Effects of Supplementation with Chlorogenic Acid-Rich Extract from Eucommia ulmoides Oliver During Peri-Implantation on the Reproductive Performance and Gut Microbiota of Sows
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
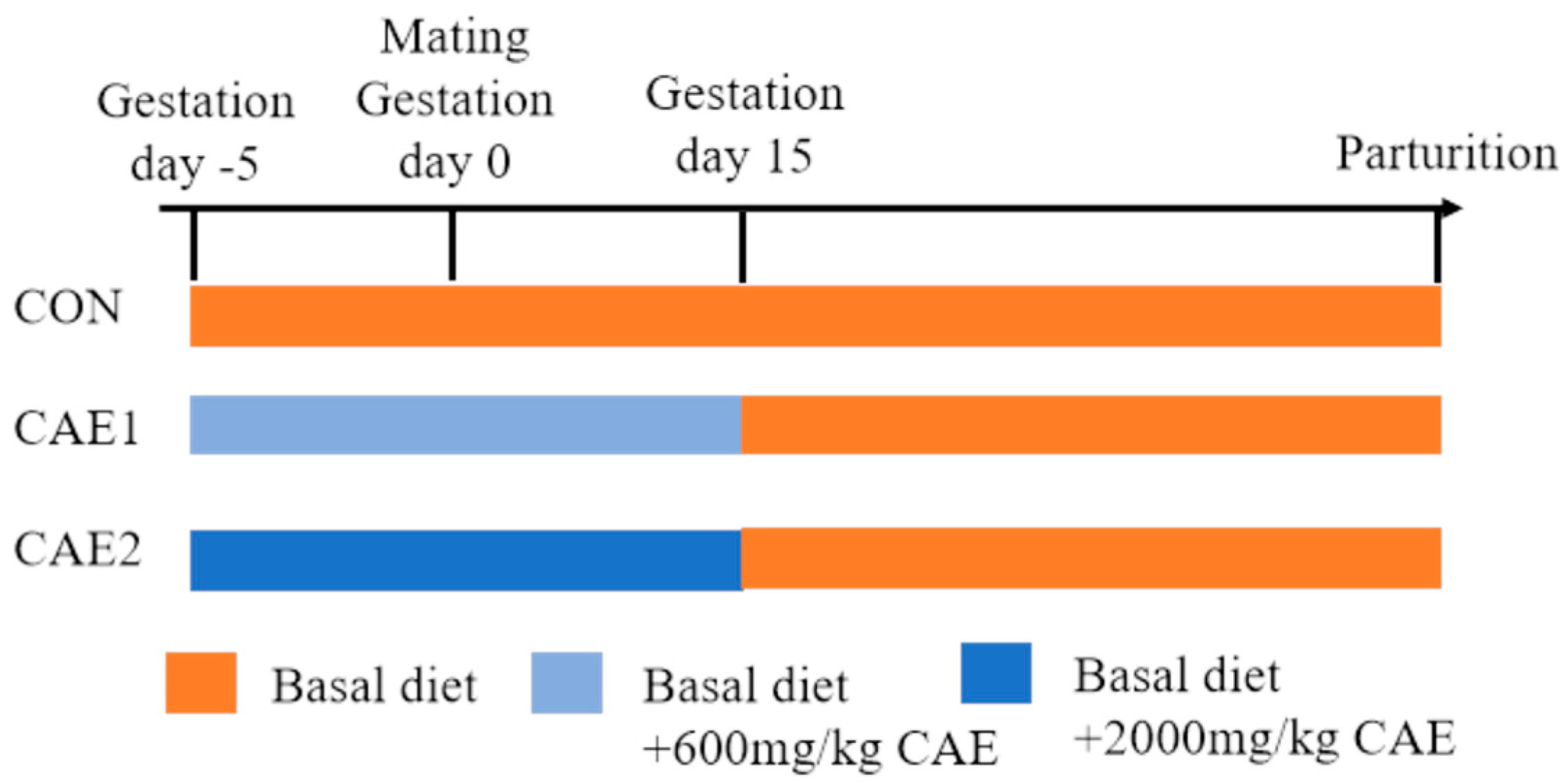
2.1. Experimental Animals and Design
2.2. Sample Collection
2.3. Reproductive Performance Analysis
2.4. Serum Biochemical Parameters Analysis
2.5. DNA Extraction and Microbiota Analysis
2.6. Short-Chain Fatty Acid (SCFA) Analysis
2.7. Statistical Analysis
3. Results
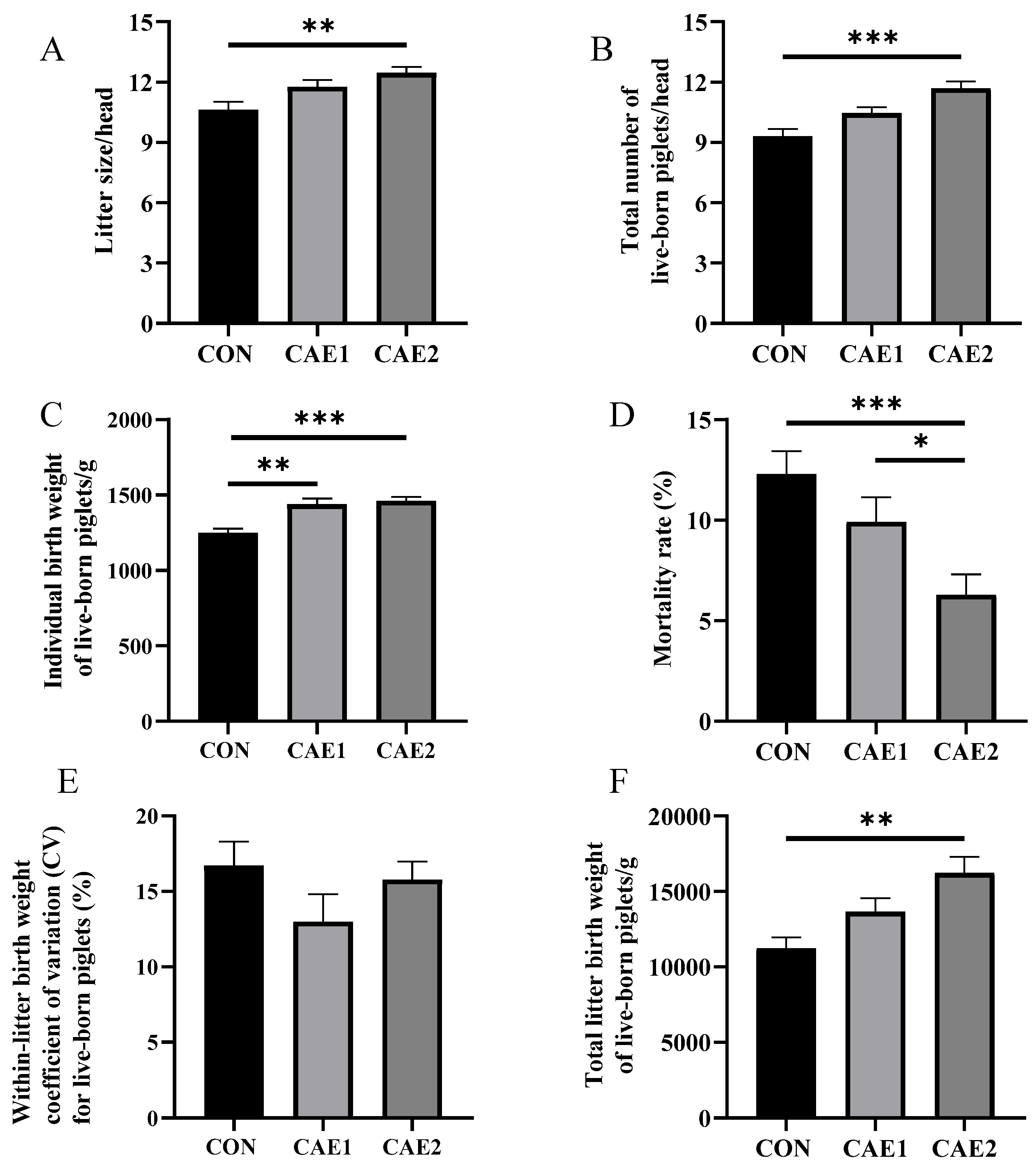
3.1. Reproductive Performance
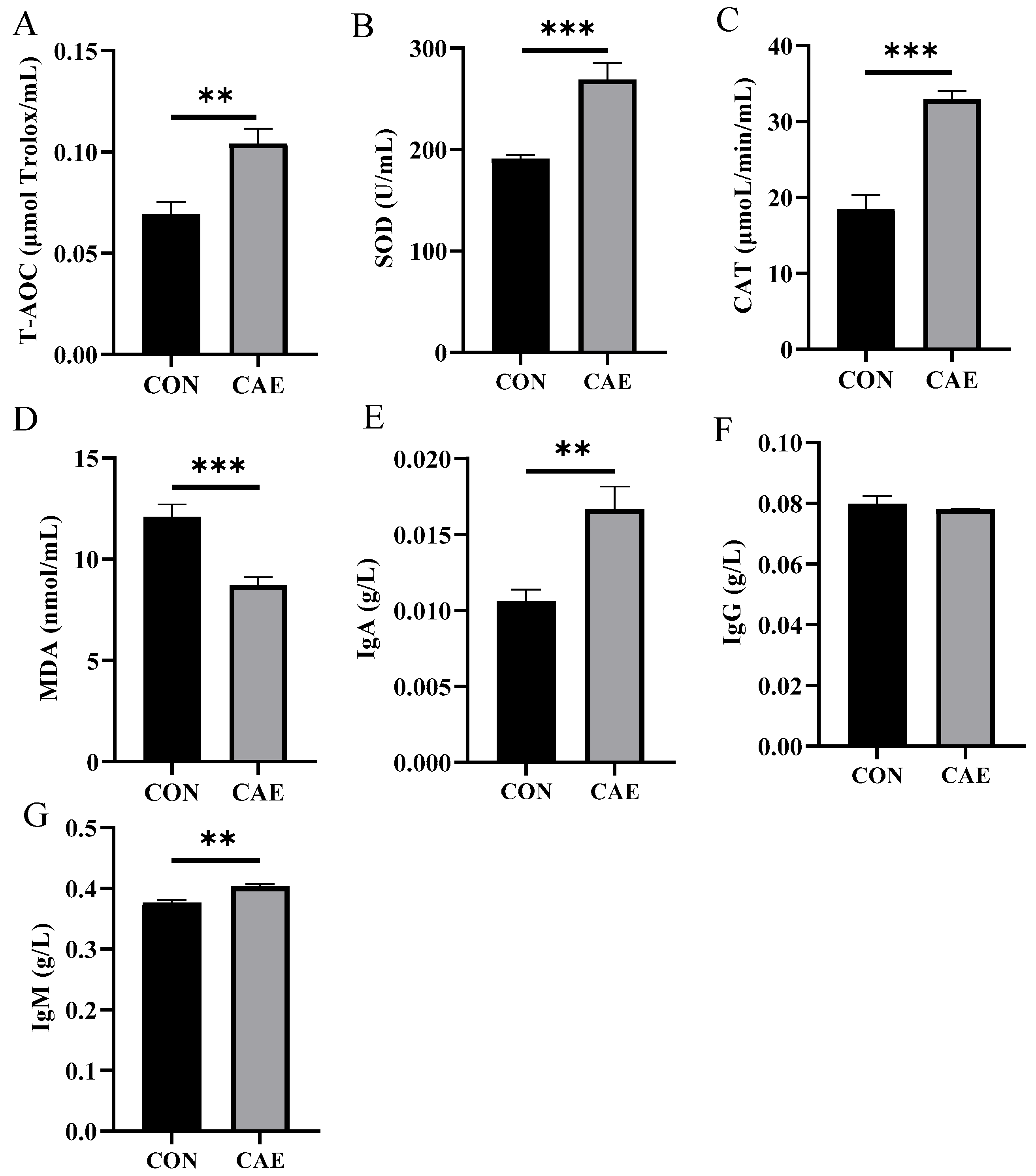
3.2. Serum Antioxidant and Immune Indices
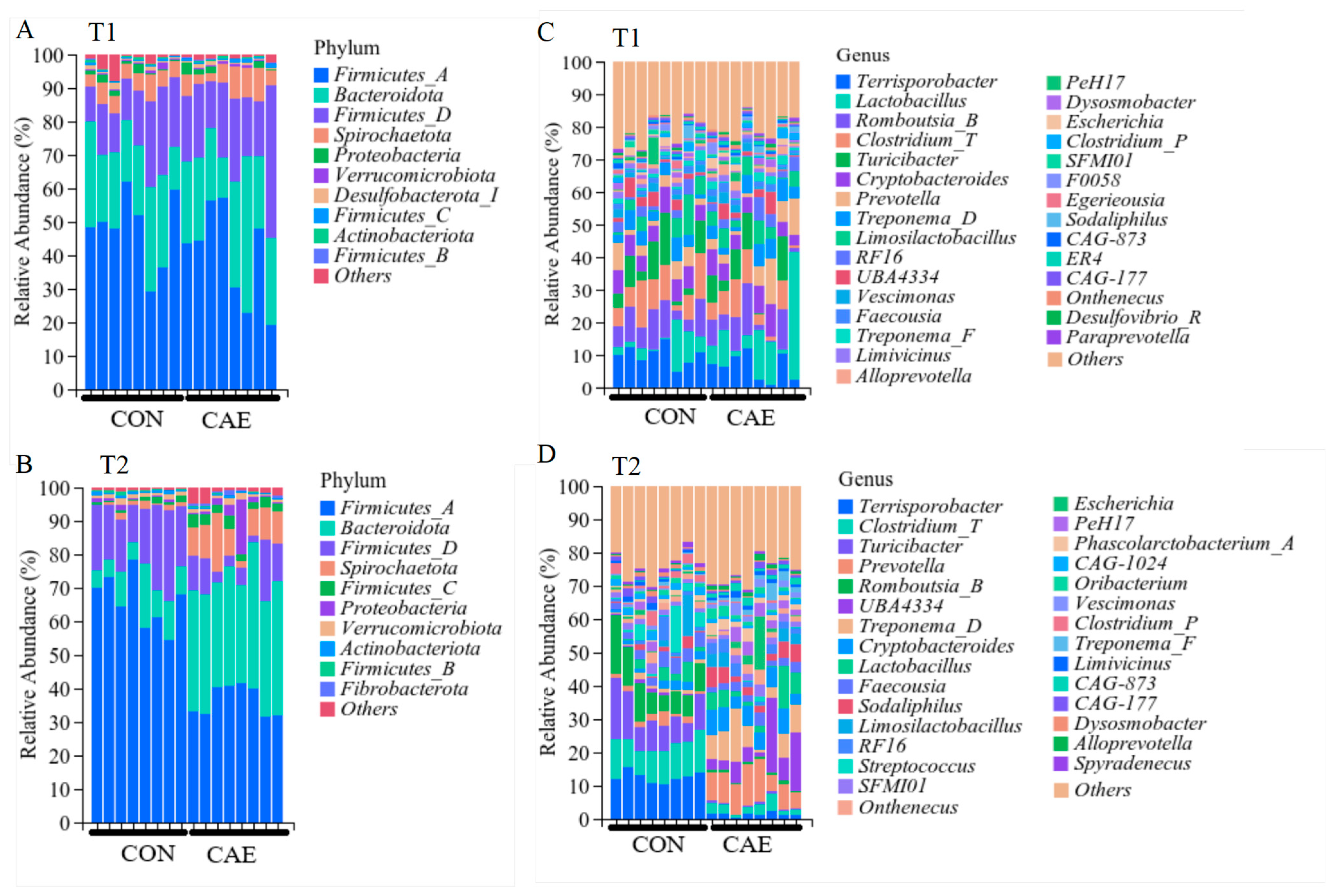
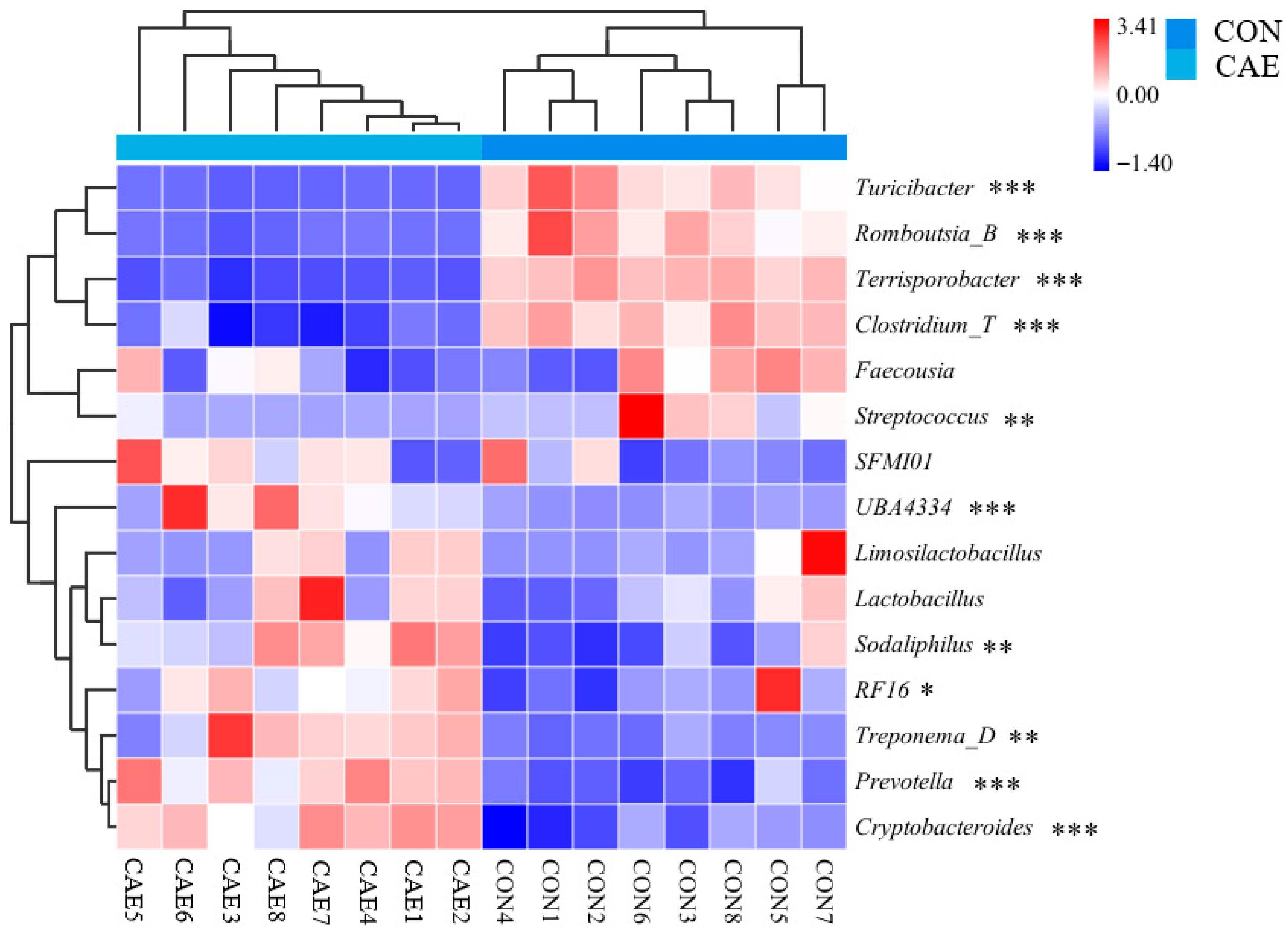
3.3. Fecal Microbial Analysis
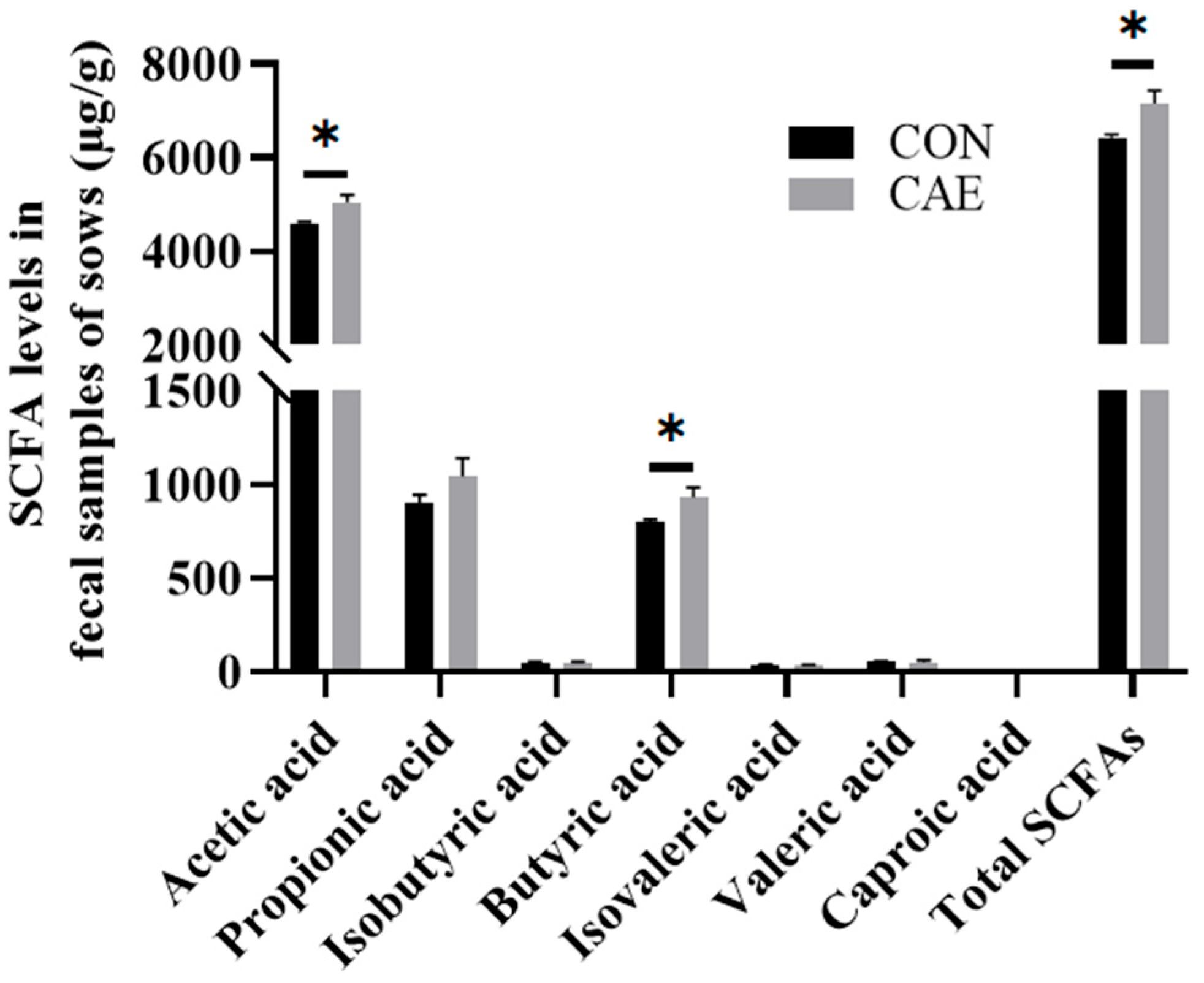
3.4. Fecal SCFA Analysis
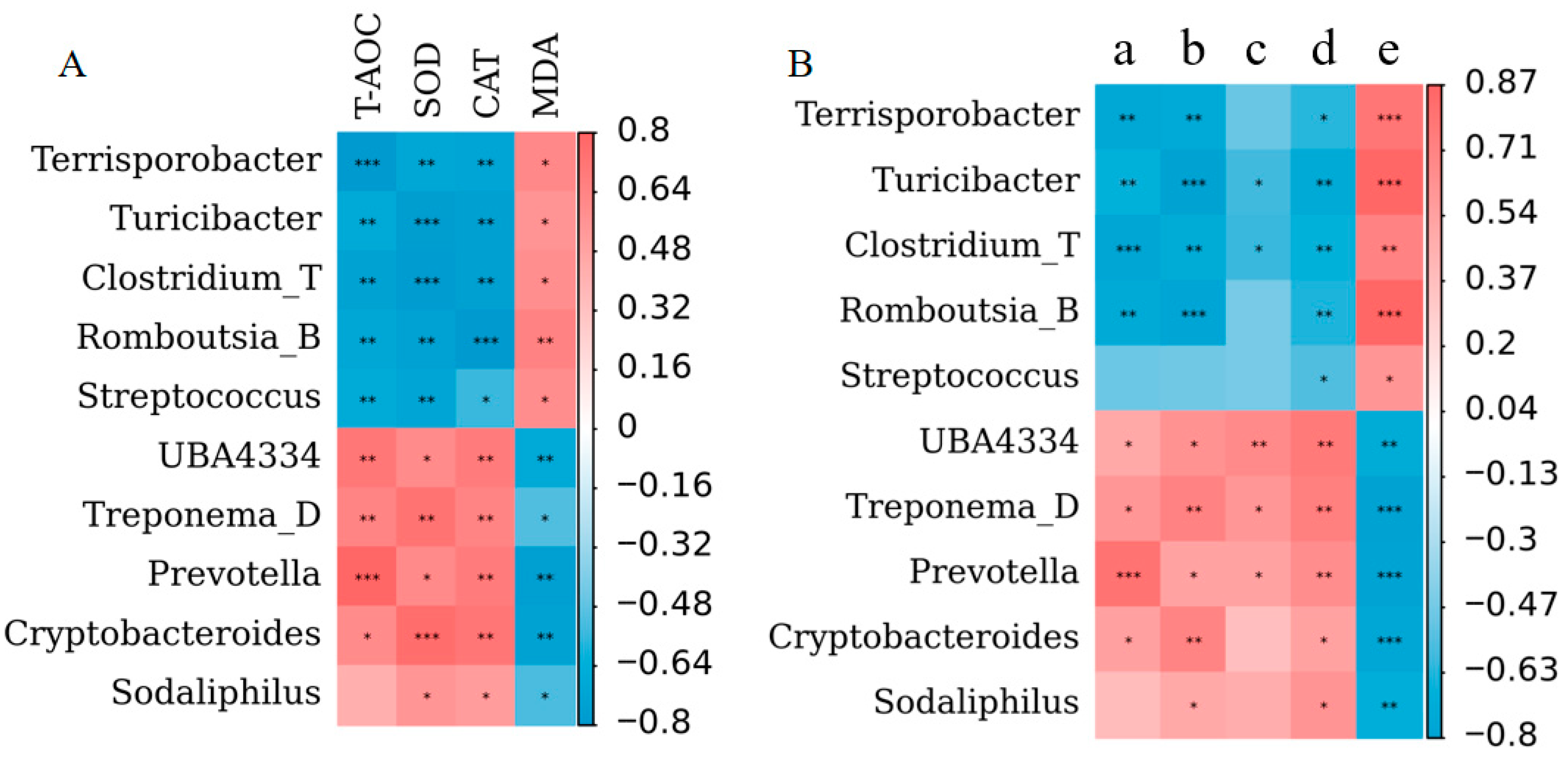
3.5. Correlations Between the Microbiota and Antioxidant Indices and Between Microbiota and Reproductive Performance Indices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, X.; Hu, R.; Shi, M.; Wang, L.; Yan, J.; Gong, J.; Zhang, Q.; He, J.; Wu, S. Placental Malfunction, Fetal Survival and Development Caused by Sow Metabolic Disorder: The Impact of Maternal Oxidative Stress. Antioxidants 2023, 12, 360. [Google Scholar] [CrossRef]
