Prevalence and Risk Factors of Mycoplasma Hyopneumoniae in Swine Farms, Mainland China, 2003–2024: A Meta-Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Quality Assessment and Data Extraction
2.4. Statistical Analysis
3. Results
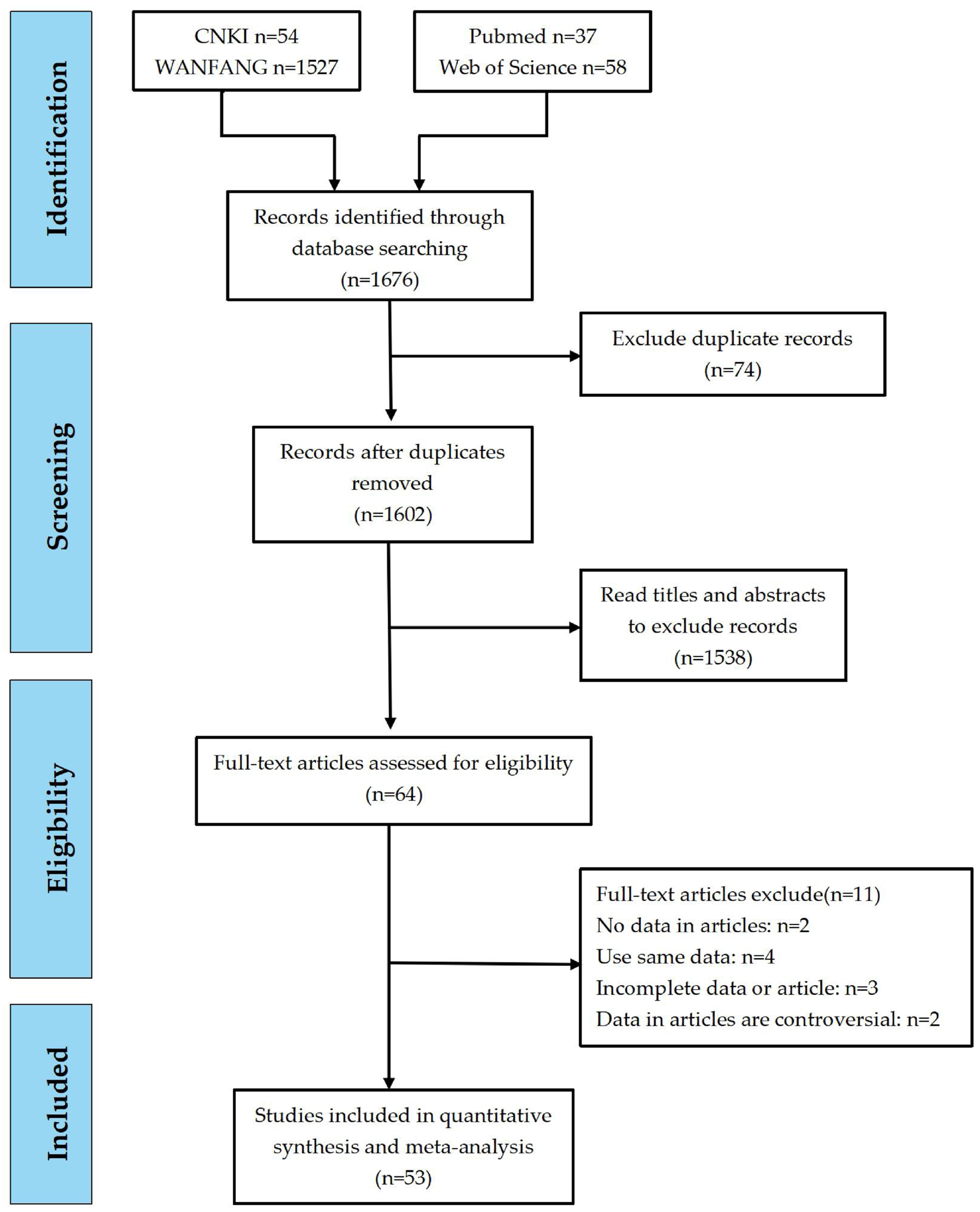
3.1. Literature Search Results
3.2. Data Extraction and Quality Analysis
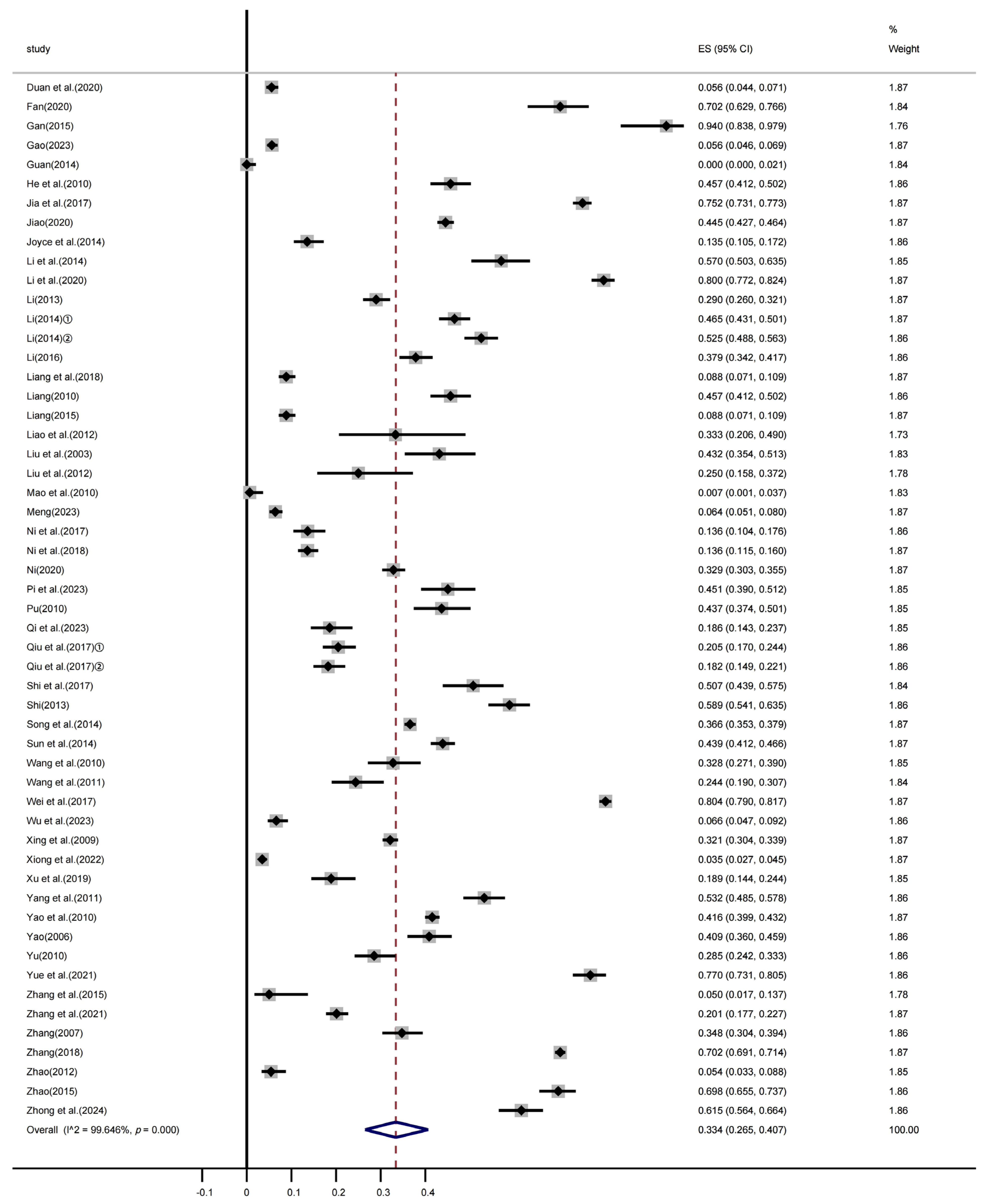
3.3. Heterogeneity Analysis
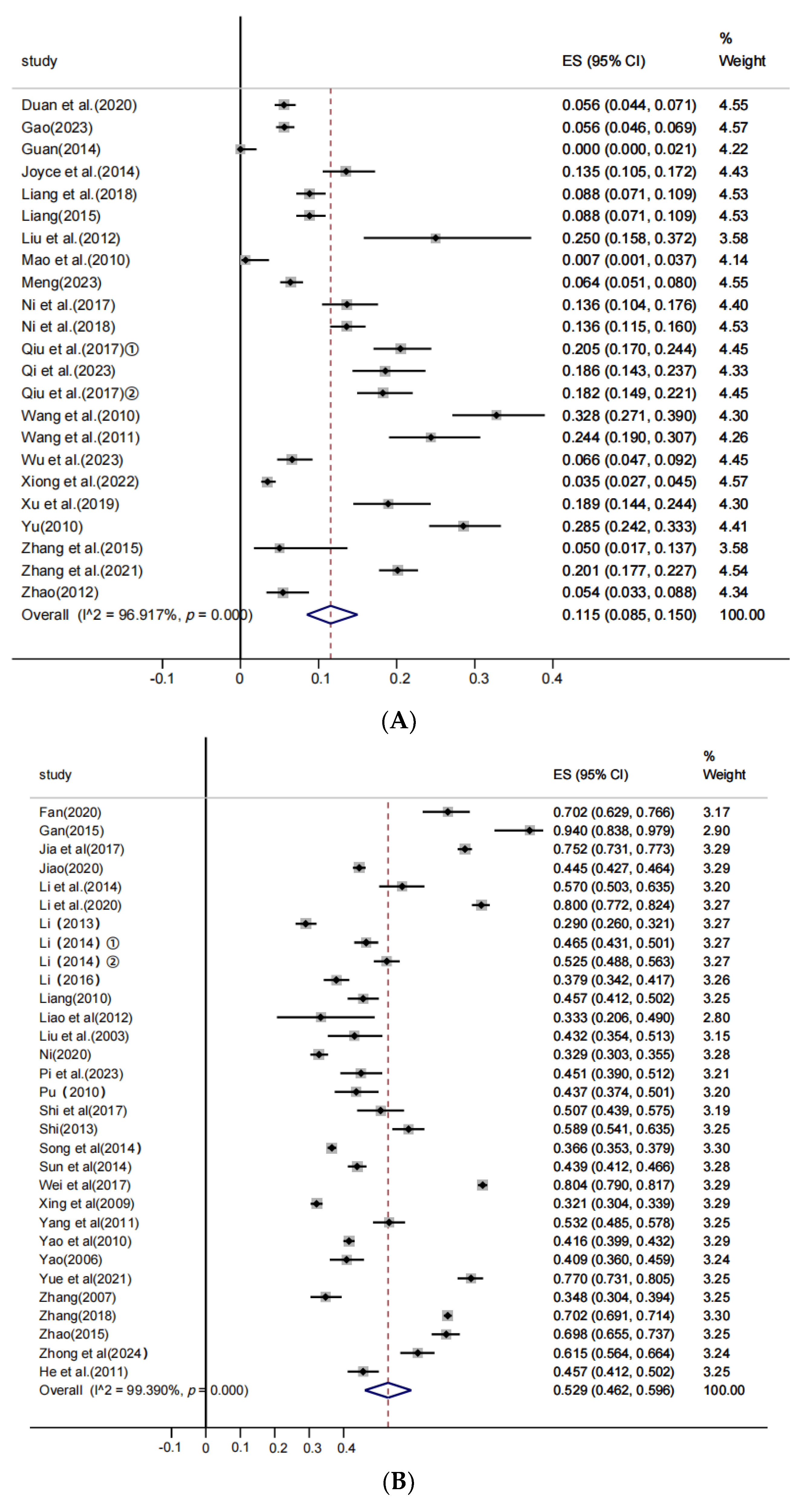
3.4. Subgroup Analysis
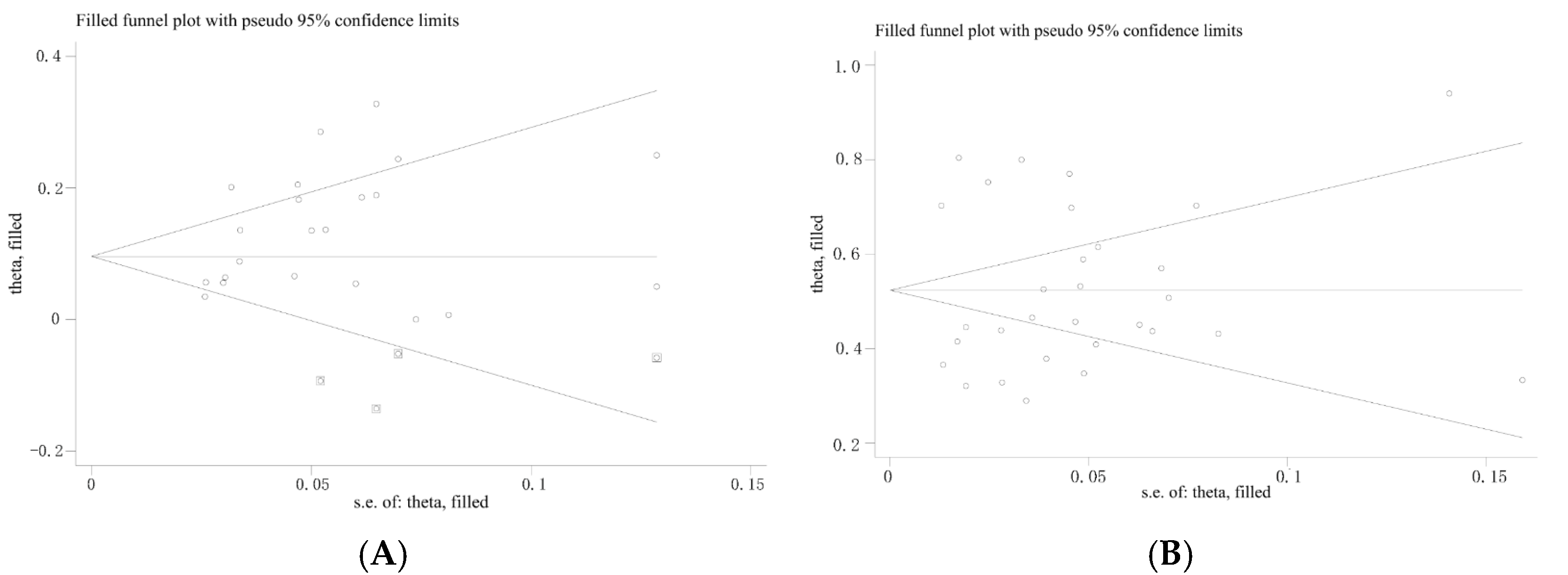
3.5. Publication Bias and Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thacker, E.; Minion, F. Mycoplasmosis. In Diseases of Swine, 10th ed.; Zimmerman, J.J., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Eds.; Wiley-Blackwell Publishing: Ames, IA, USA, 2012; pp. 779–798. [Google Scholar]
- Ferraz, M.E.S.; Almeida, H.M.S.; Storino, G.Y.; Sonálio, K.; Souza, M.R.; Moura, C.A.A.; Oliveira, S.; Pieters, M. Lung consolidation caused by Mycoplasma hyopneumoniae has a negative effect on productive performance and economic revenue in finishing pigs. Prev. Vet. Med. 2020, 182, 105091. [Google Scholar] [CrossRef]
- Holst, S.; Yeske, P.; Pieters, M. Elimination of Mycoplasma hyopneumoniae from breed-to-wean farms: A review of current protocols with emphasis on herd closure and medication. J. Swine Health Prod. 2015, 23, 321–330. [Google Scholar] [CrossRef]
- Jafari Jozani, R.; Khallawi, M.F.H.A.; Trott, D.; Petrovski, K.; Low, W.Y.; Hemmatzadeh, F. Unravelling Antimicrobial Resistance in Mycoplasma hyopneumoniae: Genetic Mechanisms and Future Directions. Vet. Sci. 2024, 11, 542. [Google Scholar] [CrossRef]
- Maes, D.; Sibila, M.; Kuhnert, P.; Segalés, J.; Haesebrouck, F.; Pieters, M. Update on Mycoplasma hyopneumoniae infections in pigs: Knowledge gaps for improved disease control. Transbound. Emerg. Dis. 2018, 65 (Suppl. S1), 110–124. [Google Scholar] [CrossRef]
- Garcia-Morante, B.; De Abreu, C.; Underwood, G.; Lara Puente, J.H.; Pieters, M. Characterization of a Mycoplasma hyopneumoniae aerosol infection model in pigs. Vet. Microbiol. 2024, 299, 110296. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Zhang, X.; Zhou, Y.; Tang, H.; Zhao, D.; Liu, F. Immunosuppression Reduces Lung Injury Caused by Mycoplasma pneumoniae Infection. Sci. Rep. 2019, 9, 7147. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Zhang, X.; Zhou, Y.; Tang, H.; Zhao, D.; Liu, F. Control of Mycoplasma hyopneumoniae infections in pigs. Vet. Microbiol. 2018, 225, 104–109. [Google Scholar]
- Poeta Silva, A.P.S.; Marostica, T.P.; McDaniel, A.; Minion, F.C.; Oliveira, S.; Pieters, M. Comparison of Mycoplasma hyopneumoniae response to infection by route of exposure. Vet. Microbiol. 2021, 258, 109118. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, Y.; Zhao, H.; Wu, Q.; Xin, J.; Pan, Q. Mycoplasma hyopneumoniae inhibits the unfolded protein response to prevent host macrophage apoptosis and M2 polarization. Infect. Immun. 2024, 92, e00051-24. [Google Scholar] [CrossRef]
- Maes, D.; Boyen, F.; Haesebrouck, F.; Gautier-Bouchardon, A.V. Antimicrobial treatment of Mycoplasma hyopneumoniae infections. Vet. J. 2020, 259–260, 105474. [Google Scholar] [CrossRef]
- Yu, M.; Wang, H.L.; Wang, X.F.; Wang, T.; Wang, K.W.; Yu, J.; Zhang, Y.Y.; Han, H.; Wu, J.Q. Investigation on Mycoplasma hyopneumoniae, Mycoplasma hyorhinis and PRRSV infections in large-scale swine farms. China Anim. Health Inspect. 2015, 32, 8–10, 18. [Google Scholar]
- Gao, Y.M.; Chen, G.S.; Ni, S.T.; Tong, Z.; Wang, H.; Yang, F.; Yang, L.J.; Mo, Y.P.; Tan, C. Survey and analysis of Mycoplasma hyopneumoniae infection in some areas of southern China in 2022. Acta Vet. Zootech. Sin. 2024, 55, 3064–3074. [Google Scholar]
