Simvastatin Combined with CpG Enhances the Immunogenicity of the H9N2 Inactivated Vaccine
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Vaccine Preparation
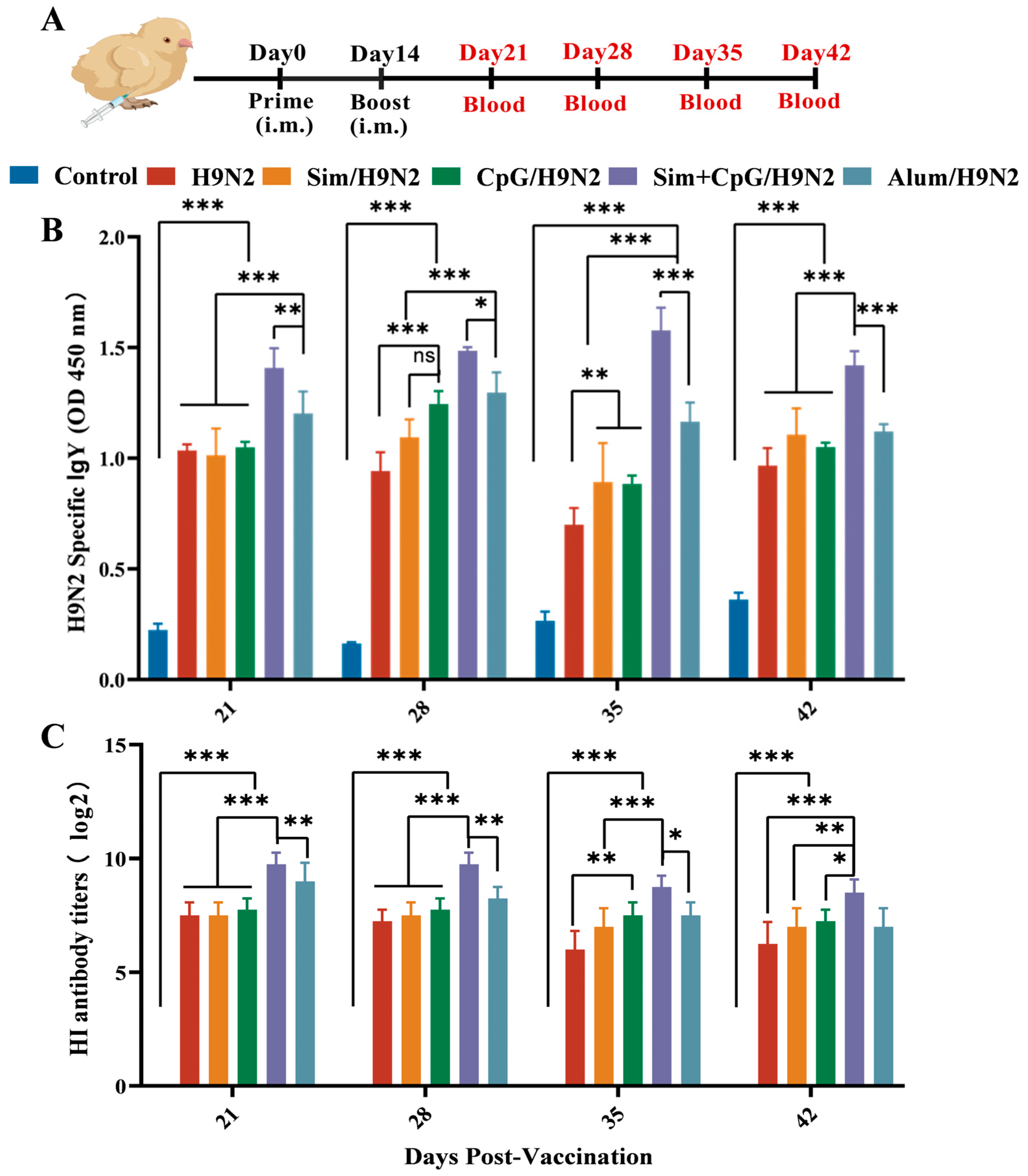
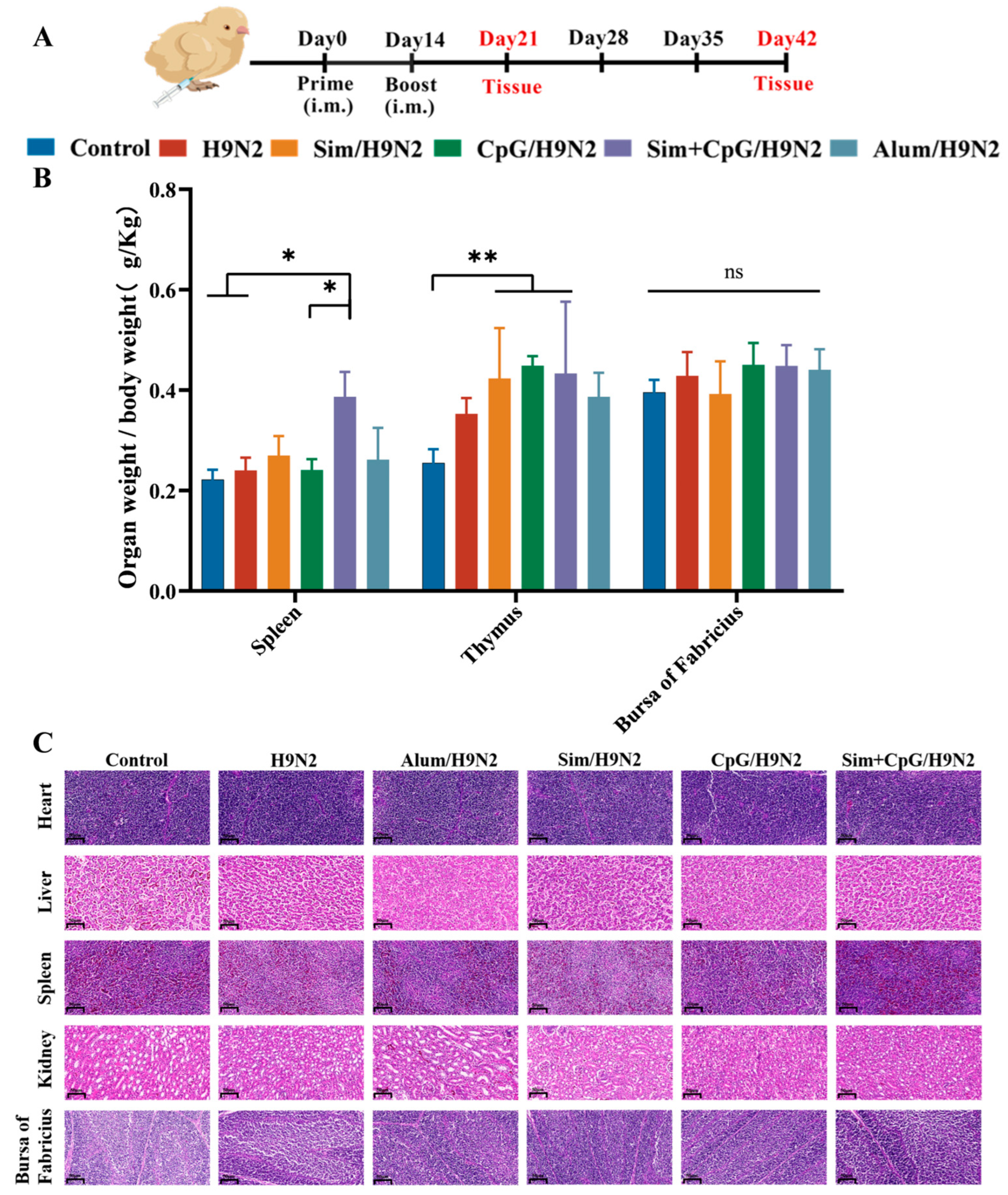
2.3. Animals and Immunization Programmes
2.4. Antibody Detection and Hemagglutination Inhibition Assay
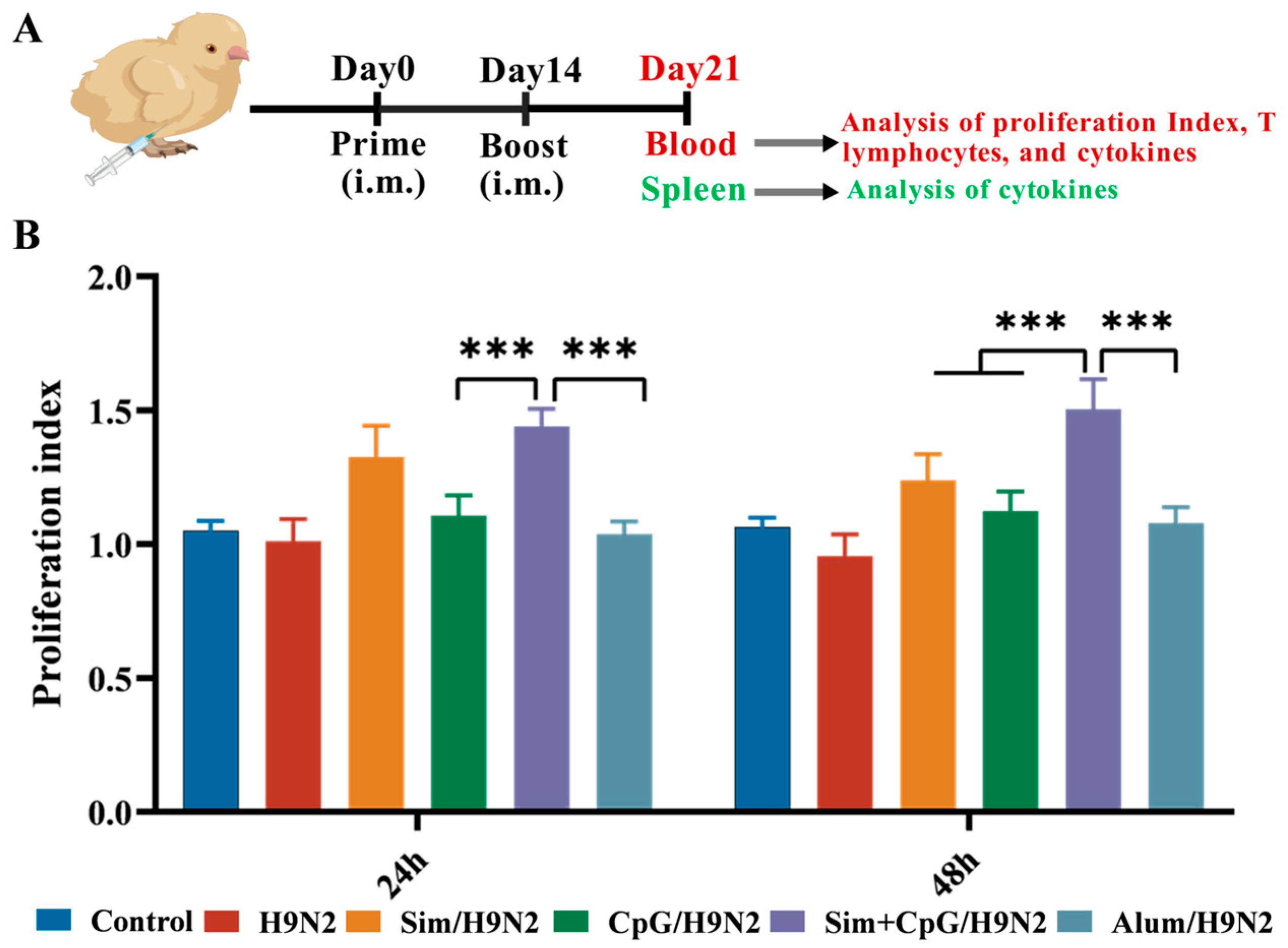
2.5. Determination of the Proliferation Index of Peripheral Blood Lymphocytes
2.6. Assessment of Peripheral T Lymphocyte Populations
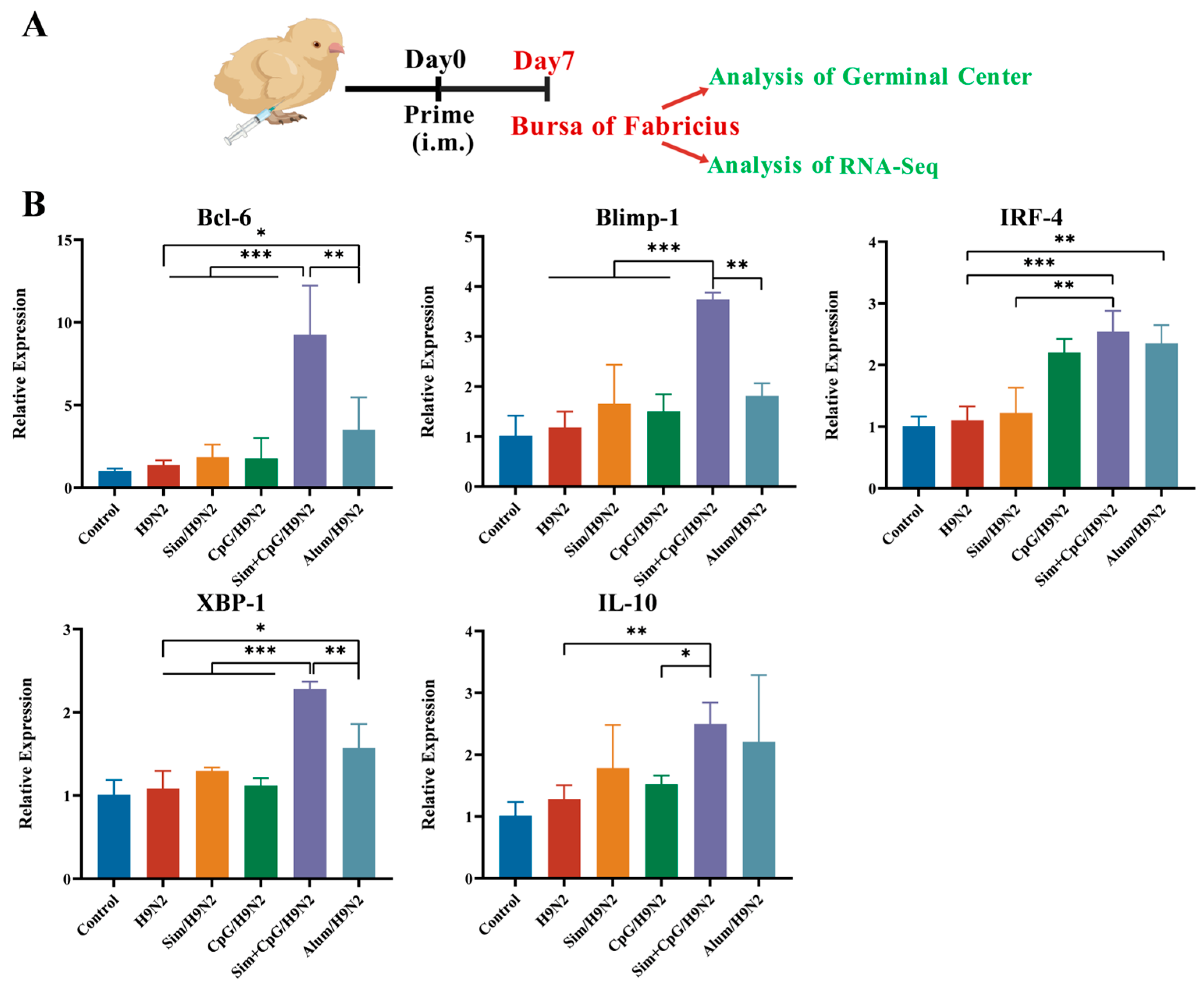
2.7. Quantitative Real-Time PCR (qPCR) Analysis
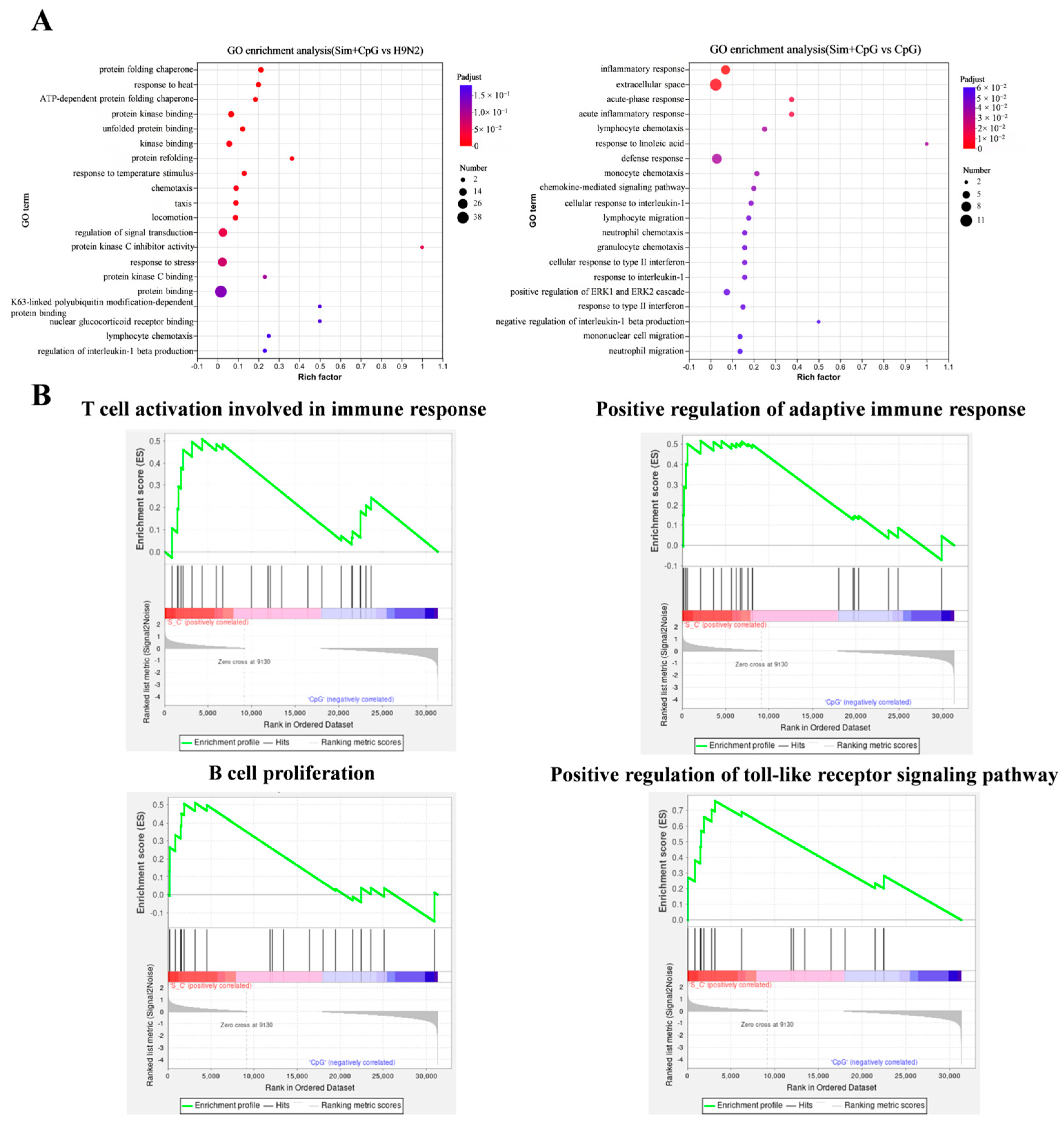
2.8. RNA-Sequencing Analysis
2.9. Determination of Immune Organ Index
2.10. Safety Assessment
2.11. Statistical Analysis
3. Results and Discussion
3.1. Sim + CpG/H9N2 Enhanced the Antibody Response of the H9N2 Inactivated Vaccine
3.2. Sim + CpG/H9N2 Promotes the Cellular Immune Response of the H9N2 Inactivated Vaccine
3.3. Sim + CpG/H9N2 Promotes the Expression of Genes Related to Germinal Centers
3.4. The Immune Response Mechanism of Sim + CpG/H9N2
3.5. Development of Immune Organs and Biosafety After Vaccination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peacock, T.P.; James, J.; Sealy, J.E.; Iqbal, M. A Global Perspective on H9N2 Avian Influenza Virus. Viruses 2019, 11, 620. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Zeng, X.; Xie, Y.; Li, X.; Liu, J.; Yang, J.; Yang, L.; Wang, D. Reported Human Infections of H9N2 Avian Influenza Virus in China in 2021. Front. Public Health 2023, 11, 1255969. [Google Scholar] [CrossRef] [PubMed]