- Park, W.; Park, J.; Park, S.; Lim, W.; Song, G. Picolinafen exposure induces ROS accumulation and calcium depletion, leading to apoptosis in porcine embryonic trophectoderm and uterine luminal epithelial cells during the peri-implantation period. Theriogenology 2023, 201, 12–23. [Google Scholar] [CrossRef]
- Bazer, F.W.; Wang, X.; Johnson, G.A.; Wu, G. Select nutrients and their effects on conceptus development in mammals. Anim. Nutr. 2015, 1, 85–95. [Google Scholar] [CrossRef]
- Gao, D.; Cao, X.; Ren, H.; Wu, L.; Yan, Y.; Hua, R.; Xing, W.; Lei, M.; Liu, J. Immunotoxicity and uterine transcriptome analysis of the effect of zearalenone (ZEA) in sows during the embryo attachment period. Toxicol. Lett. 2022, 357, 33–42. [Google Scholar] [CrossRef]
- Qi, X.; Yun, C.; Pang, Y.; Qiao, J. The impact of the gut microbiota on the reproductive and metabolic endocrine system. Gut Microbes 2021, 13, 1–21. [Google Scholar] [CrossRef]
- Tomaiuolo, R.; Veneruso, I.; Cariati, F.; D’Argenio, V. Microbiota and Human Reproduction: The Case of Female Infertility. High. Throughput 2020, 9, 12. [Google Scholar] [PubMed]
- Zhang, S.X.; Wang, D.L.; Qi, J.J.; Yang, Y.W.; Sun, H.; Sun, B.X.; Liang, S. Chlorogenic acid ameliorates the heat stress-induced impairment of porcine Sertoli cells by suppressing oxidative stress and apoptosis. Theriogenology 2024, 214, 148–156. [Google Scholar] [PubMed]