- Maes, D.; Boyen, F.; Devriendt, B.; Verheyen, A.; Haesebrouck, F. Perspectives for improvement of Mycoplasma hyopneumoniae vaccines in pigs. Vet. Res. 2021, 52, 67. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Patel, A.; Shah, P. Investigating antimicrobial resistance mechanisms in Mycoplasma hyopneumoniae and implications for treatment. Antimicrob. Agents Chemother. 2023, 67, 301–309. [Google Scholar]
- Maes, D.; Segalés, J.; Meyns, T.; Sibila, M.; Pieters, M.; Haesebrouck, F. Control of Mycoplasma hyopneumoniae infections in pigs. Vet. Microbiol. 2008, 126, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Doi, S.A.; Xu, C. The Freeman-Tukey double arcsine transformation for the meta-analysis of proportions: Recent criticisms were seriously misleading. J. Evid.-Based Med. 2021, 14, 259–261. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, H.; Ye, L.; Shi, J.; Zhang, H.; Zhang, T. The Occurrence and Meta-Analysis of Investigations on Intestinal Parasitic Infections Among Captive Wild Mammals in Mainland China. Vet. Sci. 2025, 12, 182. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; Version 6.2; Wiley: Hoboken, NJ, USA, 2021. [Google Scholar]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Shi, L.Y.; Lin, L.F. The trim-and-fill method for publication bias: Practical guidelines and recommendations based on a large database of meta-analyses. Medicine 2019, 98, e15987. [Google Scholar] [CrossRef]
- Duan, Q.P.; Zhang, Y.J.; Zhao, S. Epidemiological investigation of major respiratory diseases in some large-scale pig farms in Guangxi from 2015 to 2019. J. Anim. Vet. Sci. 2020, 52, 120–127. [Google Scholar]
- Fan, C.M. Epidemiological investigation and analysis of respiratory diseases in some household pig farms in Hunan Province. China Swine Ind. 2020, 15, 80–83. [Google Scholar] [CrossRef]
- Gan, Y. The Diagnosis of Mycoplasmal Pneumonia and the Establishment of Negative Pigs. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2015. [Google Scholar]
- Gao, Y.M. Epidemiological Investigation and Immunization Efficacy Evaluation of Mycoplasma hyopneumoniae in a Breeding Pig Farm of a Company in Guangxi. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2023. [Google Scholar] [CrossRef]
- Guan, Z. Epidemiological Investigation of Mixed Respiratory Infections in Pigs in Some Large-Scale Farms in Eastern Sichuan. Master’s Thesis, Sichuan Agricultural University, Ya’an, China, 2014. [Google Scholar]
- He, Y.; Xu, M.J.; Zhou, D.H.; Zou, F.C.; Lin, R.Q.; Yin, C.C.; He, X.H.; Liang, R.; Liang, M.; Zhu, X.Q. Seroprevalence of Mycoplasma hyopneumoniae in pigs in subtropical southern China. Trop. Anim. Health Prod. 2011, 43, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.Y.; Wei, Y.M.; Qian, Z.B. Epidemiological and serological investigation of major swine diseases in Zhangye City. J. Anim. Husb. Vet. Med. 2017, 36, 73–75. [Google Scholar]
- Jiao, Q.L. Epidemiological Investigation and Immunoprevention Research on Mycoplasmal Pneumonia and Infectious Atrophic Rhinitis in Large-Scale Pig Farms in Shandong Province. Master’s Thesis, Shandong Agricultural University, Tai’an, China, 2020. [Google Scholar] [CrossRef]
- Maingi, W.J.; Xiong, Q.Y.; Wei, Y.N. Detection of Mycoplasma hyorhinis and Mycoplasma hyopneumoniae infection in pig farms in Jiangsu Province, China, by nested PCR. Chin. J. Zoonoses 2014, 30, 800–805. [Google Scholar]
- Li, Z.C. Epidemiological Investigation of Mycoplasma hyopneumoniae (MPS) and Clinical Immunization Efficacy Experiment in Ningbo Region. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2016. [Google Scholar]
- Li, P.F. Epidemiological Investigation and Immunization Comparison Test of Mycoplasma hyopneumoniae in Some Areas of Shanxi Province. Master’s Thesis, Shanxi Agricultural University, Taiyuan, China, 2013. [Google Scholar]
- Li, X.P.; Bian, S.S.; Cao, J. Detection and analysis of Mycoplasma hyopneumoniae infection in pigs from large-scale slaughterhouses in Xinjiang. J. Anim. Vet. Sci. 2020, 52, 121–125. [Google Scholar]