- Sagong, M.; Lee, K.-N.; Lee, E.-K.; Kang, H.; Choi, Y.K.; Lee, Y.-J. Current Situation and Control Strategies of H9N2 Avian Influenza in South Korea. J. Vet. Sci. 2023, 24, e5. [Google Scholar] [CrossRef]
- Pinotti, F.; Kohnle, L.; Lourenço, J.; Gupta, S.; Hoque, M.A.; Mahmud, R.; Biswas, P.; Pfeiffer, D.; Fournié, G. Modelling the Transmission Dynamics of H9N2 Avian Influenza Viruses in a Live Bird Market. Nat. Commun. 2024, 15, 3494. [Google Scholar] [CrossRef]
- Yang, W.; Ding, N.; Luo, R.; Zhang, Q.; Li, Z.; Zhao, F.; Zhang, S.; Zhang, X.; Zhou, T.; Wang, H.; et al. Exosomes from Young Healthy Human Plasma Promote Functional Recovery from Intracerebral Hemorrhage via Counteracting Ferroptotic Injury. Bioact. Mater. 2023, 27, 1–14. [Google Scholar] [CrossRef]
- Johnson, R.F.; Kurup, D.; Hagen, K.R.; Fisher, C.; Keshwara, R.; Papaneri, A.; Perry, D.L.; Cooper, K.; Jahrling, P.B.; Wang, J.T.; et al. An Inactivated Rabies Virus-Based Ebola Vaccine, FILORAB1, Adjuvanted with Glucopyranosyl Lipid A in Stable Emulsion Confers Complete Protection in Nonhuman Primate Challenge Models. J. Infect. Dis. 2016, 214, S342–S354. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Chen, Y.; Xie, J.; Du, S.; Chen, R.; Zheng, Y.; You, B.; Feng, M.; Liao, M.; Dai, M. Construction with Recombinant Epitope-Expressing Baculovirus Enhances Protective Effects of Inactivated H9N2 Vaccine against Heterologous Virus. Vet. Microbiol. 2025, 300, 110337. [Google Scholar] [CrossRef]
- Shichinohe, S.; Watanabe, T. Advances in Adjuvanted Influenza Vaccines. Vaccines 2023, 11, 1391. [Google Scholar] [CrossRef]
- Jiao, L.; Song, Z.; Zhou, Y.; Zhu, T.; Yu, R.; Wang, Z.; Qiu, Y.; Miao, J.; Zhang, S.; Liu, Z.; et al. Naringenin as a Phytogenic Adjuvant Systematically Enhances the Protective Efficacy of H9N2 Inactivated Vaccine through Coordinated Innate-Adaptive Immune Priming in Chickens. Poult. Sci. 2025, 104, 105257. [Google Scholar] [CrossRef]
- Gruenbacher, G.; Thurnher, M. Mevalonate Metabolism in Immuno-Oncology. Front. Immunol. 2017, 8, 1714. [Google Scholar] [CrossRef]
- Xia, Y.; Xie, Y.; Yu, Z.; Xiao, H.; Jiang, G.; Zhou, X.; Yang, Y.; Li, X.; Zhao, M.; Li, L.; et al. The Mevalonate Pathway Is a Druggable Target for Vaccine Adjuvant Discovery. Cell 2018, 175, 1059–1073.e21. [Google Scholar] [CrossRef]
- Xu, S.; Wu, Z.; Cai, G.; Zhang, Y.; Peng, S.; Jiao, L.; Liu, Z.; Yang, Y.; Wang, D. Astragalus Polysaccharides Combined with Simvastatin as an Immunostimulant Enhances the Immune Adjuvanticity of Oil-in-Water Emulsion and Immune Responses in Mice. Vaccine 2023, 41, 1684–1693. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Liu, Q.; de Jong, M.C.M.; Forlenza, M.; Niu, S.; Yan, D.; Teng, Q.; Li, X.; Beerens, N.; Li, Z. Immunoadjuvant Efficacy of CpG Plasmids for H9N2 Avian Influenza Inactivated Vaccine in Chickens with Maternal Antibodies. Vet. Immunol. Immunopathol. 2023, 259, 110590. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Wang, G.; Yu, H.; Huang, M.; Zhang, Y.; Liu, R.; Wang, S.; Cui, H.; Zhang, Y.; et al. Development and Application of Indirect ELISA for IBDV VP2 Antibodies Detection in Poultry. Viruses 2025, 17, 871. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.; Zhang, H.; Yangzong, X.; Li, Z.; Liu, Z.; Dan, M.; Li, X.; Sun, X.; Zhao, Q.; Sun, Y. A Highly Sensitive One-Step Nanobody-Based Immunoassay to Specifically Detect Antibodies against Fowl Adenovirus Serotype 4. Poult. Sci. 2025, 104, 104970. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, T.; He, J.; Zhang, Y.; Gu, P.; Qiu, T.; Bo, R.; Hu, Y.; Liu, J.; Wang, D. Adjuvanticity of Ganoderma Lucidum Polysaccharide Liposomes on Porcine Circovirus Type-II in Mice. Int. J. Biol. Macromol. 2019, 141, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.M.; Hassan, M.A.E.M.; Hussein, H.A.; Ahmed, B.M.; El Sanousi, A.A.E.-G. Protective Efficacy of Combined Trivalent Inactivated ISA 71 Oil Adjuvant Vaccine against Avian Influenza Virus Subtypes (H9N2 and H5N1) and Newcastle Disease Virus. Vet. World 2017, 10, 1212–1220. [Google Scholar] [CrossRef]