- Zheng, Y.; Li, L.; Chen, B.; Fang, Y.; Lin, W.; Zhang, T.; Feng, X.; Tao, X.; Wu, Y.; Fu, X.; et al. Chlorogenic acid exerts neuroprotective effect against hypoxia-ischemia brain injury in neonatal rats by activating Sirt1 to regulate the Nrf2-NF-κB signaling pathway. Cell Commun. Signal 2022, 20, 84. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Xu, X.; Fotina, H.; Fotina, T. Anti-inflammatory effects of chlorogenic acid from Taraxacum officinale on LTA-stimulated bovine mammary epithelial cells via the TLR2/NF-κB pathway. PLoS ONE 2023, 18, e0282343. [Google Scholar]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Author Correction: Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 1091. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- McDonald, D.; Jiang, Y.; Balaban, M.; Cantrell, K.; Zhu, Q.; Gonzalez, A.; Morton, J.T.; Nicolaou, G.; Parks, D.H.; Karst, S.M.; et al. Greengenes2 unifies microbial data in a single reference tree. Nat. Biotechnol. 2024, 42, 715–718. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.; Zhu, M.J. A sensitive GC/MS detection method for analyzing microbial metabolites short chain fatty acids in fecal and serum samples. Talanta 2019, 196, 249–254. [Google Scholar] [CrossRef]
- Han, X.; Guo, J.; You, Y.; Yin, M.; Ren, C.; Zhan, J.; Huang, W. A fast and accurate way to determine short chain fatty acids in mouse feces based on GC-MS. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2018, 1099, 73–82. [Google Scholar] [CrossRef]