- Li, Y.Y. Epidemiological Investigation of Respiratory Disease Syndrome in Luchuan Pigs in Guangxi and Isolation and Identification of Porcine Circovirus Type 2. Master’s Thesis, Guangxi University, Nanning, China, 2014. [Google Scholar]
- Li, S. Epidemiological Investigation of Mycoplasma hyopneumoniae in Northern Xinjiang. Master’s Thesis, Shihezi University, Shihezi, China, 2014. [Google Scholar]
- Li, S.; Li, J.; Zhou, X. Molecular epidemiological investigation of Mycoplasma hyopneumoniae in parts of Northern Xinjiang. J. Anim. Vet. Sci. 2014, 46, 107–109. [Google Scholar]
- Liang, Q.L. Serological Epidemiological Investigation of Influenza A, Hepatitis E, Mycoplasma hyopneumoniae, and Haemophilus parasuis in Wild Boars. Master’s Thesis, Jilin Agricultural University, Changchun, China, 2019. [Google Scholar] [CrossRef]
- Liang, R. Toxoplasmosis, Chlamydiosis, and Mycoplasmosis in Some Pig Farms in Guangdong. Master’s Thesis, South China Agricultural University, Guangzhou, China, 2010. [Google Scholar] [CrossRef]
- Liang, Q.L.; Zou, Y.; Gao, Y.H.; Nie, L.B.; Zhang, X.X.; Hu, G.X.; Du, R.; Zhu, X.Q. First report of Mycoplasma hyopneumoniae seroprevalence in farmed wild boars in China. Acta Trop. 2018, 182, 212–214. [Google Scholar] [CrossRef]
- Liao, Q.S.; Li, Z.X.; Ding, L.C.; Chai, J.; Xu, J. Epidemiological Research of Major Infectious Disease from Partial Pig Farm from 2009 to 2010. China Anim. Husb. Vet. Med. 2012, 39, 194–199. [Google Scholar]
- Liu, M.M.; Jia, L.J.; Xue, S.J.; Liang, W.F.; Zhang, S.F. Comparison of PCR methods to different target genes of Mycoplasma suis. Anim. Husb. Vet. Med. 2012, 44, 1–4. [Google Scholar]
- Liu, B.S.; Li, Y.B.; Wang, Q.Q.; Kong, F.Y.; Shi, Z.Z.; Pu, Y.H.; Wang, J.Y.; Chang, H.R. Epidemiological investigation and diagnosis of swine diseases in Honghe Prefecture in the summer of 2002. Yunnan J. Anim. Husb. Vet. Med. 2003, 4, 16–17. [Google Scholar]
- Mao, A.M.; Wang, Y.J.; Bi, Z.S. Detection and analysis of common pathogens in cases of unknown high fever disease in pigs in Wuxi from 2007 to 2008. China Anim. Health Inspect. 2010, 27, 50–52. [Google Scholar]
- Meng, R. Epidemiological investigation of major diseases in Changbai pigs in Dushan County, Guizhou Province. China Livest. Poultry Breed. 2023, 19, 155–159. [Google Scholar]
- Ni, L.G.; Tao, Y.; Xu, P. Epidemiological investigation report on Mycoplasma hyopneumoniae in Sujiang pigs. Mod. Anim. Husb. 2017, 41–42. [Google Scholar]
- Ni, L.G.; Zhao, X.T.; Tao, Y.; Chen, Z. Epidemiological investigation of Mycoplasma hyopneumoniae in different pig breeds. Heilongjiang J. Anim. Sci. Vet. Med. 2018, 91–95. [Google Scholar] [CrossRef]
- Ni, L.G. Molecular Genetic Mechanisms Underlying Differences in Susceptibility to Mycoplasma hyopneumoniae Between Jiangquhai and Duroc Pigs. Master’s Thesis, Yangzhou University, Yangzhou, China, 2020. [Google Scholar] [CrossRef]
- Pi, Z.Y.; Wang, C.F.; Wang, C.X. Epidemiological investigation of Mycoplasma hyopneumoniae in pigs around Yining City, Xinjiang. China Swine Ind. 2023, 18, 87–89. [Google Scholar] [CrossRef]
- Pu, T.C. Serological Investigation and Pathological Analysis of Mycoplasma hyopneumoniae (MPS) in a Pig farm. Master’s Thesis, Guizhou University, Guiyang, China, 2010. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, J.; Zhao, C.P.; Lu, Y.; Tan, Y.; Zhang, X.; Wang, J.; Zhou, S.; Shi, K. Epidemiological investigation of common swine diseases in Songtao County, Guizhou. Mod. Anim. Husb. Sci. Technol. 2023, 35–39. [Google Scholar] [CrossRef]
- Qiu, G.; Rui, Y.; Li, K.; Huang, S.; Han, Z.; Wang, X.; Jiang, W.; Luo, H.; Lan, Y.; Li, J. Detection and phylogenetic analysis of Mycoplasma hyopneumoniae from Tibetan pigs in western China. Trop. Anim. Health Prod. 2017, 49, 1545–1551. [Google Scholar] [CrossRef]
- Qiu, G.; Rui, Y.; Zhang, J.; Zhang, L.; Huang, S.; Wu, Q.; Li, K.; Han, Z.; Liu, S.; Li, J. Macrolide-Resistance Selection in Tibetan Pigs with a High Load of Mycoplasma hyopneumoniae. Microb Drug Resist. 2018, 24, 1043–1049. [Google Scholar] [CrossRef]
