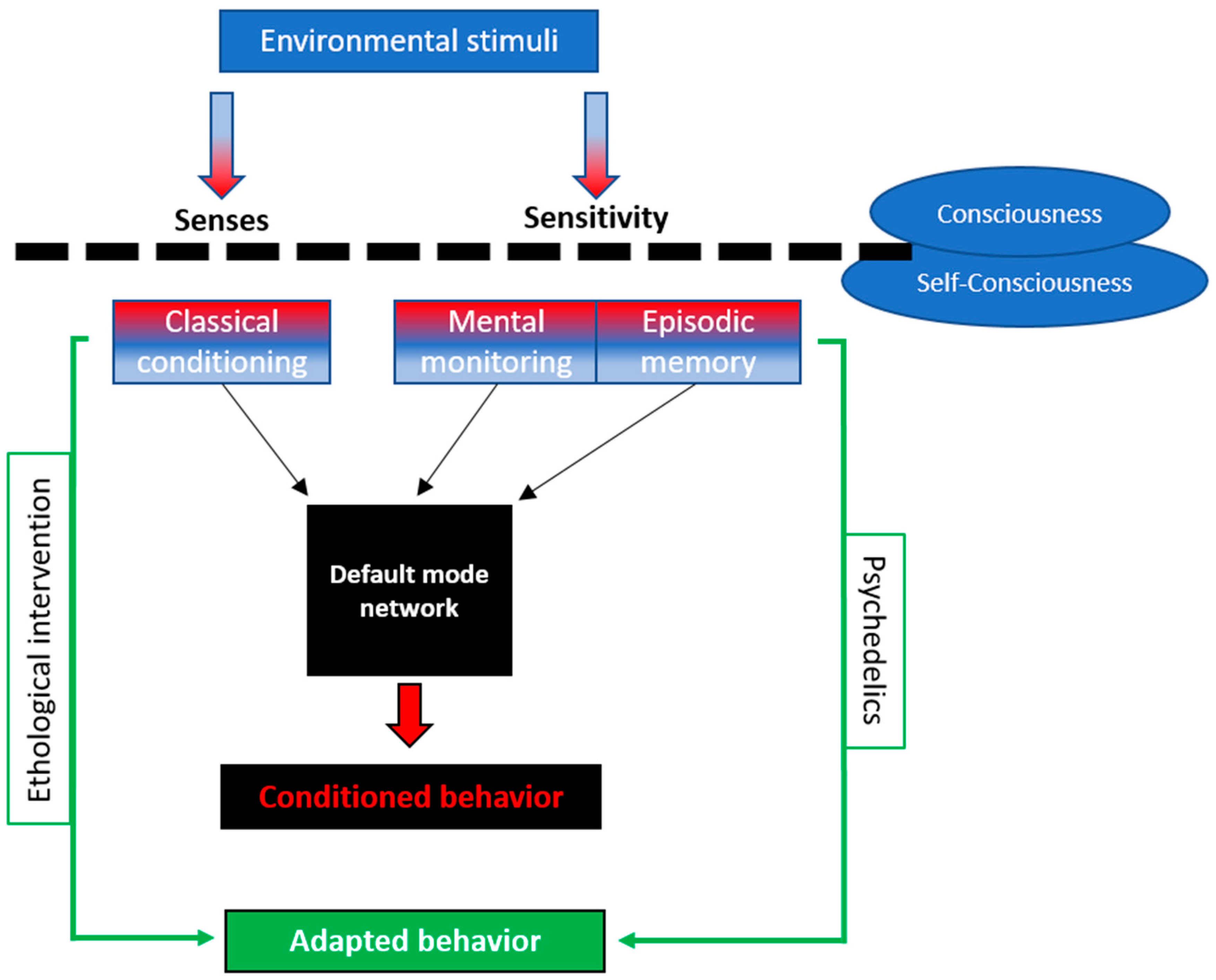
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Assessment of the Nine Dimensions Derived from the Symptom Assessment-45 Questionnaire (SA-45)
References
- Ibanez, M.; Anzola Delgado, B. Anxiety Disorders in Dogs. In Anxiety Disorders; Kalinin, V.V., Ed.; IntechOpen: Rijeka, Croatia, 2011. [Google Scholar]
- Storengen, L.; Boge, S.; Strøm, S.; Løberg, G.; Lingaas, F. A descriptive study of 215 dogs diagnosed with separation anxiety. Appl. Anim. Behav. Sci. 2014, 159, 82–89. [Google Scholar] [CrossRef]
- Salonen, M.; Sulkama, S.; Mikkola, S.; Puurunen, J.; Hakanen, E.; Tiira, K.; Araujo, C.; Lohi, H. Prevalence, comorbidity, and breed differences in canine anxiety in 13,700 Finnish pet dogs. Sci. Rep. 2020, 10, 2962. [Google Scholar] [CrossRef]
- Pierantoni, L.; Albertini, M.; Piotti, P.; Ripamonti, G.; Pocar, P.; Borromeo, V.; Pirrone, F. Signs of Anxiety and Salivary Copeptin Levels in Dogs Diagnosed with Separation-Related Problems in a Short Separation Test. Animals 2022, 12, 1974. [Google Scholar] [CrossRef]
- Sherman, B.L. Separation anxiety in dogs. Compend. Contin. Educ. Vet. 2008, 30, 27–42. [Google Scholar]
- Crespo-Garay, C. España, líder europea en abandono de animales: 700 cada día. Natl. Geogr. 2021. Available online: https://www.antena3.com/noticias/sociedad/espana-pais-europeo-que-mas-animales-abandonados-700-dia_2022082063013cba142d7b000190a15e.html (accessed on 14 July 2025).
- Hernández-Garzón, P. Ansiedad por separación. In Manual de Etología Canina; Grupo Asís Biomedia: Zaragoza, Spain, 2012; pp. 89–117. [Google Scholar]
- Sundman, A.S.; Van Poucke, E.; Svensson Holm, A.C.; Faresjo, A.; Theodorsson, E.; Jensen, P.; Roth, L.S.V. Long-term stress levels are synchronized in dogs and their owners. Sci. Rep. 2019, 9, 7391. [Google Scholar] [CrossRef]
- Gobbo, E.; Zupan, M. Dogs’ Sociability, Owners’ Neuroticism and Attachment Style to Pets as Predictors of Dog Aggression. Animals 2020, 10, 315. [Google Scholar] [CrossRef] [PubMed]
- Turcsán, B.; Range, F.; Virányi, Z.; Miklósi, Á.; Kubinyi, E. Birds of a feather flock together? Perceived personality matching in owner–dog dyads. Appl. Anim. Behav. Sci. 2012, 140, 154–160. [Google Scholar] [CrossRef]
- McCrae, R.R.; Costa, P.T., Jr. Personality trait structure as a human universal. Am. Psychol. 1997, 52, 509–516. [Google Scholar] [CrossRef]
- Salonen, M.; Mikkola, S.; Hakanen, E.; Sulkama, S.; Puurunen, J.; Lohi, H. Personality traits associate with behavioral problems in pet dogs. Transl. Psychiatry 2022, 12, 78. [Google Scholar] [CrossRef]