- Hivroz, C.; Saitakis, M. Biophysical Aspects of T Lymphocyte Activation at the Immune Synapse. Front. Immunol. 2016, 7, 46. [Google Scholar] [CrossRef]
- Santosa, E.K.; Sun, J.C. Cardinal Features of Immune Memory in Innate Lymphocytes. Nat. Immunol. 2023, 24, 1803–1812. [Google Scholar] [CrossRef]
- Pereira, M.V.A.; Galvani, R.G.; Gonçalves-Silva, T.; de Vasconcelo, Z.F.M.; Bonomo, A. Tissue Adaptation of CD4 T Lymphocytes in Homeostasis and Cancer. Front. Immunol. 2024, 15, 1379376. [Google Scholar] [CrossRef]
- Hirakawa, R.; Nurjanah, S.; Furukawa, K.; Murai, A.; Kikusato, M.; Nochi, T.; Toyomizu, M. Heat Stress Causes Immune Abnormalities via Massive Damage to Effect Proliferation and Differentiation of Lymphocytes in Broiler Chickens. Front. Vet. Sci. 2020, 7, 46. [Google Scholar] [CrossRef]
- Cao, L.; Li, J.; Zhang, J.; Huang, H.; Gui, F.; Xu, W.; Zhang, L.; Bi, S. Beta-Glucan Enhanced Immune Response to Newcastle Disease Vaccine and Changed mRNA Expression of Spleen in Chickens. Poult. Sci. 2023, 102, 102414. [Google Scholar] [CrossRef]
- Seyed, N.; Rafati, S. Th1 Concomitant Immune Response Mediated by IFN-γ Protects against Sand Fly Delivered Leishmania Infection: Implications for Vaccine Design. Cytokine 2021, 147, 155247. [Google Scholar] [CrossRef]