- Nakajima, A.; Kaga, N.; Nakanishi, Y.; Ohno, H.; Miyamoto, J.; Kimura, I.; Hori, S.; Sasaki, T.; Hiramatsu, K.; Okumura, K.; et al. Maternal High Fiber Diet during Pregnancy and Lactation Influences Regulatory T Cell Differentiation in Offspring in Mice. J. Immunol. 2017, 199, 3516–3524. [Google Scholar] [CrossRef] [PubMed]
- Langendijk, P. Latest Advances in Sow Nutrition during Early Gestation. Animals 2021, 11, 1720. [Google Scholar] [CrossRef]
- Artini, P.G.; Scarfò, G.; Marzi, I.; Fusi, J.; Obino, M.E.; Franzoni, F.; Zappelli, E.; Chelucci, E.; Martini, C.; Cela, V.; et al. Oxidative Stress-Related Signaling Pathways Predict Oocytes’ Fertilization In Vitro and Embryo Quality. Int. J. Mol. Sci. 2022, 23, 13442. [Google Scholar] [CrossRef]
- Mauchart, P.; Vass, R.A.; Nagy, B.; Sulyok, E.; Bódis, J.; Kovács, K. Oxidative Stress in Assisted Reproductive Techniques, with a Focus on an Underestimated Risk Factor. Curr. Issues Mol. Biol. 2023, 45, 1272–1286. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Y.; Xiong, E.; Hong, R.; Lu, Q.; Ohno, H.; Wang, J.Y. Role of the IgM Fc Receptor in Immunity and Tolerance. Front. Immunol. 2019, 10, 529. [Google Scholar] [CrossRef]