- Shi, X.C. Serological Epidemiological Investigation of Six Infectious Diseases in Tibetan Pig Populations in Nyingchi, Tibet. Master’s Thesis, Northwest A&F University, Xianyang, China, 2013. [Google Scholar]
- Shi, L.M.; Feng, B.K.; Du, X.Y.; Sun, Q.; Zhang, Y. Epidemiological investigation and serological detection of Mycoplasma hyopneumoniae in pigs. Hubei J. Anim. Husb. Vet. Med. 2017, 38, 13–15. [Google Scholar] [CrossRef]
- Song, Q.; Zhang, W.; Song, W.; Liu, Z.; Khan, M.K.; He, L.; Fang, R.; Li, P.; Zhou, Y.; Hu, M.; et al. Seroprevalence and risk factors of Mycoplasma suis infection in pig farms in central China. Prev. Vet. Med. 2014, 117, 215–221. [Google Scholar] [CrossRef]
- Sun, Q.; Wei, J.; Gan, Y.Q.; Xia, L.M.; Lu, J. Investigation on the infection of swine asthma in large-scale pig farms in Shanghai. Livest. Poult. Ind. 2014, 7, 68–69. [Google Scholar]
- Wang, G.P.; Dai, W.; Jia, X.Y. Detection of Mycoplasma hyopneumoniae and Mycoplasma hyorhinis infection in pigs in Changsha by PCR. Swine Prod. 2011, 03, 81–82. [Google Scholar] [CrossRef]
- Wang, G.P.; Hu, S.F.; Jiang, F.F.; Li, X.Y.; Yu, X.L.; Yang, X.D. Detection of Mycoplasma hyopneumoniae and Mycoplasma hyorhinis in subclinically infected pigs. In Proceedings of the 11th National Symposium on Surveillance and Eradication of Major Diseases in Large-Scale Pig Farms; Huazhong Agricultural University Animal Medical College: Wuhan, China, 2010. [Google Scholar]
- Wei, Y.M.; Qian, Z.B. Epidemiological and serological investigation report on major diseases in large-scale pig farms. J. Anim. Husb. Vet. Med. 2017, 36, 97–101. [Google Scholar]
- Wu, Y.; Li, N.M.; Zhang, Y.X.; Zhang, W.; Chen, S.; Huang, Y.; Tian, Y.; Zeng, Z.; Wu, X.; Wang, B. Establishment and application of a multiplex PCR detection method for porcine respiratory bacterial pathogens. Prog. Vet. Med. 2023, 44, 82–87. [Google Scholar] [CrossRef]
- Xing, F.S.; Wang, W.H.; Zhang, Y.M.; Cheng, X.Y. Epidemiological investigation and pathological diagnosis of Mycoplasma hyopneumoniae in pigs. J. Northwest A&F Univ. (Agric. Sci.) 2009, 18, 67–70. [Google Scholar]
- Xiong, J.P.; Liang, S.H. Epidemiological investigation and analysis of major swine diseases in Yongchang County. J. Anim. Husb. Vet. Med. 2022, 41, 180–186. [Google Scholar]
- Xu, L.; Lan, S.; Li, J.; Yu, B.; Zhang, P.; Li, B.; Yuan, X. Epidemiological investigation of three Mycoplasma species in pigs in Zhejiang Province from 2014 to 2018. Zhejiang Agric. Sci. 2019, 60, 2368–2370. [Google Scholar] [CrossRef]
- Yang, J.J.; Li, S.; Li, J.; Lu, X.G. Epidemiological investigation of Mycoplasma hyopneumoniae in large-scale pig farms in northern Xinjiang. China Anim. Health Inspect. 2011, 28, 44–46. [Google Scholar]
- Yao, R.Y. Study on ELISA Antibody Detection Method for Mycoplasma hyopneumoniae and Cloning of Nucleotide Reductase Gene. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2006. [Google Scholar]
- Yao, J.; Zhang, L.; Wu, X.; Meng, F.; Wang, J.; Han, Y.; Fan, Z.; Lei, Y. Epidemiological survey of Mycoplasma hyopneumoniae in breeding pig farms. Swine Prod. 2010, 2, 57–58. [Google Scholar] [CrossRef]
- Yu, J. Serological Epidemiological Investigation and Comprehensive Prevention and Control Strategies for Mycoplasma hyopneumoniae Infection in a Certain District. Master’s Thesis, Shanghai Jiao Tong University, Shanghai, China, 2010. [Google Scholar]
- Yue, W.; Liu, Y.; Meng, Y.; Ma, H.; He, J. Prevalence of porcine respiratory pathogens in slaughterhouses in Shanxi Province, China. Vet. Med. Sci. 2021, 7, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Y. Epidemiological survey of Mycoplasma hyopneumoniae in breeding pig farms. Today’s Anim. Husb. Vet. Med. 2018, 34, 65. [Google Scholar]