- Gao, W.; Sun, X.; Li, D.; Sun, L.; He, Y.; Wei, H.; Jin, F.; Cao, Y. Toll-like Receptor 4, Toll-like Receptor 7 and Toll-like Receptor 9 Agonists Enhance Immune Responses against Blood-Stage Plasmodium Chabaudi Infection in BALB/c Mice. Int. Immunopharmacol. 2020, 89, 107096. [Google Scholar] [CrossRef] [PubMed]
- Piro, G.; Simionato, F.; Carbone, C.; Frizziero, M.; Malleo, G.; Zanini, S.; Casolino, R.; Santoro, R.; Mina, M.M.; Zecchetto, C.; et al. A Circulating TH2 Cytokines Profile Predicts Survival in Patients with Resectable Pancreatic Adenocarcinoma. Oncoimmunology 2017, 6, e1322242. [Google Scholar] [CrossRef] [PubMed]
- Merino Tejero, E.; Lashgari, D.; García-Valiente, R.; Gao, X.; Crauste, F.; Robert, P.A.; Meyer-Hermann, M.; Martínez, M.R.; van Ham, S.M.; Guikema, J.E.J.; et al. Multiscale Modeling of Germinal Center Recapitulates the Temporal Transition From Memory B Cells to Plasma Cells Differentiation as Regulated by Antigen Affinity-Based Tfh Cell Help. Front. Immunol. 2021, 11, 620716. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, X.; Liu, X.; Zhong, B.; Tang, H.; Jin, W.; Clevers, H.; Wang, H.; Wang, X.; Dong, C. Transcription Factor Ascl2 Promotes Germinal Center B Cell Responses by Directly Regulating AID Transcription. Cell Rep. 2021, 35, 109188. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Huang, T.; Li, S.; Ma, A.; Pérez, J.L.; Sander, C.; Keskin, D.B.; Wu, C.J.; Fraenkel, E.; Hacohen, N. Dictionary of Immune Responses to Cytokines at Single-Cell Resolution. Nature 2024, 625, 377–384. [Google Scholar] [CrossRef]