- Pabst, O.; Slack, E. IgA and the intestinal microbiota: The importance of being specific. Mucosal Immunol. 2020, 13, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Li, F.; Song, W.; Lei, Z.L.; Sha, Q.Q.; Liu, S.Y.; Zhou, C.Y.; Zhang, X.; Li, X.Z.; Schatten, H.; et al. Gut microbiota-bile acid-vitamin D axis plays an important role in determining oocyte quality and embryonic development. Clin. Transl. Med. 2023, 13, e1236. [Google Scholar] [CrossRef]
- Grant, C.V.; Loman, B.R.; Bailey, M.T.; Pyter, L.M. Manipulations of the gut microbiome alter chemotherapy-induced inflammation and behavioral side effects in female mice. Brain Behav. Immun. 2021, 95, 401–412. [Google Scholar]
- He, Z.; Guo, M.; Zhang, X.; Wang, S.; Liu, T.; Lin, Y.; Ouyang, Q.; Hu, S.; He, H.; Li, L.; et al. 16S rRNA and transcriptome analysis revealed the regulatory mechanism of Romboutsia lituseburensis on serum immunoglobulin levels in geese. Poult. Sci. 2025, 104, 105018. [Google Scholar] [CrossRef]
- Cheng, M.P.; Domingo, M.C.; Lévesque, S.; Yansouni, C.P. A case report of a deep surgical site infection with Terrisporobacter glycolicus/T. Mayombei and review of the literature. BMC Infect. Dis. 2016, 16, 529. [Google Scholar] [CrossRef]
- Cai, C.; Zhang, Z.; Morales, M.; Wang, Y.; Khafipour, E.; Friel, J. Feeding practice influences gut microbiome composition in very low birth weight preterm infants and the association with oxidative stress: A prospective cohort study. Free Radic. Biol. Med. 2019, 142, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Hu, L.; Zhao, Y.; Li, Z.; Zhuo, Y.; Jiang, X.; Li, J.; Zhao, X.; Che, L.; Feng, B.; et