- Powell, L.; Stefanovski, D.; Siracusa, C.; Serpell, J. Owner Personality, Owner-Dog Attachment, and Canine Demographics Influence Treatment Outcomes in Canine Behavioral Medicine Cases. Front. Vet. Sci. 2020, 7, 630931. [Google Scholar] [CrossRef]
- Riva, J.; Bondiolotti, G.; Michelazzi, M.; Verga, M.; Carenzi, C. Anxiety related behavioural disorders and neurotransmitters in dogs. Appl. Anim. Behav. Sci. 2008, 114, 168–181. [Google Scholar] [CrossRef]
- Akimova, E.; Lanzenberger, R.; Kasper, S. The serotonin-1A receptor in anxiety disorders. Biol. Psychiatry 2009, 66, 627–635. [Google Scholar] [CrossRef]
- Vermeire, S.T.; Audenaert, K.R.; Dobbeleir, A.A.; De Meester, R.H.; De Vos, F.J.; Peremans, K.Y. Evaluation of the brain 5-HT2A receptor binding index in dogs with anxiety disorders, measured with 123I-5I-R91150 and SPECT. J. Nucl. Med. 2009, 50, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Dantas, L.M.S.; Ogata, N. Veterinary Psychopharmacology. Vet. Clin. N. Am. Small Anim. Pract. 2024, 54, 195–205. [Google Scholar] [CrossRef]
- Lader, M. Anxiolytic drugs: Dependence, addiction and abuse. Eur. Neuropsychopharmacol. 1994, 4, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Lowe, H.; Toyang, N.; Steele, B.; Grant, J.; Ali, A.; Gordon, L.; Ngwa, W. Psychedelics: Alternative and Potential Therapeutic Options for Treating Mood and Anxiety Disorders. Molecules 2022, 27, 2520. [Google Scholar] [CrossRef]
- Henriquez-Hernandez, L.A.; Garcia-Serrano, I.; Quintana-Hernandez, D.J.; Rojas-Hernandez, J.; Hernandez-Alvarez, E.; Zumbado, M.; Fernandez-Borkel, T.; Borkel, L.F. Single-dose 1cp-LSD administration for canine anxiety: A pilot study. Vet. Res. Commun. 2024, 48, 4007–4014. [Google Scholar] [CrossRef]
- Hernandez-Alvarez, E.; Borkel, L.F.; Rojas-Hernandez, J.; Quintana-Hernandez, D.J.; Garcia-Serrano, I.; Fernandez-Borkel, T.; Zumbado, M.; Henriquez-Hernandez, L.A. Evaluating the Potential of Microdosing 1cp-LSD for the Treatment of Canine Anxiety: A One-Month Case Study. Vet. Med. Sci. 2025, 11, e70486. [Google Scholar] [CrossRef]
- Kwan, A.C.; Olson, D.E.; Preller, K.H.; Roth, B.L. The neural basis of psychedelic action. Nat. Neurosci. 2022, 25, 1407–1419. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.E. Psychedelics. Pharmacol. Rev. 2016, 68, 264–355. [Google Scholar] [CrossRef]
- Wacker, D.; Wang, S.; McCorvy, J.D.; Betz, R.M.; Venkatakrishnan, A.J.; Levit, A.; Lansu, K.; Schools, Z.L.; Che, T.; Nichols, D.E.; et al. Crystal Structure of an LSD-Bound Human Serotonin Receptor. Cell 2017, 168, 377–389.e12. [Google Scholar] [CrossRef] [PubMed]