- Cancro, M.P.; Tomayko, M.M. Memory B Cells and Plasma Cells: The Differentiative Continuum of Humoral Immunity. Immunol. Rev. 2021, 303, 72–82. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, T.; Xu, S.; Gu, P.; Cai, G.; Peng, S.; Liu, Z.; Yang, Y.; Hu, Y.; Liu, J.; et al. Cationic Nanoparticle-Stabilized Vaccine Delivery System for the H9N2 Vaccine to Promote Immune Response in Chickens. Mol. Pharm. 2023, 20, 1613–1623. [Google Scholar] [CrossRef]
- Gu, W.; An, J.; Li, Y.; Yang, Y.; Wang, S.; Shan, H.; Li, S.; Li, H.; Liu, G.; Li, K.; et al. Tuning the Organ Tropism of Polymersome for Spleen-Selective Nanovaccine Delivery to Boost Cancer Immunotherapy. Adv. Mater. 2023, 35, 2301686. [Google Scholar] [CrossRef] [PubMed]
- Kreins, A.Y.; Maio, S.; Dhalla, F. Inborn Errors of Thymic Stromal Cell Development and Function. Semin. Immunopathol. 2021, 43, 85–100. [Google Scholar] [CrossRef] [PubMed]


| Gene | Sense Strand (5′−3′) | Antisense Strand (5′−3′) |
|---|---|---|
| IL-4 | GTGCCCACGCTGTGCTTAC | AGGAAACCTCTCCCTGGATGT |
| IL-6 | AAATCCCTCCTCGCCAATCT | CCCTCACGGTCTTCTCCATAAA |
| IFN-γ | ACGACACCATCCTGGACACC | TTTGGCGTTGGCTGTCGTTC |
| IL-1β | CATCACCAACCAACCCGA | ACGAGATGGAAACCAGCAA |
| TNF-α | CAGCCCTCACATCACCTC | GCTGCCACTCCAGCAATA |
| IL-10 | CGCTGTCACCGCTTCTTCA | TCCCGTTCTCATCCATCTTCTC |