al. Effects of Dietary Choline Levels During Pregnancy on Reproductive Performance, Plasma Metabolome and Gut Microbiota of Sows. Front. Vet. Sci. 2021, 8, 771228. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Huang, J.; Li, G.; Zhang, J.; Sun, Y.; Zeng, D.; Bao, W.; Zhong, J.; Huang, Q. Clinical efficacy of intravaginal recombinant lysostaphin administration on endometritis in sows. Vet. Med. Sci. 2021, 7, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Candeliere, F.; Musmeci, E.; Amaretti, A.; Sola, L.; Raimondi, S.; Rossi, M. Profiling of the intestinal community of Clostridia: Taxonomy and evolutionary analysis. Microbiome Res. Rep. 2023, 2, 13. [Google Scholar] [CrossRef]
- Cidan, Y.; Lu, S.; Wang, H.; Wang, J.; Ali, M.; Fouad, D.; Ataya, F.S.; Zhu, Y.; Basang, W.; Li, K. Comparative Analysis of Microbiota in Jiani Yaks with Different Rib Structures. Life 2024, 14, 1458. [Google Scholar] [CrossRef]
- Nishida, A.H.; Ochman, H. Captivity and the co-diversification of great ape microbiomes. Nat. Commun. 2021, 12, 5632. [Google Scholar] [CrossRef]
- Rattigan, R.; Lawlor, P.G.; Cormican, P.; Crespo-Piazuelo, D.; Cullen, J.; Phelan, J.P.; Ranjitkar, S.; Crispie, F.; Gardiner, G.E. Maternal and/or post-weaning supplementation with Bacillus altitudinis spores modulates the microbial composition of colostrum, digesta and faeces in pigs. Sci. Rep. 2023, 13, 8900. [Google Scholar] [CrossRef] [PubMed]