- Henriquez-Hernandez, L.A.; Rojas-Hernandez, J.; Quintana-Hernandez, D.J.; Borkel, L.F. Hofmann vs. Paracelsus: Do Psychedelics Defy the Basics of Toxicology?—A Systematic Review of the Main Ergolamines, Simple Tryptamines, and Phenylethylamines. Toxics 2023, 11, 148. [Google Scholar] [CrossRef]
- Schlag, A.K.; Aday, J.; Salam, I.; Neill, J.C.; Nutt, D.J. Adverse effects of psychedelics: From anecdotes and misinformation to systematic science. J. Psychopharmacol. 2022, 36, 258–272. [Google Scholar] [CrossRef]
- Vollenweider, F.X.; Preller, K.H. Psychedelic drugs: Neurobiology and potential for treatment of psychiatric disorders. Nat. Rev. Neurosci. 2020, 21, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Arita, S.; Arita, N.; Hikasa, Y. Effects of Eplerenone on Blood Pressure and Echocardiographic and Serum Biochemical Variables in Five Healthy Dogs: A Pilot Study. Vet. Med. Int. 2020, 2020, 5193856. [Google Scholar] [CrossRef] [PubMed]
- Grant, K.L.; Long, S.N. Extended release huperzine for the treatment of idiopathic epilepsy in dogs—A Case Report. Front. Vet. Sci. 2025, 12, 1518379. [Google Scholar] [CrossRef]
- Kaka, U.; Saifullah, B.; Abubakar, A.A.; Goh, Y.M.; Fakurazi, S.; Kaka, A.; Behan, A.A.; Ebrahimi, M.; Chen, H.C. Serum concentration of ketamine and antinociceptive effects of ketamine and ketamine-lidocaine infusions in conscious dogs. BMC Vet. Res. 2016, 12, 198. [Google Scholar] [CrossRef]
- Pargatzi, G.; Bergadano, A.; Spadavecchia, C.; Theurillat, R.; Thormann, W.; Levionnois, O.L. Stereoselective Pharmacokinetics of Ketamine Administered at a Low Dose in Awake Dogs. Animals 2024, 14, 1012. [Google Scholar] [CrossRef]
- Parthasarathy, V.; Crowell-Davis, S.L. Relationship between attachment to owners and separation anxiety in pet dogs (Canis lupus familiaris). J. Vet. Behav. 2006, 1, 109–120. [Google Scholar] [CrossRef]
- Mills, D.S.; Mueller, H.W.; McPeake, K.; Engel, O. Development and Psychometric Validation of the Lincoln Canine Anxiety Scale. Front. Vet. Sci. 2020, 7, 171. [Google Scholar] [CrossRef]
- Cupani, M.; Lorenzo-Seva, U. The development of an alternative IPIP inventory measuring the Big-Five factor markers in an Argentine sample. Personal. Individ. Differ. 2016, 91, 40–46. [Google Scholar] [CrossRef]
- Sandín, B.; Valiente, R.; Chorot, P.; Santed-Germán, M.Á.; Lostao, L. SA45: Forma abreviada del SCL-90. Psicothema 2008, 20, 290–296. [Google Scholar]
