Influence of Untreated and Microbially Degraded Mangrove Sediment Microplastics on Zebrafish (Danio rerio) Intestinal Histology and Immune and Antioxidant Biomarkers
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction and Pretreatment of Microplastics from Mangrove Sediments
2.2. Zebrafish Acclimation and Rearing Conditions
2.3. Microbial Degradation of MPs
2.4. Zebrafish Exposure Experiment
2.5. Sample Collection
2.6. Histological Analysis
2.7. Relative mRNA Expression in Intestinal Tissues of Zebrafish
2.8. Statistical Analysis
3. Results
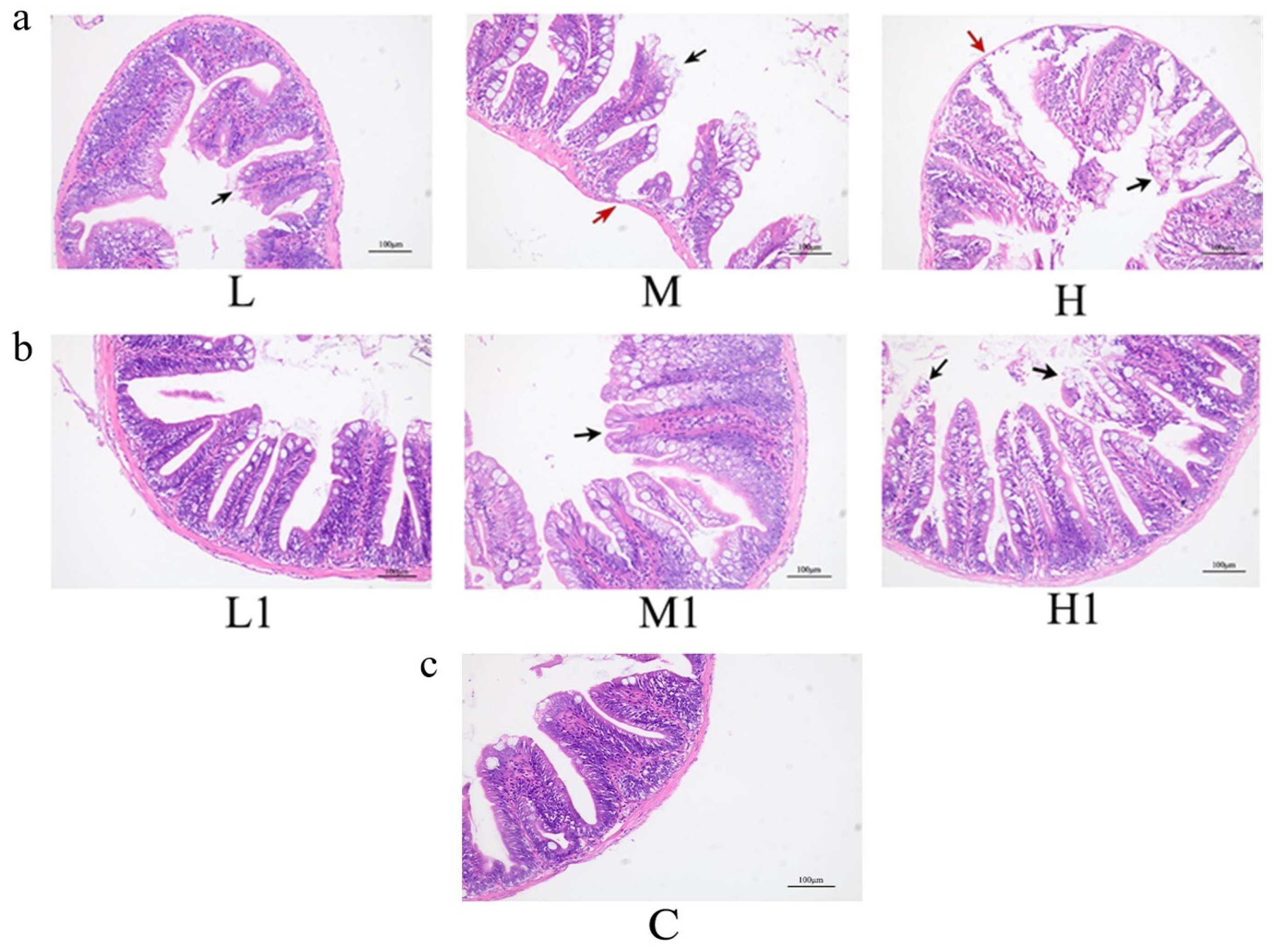
3.1. Effects of Microbial Degradation Products of Extracted MP Particles on Intestinal Tract of Zebrafish
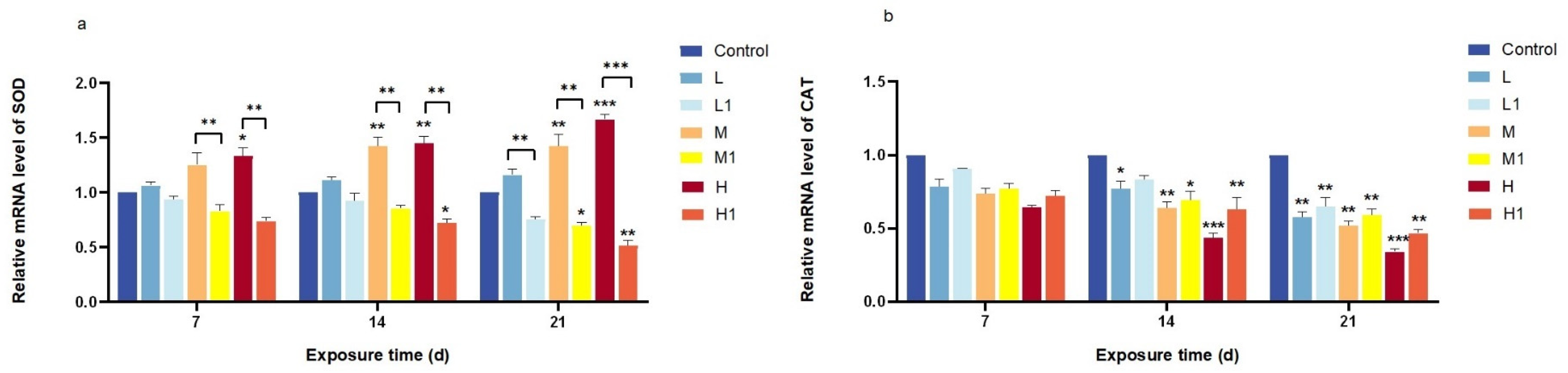
3.2. Relative mRNA Expression of Oxidative Stress-Related Genes
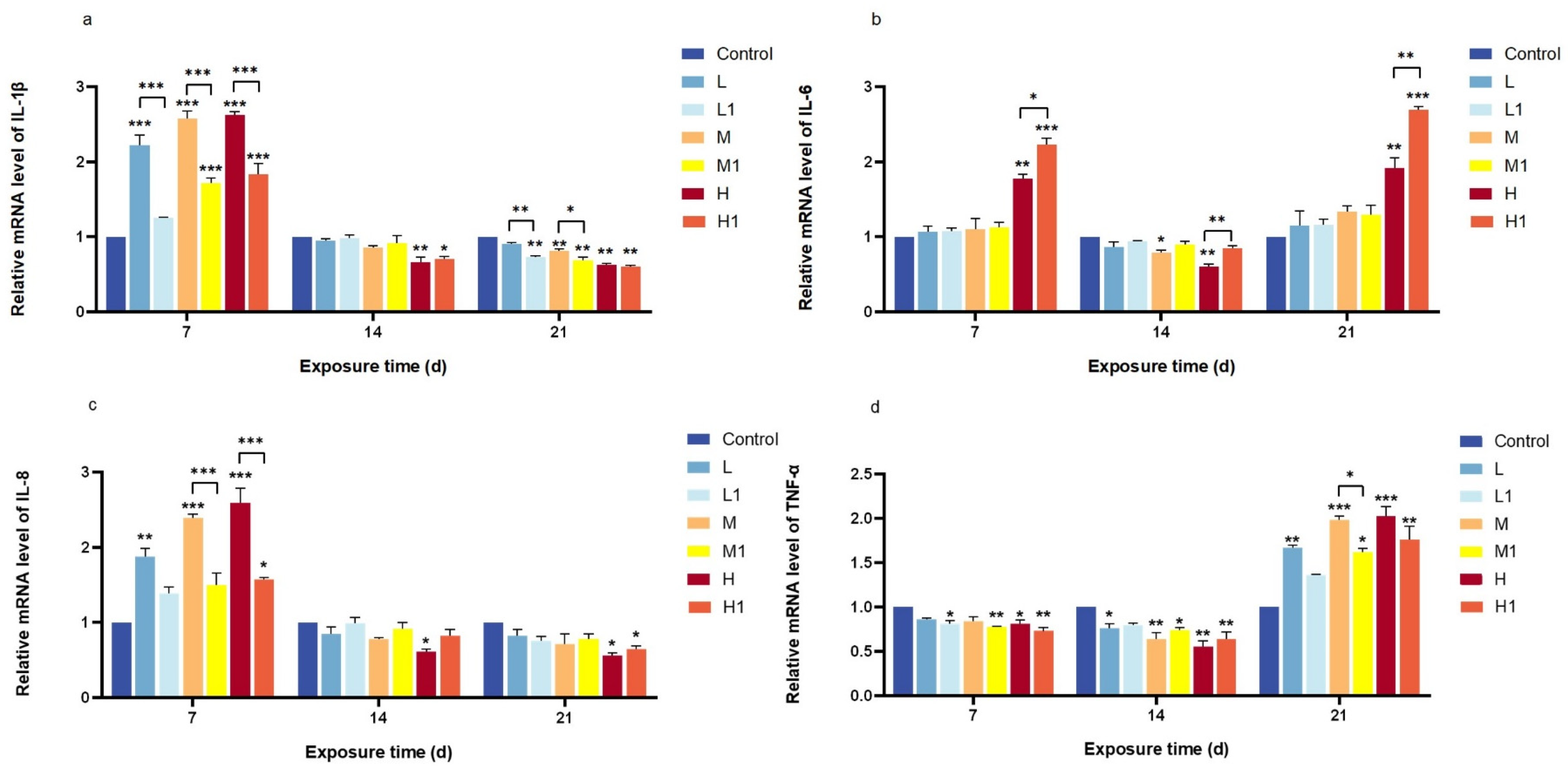
3.3. Relative mRNA Expression of Inflammatory Response-Related Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MPs | Microplastics |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| HE | Hematoxylin and eosin |