- Mann, E.R.; Lam, Y.K.; Uhlig, H.H. Short-chain fatty acids: Linking diet, the microbiome and immunity. Nat. Rev. Immunol. 2024, 24, 577–595. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Zhang, M.; Chen, S.; Tang, Y.; Cui, J.; Ding, G. Short-chain fatty acids in fetal development and metabolism. Trends Mol. Med. 2025, 31, 625–639. [Google Scholar]
- Xu, B.; Song, P.; Jiang, F.; Cai, Z.; Gu, H.; Gao, H.; Li, B.; Liang, C.; Qin, W.; Zhang, J.; et al. Large-scale metagenomic assembly provide new insights into the genetic evolution of gut microbiomes in plateau ungulates. NPJ Biofilms Microbiomes 2024, 10, 120. [Google Scholar]
- Xiao, K.; Liang, X.; Lu, H.; Li, X.; Zhang, Z.; Lu, X.; Wang, H.; Meng, Y.; Roy, A.; Luo, W.; et al. Adaptation of gut microbiome and host metabolic systems to lignocellulosic degradation in bamboo rats. Isme J. 2022, 16, 1980–1992. [Google Scholar] [CrossRef]

| Ingredient (%) | Content | Nutrient Levels § | Content |
| Corn | 74.5 | Crude protein (%) | 12.50 |
| Wheat bran | 8.8 | Calcium (%) | 0.82 |
| Soybean meal | 12 | Total phosphorus (%) | 0.58 |
| Soybean oil | 1.2 | Metabolizable Energy (MJ/kg) | 12.62 |
| Limestone | 1.2 | ||
| Dicalcium phosphate | 1.2 | ||
| Sodium chloride | 0.3 | ||
| Choline chloride (50%) | 0.1 | ||
| L-lysine | 0.26 | ||
| DL-Methionine | 0.24 | ||