- Zhang, S.H. Serological Epidemiological Investigation of Major Respiratory Pathogens in Large-Scale Pig Farms in Hainan Province and Prevention and Control of Porcine Respiratory Disease Syndrome. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2007. [Google Scholar]
- Zhang, H.; Wang, Y.; Gao, L.; Wang, Y.; Wei, R. Genotype diversity of Mycoplasma hyopneumoniae in Chinese swine herds based on multilocus sequence typing. BMC Vet. Res. 2021, 17, 347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, P.; Li, S.Z.; Fu, Y.F.; Xu, Z.; Liang, T.; Yang, S.F.; Chen, X.H.; Qi, Y.V.; Sheng, J.L. Establishment and Preliminary Application of the Multiplex PCR Method for Detection of 4 Kinds of Swine Diseases. China Anim. Husb. Vet. Med. 2015, 42, 2612–2618. [Google Scholar]
- Zhao, H.W. Establishment and Application of a Multiplex PCR Method for Four Pathogens of Porcine Respiratory Disease Syndrome. Master’s Thesis, Anhui Agricultural University, Hefei, China, 2012. [Google Scholar]
- Zhao, X.P. Diagnosis and treatment of Mycoplasma hyopneumoniae pneumonia in pigs. Hubei Anim. Husb. Vet. Med. 2015, 36, 24–25. [Google Scholar] [CrossRef]
- Zhong, D.; Zheng, J.; Guan, Z.; Wahaab, A.; Zhang, J.; Anwar, M.N.; Wei, J. Prevalence and phylogenetic analysis of Mycoplasma hyopneumoniae in native pigs from Shanghai, China. Pak. Vet. J. 2024, 44, 954–956. [Google Scholar]
- Shen, S.; Zhang, J. Scientific Scoring of Pig Lungs Effectively Monitors Respiratory Diseases in Pig Farms. Swine Ind. Sci. 2023, 40, 94–96. [Google Scholar]
- Barendregt, J.J.; Doi, S.A.; Lee, Y.Y.; Norman, R.E.; Vos, T. Meta-analysis of prevalence. J. Epidemiol. Commun. Health 2013, 67, 974–978. [Google Scholar] [CrossRef]
- Pallarés, F.J.; Añón, J.A.; Rodríguez-Gómez, I.M.; Gómez-Laguna, J.; Fabré, R.; Sánchez-Carvajal, J.M.; Ruedas-Torres, I.; Carrasco, L. Prevalence of mycoplasma-like lung lesions in pigs from commercial farms from Spain and Portugal. Porc. Health Manag. 2021, 7, 26. [Google Scholar] [CrossRef]
- Coppola, F.; D’Addio, E.; Casini, L.; Sagona, S.; Aloisi, M.; Felicioli, A. Hematological and serum biochemistry values in free-ranging crested porcupine. Vet. Sci. 2020, 7, 171. [Google Scholar] [CrossRef]
- Vangroenweghe, F.A.; Labarque, G.G.; Piepers, S.; Strutzberg-Minder, K.; Maes, D. Mycoplasma hyopneumoniae infections in peri-weaned and post-weaned pigs in Belgium and The Netherlands: Prevalence and associations with climatic conditions. Vet. J. 2015, 205, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Cezar, G.; Magalhães, E.; Rupasinghe, K.; Chandra, S.; Silva, G.; Almeida, M.; Crim, B.; Burrough, E.; Gauger, P.; Siepker, C.; et al. Using diagnostic data from veterinary diagnostic laboratories to unravel macroepidemiological aspects of porcine circoviruses 2 and 3 in the United States from 2002–2023. PLoS ONE 2024, 19, e0311807. [Google Scholar] [CrossRef]
- Pieters, M.; Maes, D. Mycoplasmosis. In Diseases of Swine, 11th ed.; Zimmermann, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Zhang, J., Eds.; Wiley: New York, NY, USA, 2019; pp. 863–883. [Google Scholar]
- Clavijo, M.J.; Pantoja, L.G.; Holtkamp, D.I.; Yeske, P.; Johnson, C.; Sprague, M.; Fano, E.; Main, R.; McDowell, E.; Painter, T.; et al. Herd classification system for Mycoplasma hyopneumoniae infection status in breeding herds. J. Swine Health Prod. 2021, 29, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Nathues, H.; Alarcon, P.; Rushton, J.; Jolie, R.; Fiebig, K.; Jimenez, M.; Geurts, V.; Nathues, C. Cost of porcine reproductive and respiratory syndrome virus at individual farm level—An economic disease model. Prev. Vet. Med. 2017, 142, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Fablet, C.; Marois-Créhan, C.; Grasland, B.; Simon, G.; Jestin, A.; Kobisch, M.; Madec, F. Infectious agents associated with respiratory diseases in 125 farrow-to-finish pig herds: A cross-sectional study. Vet. Microbiol. 2012, 157, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Calderón Díaz, J.A.; Fitzgerald, R.M.; Shalloo, L.; Rodrigues da Costa, M.; Niemi, J.; Leonard, F.C.; Kyriazakis, I.; García Manzanilla, E. Financial Analysis of Herd Status and Vaccination Practices for Porcine Reproductive and Respiratory Syndrome Virus, Swine Influenza Virus, and Mycoplasma hyopneumoniae in Farrow-to-Finish Pig Farms Using a Bio-Economic Simulation Model. Front. Vet. Sci. 2020, 7, 556674. [Google Scholar] [CrossRef]