| CD40 | GGGCTCGTGGTGAAGGTGAAAG | GGATCAGCACTGACAGCGATGAG |
| BCL6 | GCAGTTCAGAGCCCACAAAA | GTTCAGACGGGAGGTGTACA |
| Blimp-1 | ACACAGCGGAGAGAGACCAT | GCACAGCTTGCACTGGTAAG |
| IRF4 | GTGTGGGAGAATGACGAGAAG | AAGGAGATGTGATTGGGAAGG |
| XBP1 | GTGCGAGTCTACGGATGTGA | GTGCGAGTCTACGGATGTGA |
| β-actin | GAGAATTGTGCGTGACATCA | CCTGAACCTCTCATTGCCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Zhu, J.; Song, Z.; Jiao, L.; Yu, R.; Wang, Z.; Zhang, Z.; Liu, J.; Liu, Z. Simvastatin Combined with CpG Enhances the Immunogenicity of the H9N2 Inactivated Vaccine. Vet. Sci. 2025, 12, 855. https://doi.org/10.3390/vetsci12090855
Ma Y, Zhu J, Song Z, Jiao L, Yu R, Wang Z, Zhang Z, Liu J, Liu Z. Simvastatin Combined with CpG Enhances the Immunogenicity of the H9N2 Inactivated Vaccine. Veterinary Sciences. 2025; 12(9):855. https://doi.org/10.3390/vetsci12090855
Chicago/Turabian StyleMa, Yan, Jiaxi Zhu, Zuchen Song, Lina Jiao, Ruihong Yu, Zheng Wang, Zhimin Zhang, Jiaguo Liu, and Zhenguang Liu. 2025. "Simvastatin Combined with CpG Enhances the Immunogenicity of the H9N2 Inactivated Vaccine" Veterinary Sciences 12, no. 9: 855. https://doi.org/10.3390/vetsci12090855
APA StyleMa, Y., Zhu, J., Song, Z., Jiao, L., Yu, R., Wang, Z., Zhang, Z., Liu, J., & Liu, Z. (2025). Simvastatin Combined with CpG Enhances the Immunogenicity of the H9N2 Inactivated Vaccine. Veterinary Sciences, 12(9), 855. https://doi.org/10.3390/vetsci12090855