| Vitamin premix † | 0.1 | ||
| Mineral premix ‡ | 0.1 | ||
| Total | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Qu, H.; Pan, H.; Xiang, D.; Choi, S.; Liang, S. Effects of Supplementation with Chlorogenic Acid-Rich Extract from Eucommia ulmoides Oliver During Peri-Implantation on the Reproductive Performance and Gut Microbiota of Sows. Vet. Sci. 2025, 12, 857. https://doi.org/10.3390/vetsci12090857
Zhang Y, Qu H, Pan H, Xiang D, Choi S, Liang S. Effects of Supplementation with Chlorogenic Acid-Rich Extract from Eucommia ulmoides Oliver During Peri-Implantation on the Reproductive Performance and Gut Microbiota of Sows. Veterinary Sciences. 2025; 12(9):857. https://doi.org/10.3390/vetsci12090857
Chicago/Turabian StyleZhang, Yan, Hexuan Qu, Hongda Pan, Dao Xiang, Seongho Choi, and Shuang Liang. 2025. "Effects of Supplementation with Chlorogenic Acid-Rich Extract from Eucommia ulmoides Oliver During Peri-Implantation on the Reproductive Performance and Gut Microbiota of Sows" Veterinary Sciences 12, no. 9: 857. https://doi.org/10.3390/vetsci12090857
APA StyleZhang, Y., Qu, H., Pan, H., Xiang, D., Choi, S., & Liang, S. (2025). Effects of Supplementation with Chlorogenic Acid-Rich Extract from Eucommia ulmoides Oliver During Peri-Implantation on the Reproductive Performance and Gut Microbiota of Sows. Veterinary Sciences, 12(9), 857. https://doi.org/10.3390/vetsci12090857