- Sibila, M.; Pieters, M.; Molitor, T.; Maes, D.; Haesebrouck, F.; Segalés, J. Current perspectives on the diagnosis and epidemiology of Mycoplasma hyopneumoniae infection. Vet. J. 2009, 181, 221–231. [Google Scholar] [CrossRef]
- Pieters, M.; Daniels, J.; Rovira, A. Comparison of sample types and diagnostic methods for in vivo detection of Mycoplasma hyopneumoniae during early stages of infection. Vet. Microbiol. 2017, 203, 103–109. [Google Scholar] [CrossRef]
- Tang, Y.W. Promoting translational research in human and veterinary medical virology. Vet. Microbiol. 2013, 165, 2–6. [Google Scholar] [CrossRef]
- Palzer, A.; Ritzmann, M.; Wolf, G.; Heinritzi, K. Associations between pathogens in healthy pigs and pigs with pneumonia. Vet. Rec. 2008, 162, 267–271. [Google Scholar] [CrossRef]
- Villarreal, I.; Meyns, T.; Dewulf, J.; Vranckx, K.; Calus, D.; Pasmans, F.; Haesebrouck, F.; Maes, D. The effect of vaccination on the transmission of Mycoplasma hyopneumoniae in pigs under field conditions. Vet. J. 2011, 188, 48–52. [Google Scholar] [CrossRef]
- Pieters, M.; Daniels, J.; Rovira, A.; Murtaugh, M.P.; Morrison, R. Comparison of real-time PCR and ELISA for detection of Mycoplasma hyopneumoniae. J. Vet. Diagn. Investig. 2017, 29, 593–597. [Google Scholar]
- Villarreal, I.; Maes, D.; Vranckx, K.; Pasmans, F.; Haesebrouck, F. Serological and pathological evaluation of Mycoplasma hyopneumoniae infection in pigs. Vet. J. 2011, 188, 48–52. [Google Scholar] [CrossRef]
- Fablet, C.; Marois-Créhan, C.; Simon, G.; Grasland, B.; Jestin, A.; Kobisch, M.; Madec, F. Infectious agents in respiratory diseases of pigs: Comparison of detection methods. Vet. Microbiol. 2012, 157, 152–163. [Google Scholar] [CrossRef]



| No. | Authors | Publication Year | Diagnostic Technique | Sample Type | No. of Positive Samples | No. of Samples |
|---|---|---|---|---|---|---|
| 1 | Duan et al. [23] | 2020 | PCR | Pathological material | 62 | 1114 |
| 2 | Fan [24] | 2020 | AGPT, histopathological examination | Pathological material | 118 | 168 |
| 3 | Gan [25] | 2015 | PCR | Nasal swabs | 47 | 50 |
| 4 | Gao [26] | 2023 | PCR | Throat swabs | 83 | 1478 |
| 5 | Guan [27] | 2014 | PCR | Lung | 0 | 183 |
| 6 | He et al. [28] | 2010 | ELISA | Serum | 210 | 460 |
| 7 | Jia et al. [29] | 2017 | Agglutination test | Serum | 1233 | 1639 |
| 8 | Jiao [30] | 2020 | Histopathological examination, PCR | Lung, throat swabs | 1119 | 1375 |
| 9 | Joyce et al. [31] | 2014 | PCR | Nasal swabs | 54 | 399 |
| 10 | Li [32] | 2016 | PCR, ELISA | Lung, nasal swabs | 245 | 647 |
| 11 | Li [33] | 2013 | PCR, ELISA | Pathological material, serum, nasopharyngeal swabs | 247 | 852 |
| 12 | Li et al. [34] | 2020 | Histopathological examination | Lung | 730 | 917 |
| 13 | Li, Y.Y. [35] | 2014 | PCR | Lung | 364 | 782 |
| 14 | Li, S. [36] | 2014 | ELISA, PCR | Serum, lung | 352 | 670 |
| 15 | Li et al. [37] | 2014 | PCR | Lung | 122 | 214 |
| 16 | Liang [38] | 2015 | ELISA | Serum | 78 | 882 |
| 17 | Liang [39] | 2010 | ELISA | Serum | 210 | 460 |
| 18 | Liang et al. [40] | 2018 | ELISA | Serum | 49 | 619 |
| 19 | Liao et al. [41] | 2012 | ELISA | Serum | 13 | 39 |
| 20 | Liu et al. [42] | 2012 | PCR | Serum | 15 | 60 |
| 21 | Liu et al. [43] | 2003 | ELISA | Serum | 63 | 146 |
| 22 | Mao et al. [44] | 2010 | PCR | Pathological material | 1 | 151 |
| 23 | Meng [45] | 2023 | PCR | Serum | 69 | 1080 |
| 24 | Ni et al. [46] | 2017 | PCR | Nasal swabs | 48 | 352 |