- Brandt, S.D.; Kavanagh, P.V.; Westphal, F.; Stratford, A.; Odland, A.U.; Klein, A.K.; Dowling, G.; Dempster, N.M.; Wallach, J.; Passie, T.; et al. Return of the lysergamides. Part VI: Analytical and behavioural characterization of 1-cyclopropanoyl-d-lysergic acid diethylamide (1CP-LSD). Drug Test. Anal. 2020, 12, 812–826. [Google Scholar] [CrossRef]
- Liechti, M.E.; Holze, F. Dosing Psychedelics and MDMA. Curr. Top. Behav. Neurosci. 2022, 56, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Du Bois, D.; Du Bois, E.F. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989, 5, 303–311; discussion 303–312. [Google Scholar] [PubMed]
- Borkel, L.F.; Rojas-Hernandez, J.; Henriquez-Hernandez, L.A.; Santana Del Pino, A.; Quintana-Hernandez, D.J. Set and setting predict psychopathology, wellbeing and meaningfulness of psychedelic experiences: A correlational study. Expert. Rev. Clin. Pharmacol. 2024, 17, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Hernández, J.; Borkel, L.F.; Quintana-Hernández, D.J.; del Pino, Á.S.; Henríquez-Hernández, L.A. Pattern of psychedelic substance use: A comparison between populations in Spain and South America using the Psychedelic Use Scale (PUS). Curr. Psychol. 2024, 43, 35083–35098. [Google Scholar] [CrossRef]
- Taylor, J.D.; Richards, R.K.; Wiegand, R.G. Toxicological studies with sodium cyclamate and saccharin. Food Cosmet. Toxicol. 1968, 6, 313–327. [Google Scholar] [CrossRef]
- Fernández-Borkel, T.; Borkel, L.F.; Rojas-Hernández, J.; Hernández-Álvarez, E.; Quintana-Hernández, D.J.; Ponti, L.G.; Henríquez-Hernández, L.A. The Causal Role of Consciousness in Psychedelic Therapy for Treatment-Resistant Depression: Hypothesis and Proposal. ACS Pharmacol. Transl. Sci. 2025, 8, 2839–2847. [Google Scholar] [CrossRef]
- Syed, O.A.; Petranker, R.; Tsang, B. The effect of psychedelic microdosing on animal behavior: A review with recommendations for the field. Neurosci. Biobehav. Rev. 2025, 174, 106204. [Google Scholar] [CrossRef]
- Low, P. The Cambridge Declaration on Consciousness. In Proceedings of the Francis Crick Memorial Conference on Consciousness in Human and Non-Human Animals, Cambridge, UK, 7 July 2012. [Google Scholar]
- Carruthers, P. Meta-cognition in animals: A skeptical look. Mind Lang. 2008, 23, 58–89. [Google Scholar] [CrossRef]
- Andrews, K. Consciousness. In The Animal Mind: An Introduction to the Philosophy of Animal Cognition; Routledge: New York, NY, USA, 2020; pp. 73–106. [Google Scholar]
- Pavlov, P.I. Conditioned reflexes: An investigation of the physiological activity of the cerebral cortex. Ann. Neurosci. 2010, 17, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Beran, M.J.; Smith, J.D.; Redford, J.S.; Washburn, D.A. Rhesus macaques (Macaca mulatta) monitor uncertainty during numerosity judgments. J. Exp. Psychol. Anim. Behav. Process 2006, 32, 111–119. [Google Scholar] [CrossRef]
- Smith, J.D.; Schull, J.; Strote, J.; McGee, K.; Egnor, R.; Erb, L. The uncertain response in the bottlenosed dolphin (Tursiops truncatus). J. Exp. Psychol. Gen. 1995, 124, 391–408. [Google Scholar] [CrossRef] [PubMed]
- Foote, A.L.; Crystal, J.D. Metacognition in the rat. Curr. Biol. 2007, 17, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Royka, A.L.; Johnston, A.M.; Santos, L.R. Metacognition in canids: A comparison of dogs (Canis familiaris) and dingoes (Canis dingo). J. Comp. Psychol. 2020, 134, 303–317. [Google Scholar] [CrossRef]
- Fugazza, C.; Pongracz, P.; Pogany, A.; Lenkei, R.; Miklosi, A. Mental representation and episodic-like memory of own actions in dogs. Sci. Rep. 2020, 10, 10449. [Google Scholar] [CrossRef]
- Dohmatob, E.; Dumas, G.; Bzdok, D. Dark control: The default mode network as a reinforcement learning agent. Hum. Brain Mapp. 2020, 41, 3318–3341. [Google Scholar] [CrossRef]
- Kyathanahally, S.P.; Jia, H.; Pustovyy, O.M.; Waggoner, P.; Beyers, R.; Schumacher, J.; Barrett, J.; Morrison, E.E.; Salibi, N.; Denney, T.S.; et al. Anterior-posterior dissociation of the default mode network in dogs. Brain Struct. Funct. 2015, 220, 1063–1076. [Google Scholar] [CrossRef]
- Szabo, D.; Czeibert, K.; Kettinger, A.; Gacsi, M.; Andics, A.; Miklosi, A.; Kubinyi, E. Resting-state fMRI data of awake dogs (Canis familiaris) via group-level independent component analysis reveal multiple, spatially distributed resting-state networks. Sci. Rep. 2019, 9, 15270. [Google Scholar] [CrossRef]
- Beckmann, K.M.; Wang-Leandro, A.; Dennler, M.; Carrera, I.; Richter, H.; Bektas, R.N.; Steiner, A.; Haller, S. Resting state networks of the canine brain under sevoflurane anaesthesia. PLoS ONE 2020, 15, e0231955. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.L.; Baxi, M.; Katz, J.S.; Waggoner, P.; Beyers, R.; Morrison, E.; Salibi, N.; Denney, T.S.; Vodyanoy, V.; Deshpande, G. Characterization of Structural Connectivity of the Default Mode Network in Dogs using Diffusion Tensor Imaging. Sci. Rep. 2016, 6, 36851. [Google Scholar] [CrossRef] [PubMed]
- Gattuso, J.J.; Perkins, D.; Ruffell, S.; Lawrence, A.J.; Hoyer, D.; Jacobson, L.H.; Timmermann, C.; Castle, D.; Rossell, S.L.; Downey, L.A.; et al. Default Mode Network Modulation by Psychedelics: A Systematic Review. Int. J. Neuropsychopharmacol. 2022, 26, 155–188. [Google Scholar] [CrossRef]