| 25 | Ni et al. [47] | 2018 | PCR, ELISA | Nasal swabs, serum | 119 | 876 |
| 26 | Ni [48] | 2020 | PCR, ELISA | Nasal swabs, serum, lung, tracheal swab | 414 | 1260 |
| 27 | Pi et al. [49] | 2023 | PCR | Nasal swabs, lung | 114 | 253 |
| 28 | Pu [50] | 2010 | ELISA | Serum | 100 | 229 |
| 29 | Qi et al. [51] | 2023 | PCR | Lung, nasal swabs | 49 | 264 |
| 30 | Qiu et al. [52] | 2017 | ELISA | Serum | 93 | 454 |
| 31 | Qiu et al. [53] | 2017 | PCR | Nasal swabs, tracheal swab, alveolar lavage fluid | 82 | 450 |
| 32 | Shi [54] | 2013 | ELISA | Serum | 249 | 423 |
| 33 | Shi et al. [55] | 2017 | ELISA | Serum | 103 | 203 |
| 34 | Song et al. [56] | 2014 | ELISA | Serum | 2056 | 5619 |
| 35 | Sun et al. [57] | 2014 | ELISA | Serum | 562 | 1280 |
| 36 | Wang et al. [58] | 2011 | PCR | Lung | 50 | 205 |
| 37 | Wang et al. [59] | 2010 | PCR | Lung, nasal swabs, tracheal swab, alveolar lavage fluid | 78 | 238 |
| 38 | Wei et al. [60] | 2017 | Agglutination test | Serum | 2687 | 3343 |
| 39 | Wu et al. [61] | 2023 | PCR | Lung, nasal swabs | 31 | 469 |
| 40 | Xing et al. [62] | 2009 | AGPT | Pathological material | 886 | 2757 |
| 41 | Xiong et al. [63] | 2022 | PCR | Serum | 52 | 1500 |
| 42 | Xu et al. [64] | 2019 | PCR | Pathological material | 45 | 238 |
| 43 | Yang et al. [65] | 2011 | ELISA, PCR | Serum, lung | 232 | 436 |
| 44 | Yao [66] | 2006 | ELISA | Serum | 152 | 372 |
| 45 | Yao et al. [67] | 2010 | Histopathological examination, PCR, ELISA | Lung, serum, nasopharyngeal swabs | 1451 | 3492 |
| 46 | Yu [68] | 2010 | ELISA | Serum | 105 | 368 |
| 47 | Yue et al. [69] | 2021 | PCR | Lung | 378 | 491 |
| 48 | Zhang [70] | 2018 | Histopathological examination, PCR | Serum, lung | 4214 | 6000 |
| 49 | Zhang [71] | 2007 | ELISA | Serum | 146 | 420 |
| 50 | Zhang et al. [72] | 2021 | PCR | Pathological material | 199 | 989 |
| 51 | Zhang et al. [73] | 2015 | PCR | Pathological material | 3 | 60 |
| 52 | Zhao [74] | 2012 | PCR | Lung, nasal swabs | 16 | 276 |
| 53 | Zhao [75] | 2015 | Histopathological examination | Lung | 335 | 480 |
| 54 | Zhong et al. [76] | 2024 | ELISA | Serum | 224 | 364 |
| Subgroup | No. of Studies | No. of Positive Samples | No. of Samples | Infection Rate | Heterogeneity | P | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimates | 95% CI | X2 | p-Value | I2/% | ||||||
| Age | 39.80% | [0.341; 0.456] | ||||||||
| Subclinical infection swine farms | Suckling swine | 3 | 25 | 144 | 0.137 | [0.000; 0.480] | / | / | / | 0.606 |
| Nursery swine | 2 | 19 | 80 | 0.207 | [0.132; 0.305] | / | / | / | ||
| Growing swine | 3 | 45 | 387 | 0.117 | [0.052; 0.201] | / | / | / | ||
| Fattening swine | 5 | 61 | 334 | 0.169 | [0.080; 0.282] | 16.272 | 0.001 | 81.563 | ||
| Breeding swine | 3 | 36 | 220 | 0.172 | [0.085; 0.277] | 6.943 | 0.074 | 56.790 | ||
| Clinical infection swine farms | Suckling swine | 18 | 1878 | 3812 | 0.380 | [0.246; 0.523] | 1246.462 | 0.000 | 98.636 | 0.005 |
| Nursery swine | 11 | 1585 | 3625 | 0.361 | [0.161; 0.589] | 1753.059 | 0.000 | 99.430 | ||
| Growing swine | 16 | 1146 | 3996 | 0.327 | [0.235; 0.426] | 570.102 | 0.000 | 97.369 | ||
| Fattening swine | 21 | 3459 | 7683 | 0.491 | [0.364; 0.619] | 2363.728 | 0.000 | 99.154 | ||
| Breeding swine | 18 | 2378 | 4719 | 0.617 | [0.495; 0.734] | 994.129 | 0.000 | 98.189 | ||
| Period | 29.80% | [0.232; 0.367] | ||||||||
| Subclinical infection swine farms | Before 2013 | 7 | 240 | 1642 | 0.107 | [0.031; 0.218] | 223.640 | 0.000 | 97.317 | 0.944 |
| 2014–2018 | 10 | 633 | 5712 | 0.110 | [0.084; 0.139] | 158.201 | 0.000 | 89.254 | ||
| 2019–2024 | 7 | 458 | 5376 | 0.124 | [0.066; 0.197] | 342.961 | 0.000 | 97.959 | ||
| Clinical infection swine farms | Before 2013 | 16 | 9982 | 20,737 | 0.497 | [0.394; 0.600] | 3046.162 | 0.000 | 99.508 | 0.803 |
| 2014–2018 | 10 | 7830 | 12,911 | 0.546 | [0.430; 0.659] | 1329.997 | 0.000 | 99.323 | ||
| 2019–2024 | 6 | 1668 | 3560 | 0.562 | [0.234; 0.862] | 1946.302 | 0.000 | 99.743 | ||