- Rock, R.C.; Haugh, S.; Davis, K.C.; Anderson, J.L.; Johnson, A.K.; Jones, M.A.; Salekin, R.T. Predicting animal abuse behaviors with externalizing and psychopathic personality traits. Personal. Individ. Differ. 2021, 171, 110444. [Google Scholar] [CrossRef]
- Aragunde-Kohl, U.; Gomez-Galan, J.; Lazaro-Perez, C.; Martinez-Lopez, J.A. Interaction and Emotional Connection with Pets: A Descriptive Analysis from Puerto Rico. Animals 2020, 10, 2136. [Google Scholar] [CrossRef]
- Martens, P.; Enders-Slegers, M.-J.; Walker, J.K. The Emotional Lives of Companion Animals: Attachment and Subjective Claims by Owners of Cats and Dogs. Anthrozoös 2016, 29, 73–88. [Google Scholar] [CrossRef]
- Mueller, M.K.; Gee, N.R.; Bures, R.M. Human-animal interaction as a social determinant of health: Descriptive findings from the health and retirement study. BMC Public Health 2018, 18, 305. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Garzón, P. Comportamiento normal del perro. In Manual de Etología Canina; Grupo Asís Biomedia: Zaragoza, Spain, 2012; pp. 1–17. [Google Scholar]
- Xestal, C. El doble de propietarios que de perros sufren ansiedad cuando se separan. Ladridos 2022, 18–20. Available online: https://www.ladridos.es/enero2022/enero2022/la-ansiedad-por-separacion-afecta-a-mas-duenos-que-a-perros. (accessed on 27 July 2025).
- Wells, D.L.; Treacy, K.R. Pet attachment and owner personality. Front. Psychiatry 2024, 15, 1406590. [Google Scholar] [CrossRef]
- Helsly, M.; Priymenko, N.; Girault, C.; Duranton, C.; Gaunet, F. Dog behaviours in veterinary consultations: Part II. The relationship between the behaviours of dogs and their owners. Vet. J. 2022, 281, 105789. [Google Scholar] [CrossRef] [PubMed]
- Somppi, S.; Tornqvist, H.; Koskela, A.; Vehkaoja, A.; Tiira, K.; Vaataja, H.; Surakka, V.; Vainio, O.; Kujala, M.V. Dog-Owner Relationship, Owner Interpretations and Dog Personality Are Connected with the Emotional Reactivity of Dogs. Animals 2022, 12, 1338. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.; Lourenco, A.; Lima, M.; Serpell, J.; Silva, K. Evaluation of mediating and moderating effects on the relationship between owners’ and dogs’ anxiety: A tool to understand a complex problem. J. Vet. Behav. 2021, 44, 55–61. [Google Scholar] [CrossRef]
- Mills, D.; Karagiannis, C.; Zulch, H. Stress—Its Effects on Health and Behavior: A Guide for Practitioners. Vet. Clin. N. Am. Small Anim. Pract. 2014, 44, 525–541. [Google Scholar] [CrossRef]

| Owner Characteristics | Dog Characteristics | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Gender | Age | Habitat | Household Size | Gender | Age | Breed | Neutering | Cohabitation Time | Other Animals | Past Details |
| 1 | Female | 34 | Country state | 1 | Female | 2 | Andalusian Rat Terrier | No | 8 * | Yes | Adopted |
| 2 | Female | 42 | Apartment | 2 | Female | 4 | Yorkshire/Tekkel | Yes | 2 | Yes | Adopted |
| 3 | Female | 47 | Apartment | 1 | Male | 4 | Mixed | Yes | 2.5 | Yes | Shelter |
| 4 | Female | 27 | Apartment | 1 | Female | 5 | Pointer | No | 1.5 | No | Adopted |
| 5 | Female | 32 | Apartment | 1 | Female | 3 | Chihuahua | Yes | 3 | Yes ** | Shelter |
| 6 | Female | 12 | Mixed (Rat Terrier) | No | 3 | Yes | Adopted | ||||
| 7 | Female | 53 | Apartment | 4 | Female | 8 | Mixed | Yes | 8 | No | Shelter |
| 8 | Man | 50 | Rural house | 2 | Male | 12 | Shih tzu | No | 12 | No | Adopted |
| ID | Weight (kg) | Length (cm) | Dog BSA 1 (m2) | Ratio 2 | Dose 3 (µg) | HED (µg) |
|---|---|---|---|---|---|---|
| 1 | 8.2 | 61 | 0.35 | 5.8 | 5 | 28.8 |
| 2 | 11 | 70 | 0.43 | 4.6 | 5 | 23.0 |
| 3 | 7.5 | 65 | 0.35 | 5.7 | 5 | 28.5 |
| 4 | 18 | 60 | 0.48 | 4.2 | 10 | 41.7 |
| 5 | 2.7 | 41 | 0.16 | 12.3 | 2.5 | 30.7 |
| 6 | 7 | 60 | 0.32 | 6.2 | 5 | 31.1 |
| 7 | 5.5 | 73 | 0.33 | 6.0 | Placebo | — |
| 8 | 7.5 | 50 | 0.29 | 6.9 | 5 | 34.5 |
| Pre-Treatment | Post-Treatment | End of Study | p Value * | Cohen’s d † | p Value ** | Cohen’s d †† | |
|---|---|---|---|---|---|---|---|
| SA | 21.4 (8–40) | 11.3 (7–15) | 10.7 (3–17) | 0.023 ‡ | 1.20 | 0.578 | 0.14 |
| LCAS | 40.4 (30–53) | 29.9 (16–42) | 23.3 (3–39) | 0.089 | 1.04 | 0.131 | 0.52 |
| Neuroticism 1 | 1.80 (1.0–2.2) | 1.9 (1.0–3.5) | 1.9 (1.1–3.0) | 0.235 | −0.19 | 0.766 | 0.04 |
| Depression 2 | 0.66 (0–1.4) | 0.94 (0–3.4) | 0.80 (0–1.8) | 0.363 | −0.28 | 0.582 | 0.14 |
| Hostility 2 | 0.50 (0–1.0) | 0.22 (0–0.6) | 0.06 (0–0.2) | 0.017 ‡ | 0.74 | 0.248 | 0.50 |
| Interpersonal sensitivity 2 | 0.20 (0–0.4) | 0.93 (0–3.8) | 0.60 (0–3.4) | 0.250 | −0.73 | 0.245 | 0.29 |
| Somatization 2 | 1.10 (0–2.4) | 0.71 (0–3.0) | 1.17 (0.4–2.0) | 0.154 | 0.38 | 0.241 | −0.47 |
| Anxiety 2 | 1.30 (0–2.4) | 1.13 (0–3.6) | 1.06 (0–2.2) | 0.047 ‡ | 0.15 | 0.779 | 0.07 |
| Psychoticism 2 | 0 | 0.26 (0–1.4) | 0.14 (0–1.0) | 0.175 | −0.34 | 0.103 | 0.15 |
| Obsessive–compulsion 2 | 1.00 (0–2.8) | 1.08 (0–3.4) | 1.28 (0.2–2.6) | 0.483 | −0.08 | 0.412 | −0.22 |
| Phobia 2 | 0.30 (0–1.0) | 0.51 (0–2.8) | 0.23 (0–1.6) | 0.175 | −0.21 | 0.140 | 0.33 |