| Region | 33.40% | [0.256; 0.416] | ||||||||
| Subclinical infection swine farms | Northern region | 2 | 93 | 942 | 0.094 | [0.076; 0.114] | / | / | / | 0.272 |
| South region | 13 | 682 | 5716 | 0.096 | [0.056; 0.145] | 434.194 | 0.000 | 96.776 | ||
| Qinghai–Tibet Plateau region | 2 | 131 | 1069 | 0.118 | [0.099; 0.138] | / | / | / | ||
| Clinical infection swine farms | Northern region | 6 | 4416 | 9447 | 0.557 | [0.375; 0.731] | 1465.31 | 0.000 | 99.659 | 0.005 |
| South region | 13 | 2595 | 6144 | 0.474 | [0.418; 0.530] | 208.639 | 0.000 | 94.248 | ||
| Qinghai–Tibet Plateau region | 1 | 249 | 423 | 0.589 | [0.541; 0.635] | / | / | / | ||
| Northwest region | 7 | 5470 | 7468 | 0.642 | [0.538; 0.739] | 434.408 | 0.000 | 98.619 | ||
| Farming scale | 25.60% | [0.160; 0.365] | 0.000 | |||||||
| Small-scale swine farms | 8 | 362 | 1974 | 0.357 | [0.192; 0.541] | 531.799 | 0.000 | 98.308 | 0.012 | |
| Medium-scale swine farms | 3 | 243 | 1560 | 0.279 | [0.062; 0.573] | 297.666 | 0.000 | 98.992 | ||
| Large-scale swine farms | 3 | 119 | 1076 | 0.023 | [0.000; 0.169] | / | / | / | ||
| Season | 31.30% | [0.209; 0.428] | ||||||||
| Subclinical infection swine farms | Spring | 3 | 170 | 902 | 0.166 | [0.054; 0.321] | / | / | / | 0.127 |
| Summer | 2 | 33 | 720 | 0.044 | [0.030; 0.061] | / | / | / | ||
| Autumn | 2 | 29 | 570 | 0.050 | [0.034; 0.070] | / | / | / | ||
| Winter | 3 | 123 | 1024 | 0.094 | [0.020; 0.215] | / | / | / | ||
| Clinical infection swine farms | Spring | 6 | 655 | 1719 | 0.547 | [0.259; 0.819] | / | / | / | 0.105 |
| Summer | 4 | 775 | 1166 | 0.360 | [0.107; 0.664] | 765.055 | 0.000 | 99.346 | ||
| Autumn | 3 | 488 | 1215 | 0.651 | [0.564; 0.733 | 23.081 | 0.000 | 87.002 | ||
| Winter | 3 | 221 | 527 | 0.471 | [0.309; 0.636] | / | / | / | ||
| Sampling type | 31.60% | [0.260; 0.374] | ||||||||
| Subclinical infection swine farms | Serum | 8 | 544 | 5486 | 0.137 | [0.082; 0.203] | 283.042 | 0.000 | 97.527 | 0.513 |
| Swab | 7 | 295 | 2728 | 0.108 | [0.069; 0.155] | 77.43 | 0.000 | 90.96 | ||
| Tissue | 10 | 386 | 3210 | 0.083 | [0.030; 0.156] | 315.742 | 0.000 | 97.15 | ||
| Clinical infection swine farms | Serum | 21 | 9109 | 21,068 | 0.446 | [0.369; 0.525] | 2415.530 | 0.000 | 99.172 | 0.033 |
| Swab | 8 | 1570 | 4001 | 0.354 | [0.252; 0.462] | 246.880 | 0.000 | 96.760 | ||
| Tissue | 14 | 6257 | 11,199 | 0.568 | [0.449; 0.683] | 1791.307 | 0.000 | 99.274 | ||
| Diagnostic method | 24.20% | [0.192; 0.296] | ||||||||
| Antibody testing | 26 | 9619 | 20,973 | 0.402 | [0.131; 0.494] | 4408.821 | 0.000 | 99.410% | 0.000 | |
| Antigen testing | 33 | 5537 | 19,256 | 0.202 | [0.137; 0.275] | 8490.069 | 0.000 | 99.282% | ||
| Histopathological testing | 5 | 4637 | 7111 | 0.631 | [0.463; 0.783] | 811.349 | 0.000 | 99.384% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Zhang, H.; Zhang, X.; Ye, L.; Liu, X.; Zhang, T. Prevalence and Risk Factors of Mycoplasma Hyopneumoniae in Swine Farms, Mainland China, 2003–2024: A Meta-Analysis. Vet. Sci. 2025, 12, 863. https://doi.org/10.3390/vetsci12090863
Zhou H, Zhang H, Zhang X, Ye L, Liu X, Zhang T. Prevalence and Risk Factors of Mycoplasma Hyopneumoniae in Swine Farms, Mainland China, 2003–2024: A Meta-Analysis. Veterinary Sciences. 2025; 12(9):863. https://doi.org/10.3390/vetsci12090863
Chicago/Turabian StyleZhou, Hongyu, Huiling Zhang, Xueping Zhang, Lina Ye, Xinyuan Liu, and Tangjie Zhang. 2025. "Prevalence and Risk Factors of Mycoplasma Hyopneumoniae in Swine Farms, Mainland China, 2003–2024: A Meta-Analysis" Veterinary Sciences 12, no. 9: 863. https://doi.org/10.3390/vetsci12090863
APA StyleZhou, H., Zhang, H., Zhang, X., Ye, L., Liu, X., & Zhang, T. (2025). Prevalence and Risk Factors of Mycoplasma Hyopneumoniae in Swine Farms, Mainland China, 2003–2024: A Meta-Analysis. Veterinary Sciences, 12(9), 863. https://doi.org/10.3390/vetsci12090863