| Paranoid ideation 2 | 0.50 (0–1.0) | 0.60 (0–2.2) | 0.43 (0–2.2) | 0.259 | −0.11 | 0.395 | 0.18 |
| B | [CI 95%] | p Value | |
|---|---|---|---|
| ∆ Separation anxiety | |||
| Intercept | 25.6 | [−10.6, 61.8] | 0.128 |
| ∆ Dose 1 | −1.15 | [−2.29, −0.003] | 0.050 |
| Intercept | −14.6 | [−22.9, −6.37] | 0.006 |
| ∆ Psychoticism | 50.1 | [5.37, 94.8] | 0.035 |
| ∆ Lincoln Canine Anxiety Scale | |||
| Intercept | 53.2 | [8.72, 97.7] | 0.028 |
| ∆ Dose 1 | −2.05 | [−3.45, −0.64] | 0.013 |
| B | [CI 95%] | p Value | |
|---|---|---|---|
| ∆ Separation anxiety | |||
| Intercept | −0.29 | [−1.71, 1.66] | 0.968 |
| ∆ Hostility | 4.77 | [0.001, 9.54] | 0.050 |
| Intercept | 0.58 | [−0.84, 2.00] | 0.354 |
| ∆ Interpersonal sensitivity | 3.12 | [1.14, 5.10] | 0.008 |
| Intercept | 0.22 | [−1.52, 1.96] | 0.768 |
| ∆ Paranoid ideation | 3.38 | [0.14, 6.61] | 0.043 |
| ∆ Lincoln Canine Anxiety Scale | |||
| Intercept | 2.33 | [−9.64, 1.76] | 0.141 |
| ∆ Hostility | 20.4 | [4.34, 36.6] | 0.021 |
| Intercept | −1.99 | [−7.91, 3.92] | 0.441 |
| ∆ Interpersonal sensitivity | 11.6 | [3.39, 19.8] | 0.014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Álvarez, E.; Rojas-Hernández, J.; Borkel, L.F.; Quintana-Hernández, D.J.; Fernández-Borkel, T.; Henríquez-Hernández, L.A. Preliminary Findings on Low-Dose 1cp-LSD for Canine Anxiety: Exploring the Role of Owner Neuroticism and Psychopathology. Vet. Sci. 2025, 12, 872. https://doi.org/10.3390/vetsci12090872
Hernández-Álvarez E, Rojas-Hernández J, Borkel LF, Quintana-Hernández DJ, Fernández-Borkel T, Henríquez-Hernández LA. Preliminary Findings on Low-Dose 1cp-LSD for Canine Anxiety: Exploring the Role of Owner Neuroticism and Psychopathology. Veterinary Sciences. 2025; 12(9):872. https://doi.org/10.3390/vetsci12090872
Chicago/Turabian StyleHernández-Álvarez, Elisa, Jaime Rojas-Hernández, Lucas F. Borkel, Domingo J. Quintana-Hernández, Tobías Fernández-Borkel, and Luis Alberto Henríquez-Hernández. 2025. "Preliminary Findings on Low-Dose 1cp-LSD for Canine Anxiety: Exploring the Role of Owner Neuroticism and Psychopathology" Veterinary Sciences 12, no. 9: 872. https://doi.org/10.3390/vetsci12090872
APA StyleHernández-Álvarez, E., Rojas-Hernández, J., Borkel, L. F., Quintana-Hernández, D. J., Fernández-Borkel, T., & Henríquez-Hernández, L. A. (2025). Preliminary Findings on Low-Dose 1cp-LSD for Canine Anxiety: Exploring the Role of Owner Neuroticism and Psychopathology. Veterinary Sciences, 12(9), 872. https://doi.org/10.3390/vetsci12090872