| PE | polyethylene |
| LB | Luria–Bertani |
| TNF-α | tumor necrosis factor-alpha |
| IL-1β | interleukin-1 beta |
| IL-6 | interleukin-6 |
| IL-8 | interleukin-8 |
| PBS | phosphate-buffered saline |
| PS | polystyrene |
| ROS | reactive oxygen species |
References
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.; Xiang, X.; Huang, J.; Kong, L.; Wu, J.; Cheng, S. Microplastics in the Atmosphere and Water Bodies of Coastal Agglomerations: A Mini-Review. Int. J. Environ. Res. Public Health 2023, 20, 2466. [Google Scholar] [CrossRef] [PubMed]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Distribution and importance of microplastics in the marine environment: A review of the sources, fate, effects, and potential solutions. Environ. Int. 2017, 102, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Ter Halle, A.; Jeanneau, L.; Martignac, M.; Jarde, E.; Pedrono, B.; Brach, L.; Gigault, J. Nanoplastic in the North Atlantic Subtropical Gyre. Environ. Sci. Technol. 2017, 51, 13689–13697. [Google Scholar] [CrossRef]
- Phuong, N.N.; Fauvelle, V.; Grenz, C.; Ourgaud, M.; Schmidt, N.; Strady, E.; Sempéré, R. Highlights from a review of microplastics in marine sediments. Sci. Total Environ. 2021, 777, 146225. [Google Scholar] [CrossRef]
- Uddin, S.; Fowler, S.W.; Uddin, M.F.; Behbehani, M.; Naji, A. A review of microplastic distribution in sediment profiles. Mar. Pollut. Bull. 2021, 163, 111973. [Google Scholar] [CrossRef]
- Sun, X.; Li, Q.; Shi, Y.; Zhao, Y.; Zheng, S.; Liang, J.; Liu, T.; Tian, Z. Characteristics and retention of microplastics in the digestive tracts of fish from the Yellow Sea. Environ. Pollut. 2019, 249, 878–885. [Google Scholar] [CrossRef]
- Jin, Y.; Xia, J.; Pan, Z.; Yang, J.; Wang, W.; Fu, Z. Polystyrene microplastics induce microbiota dysbiosis and inflammation in the gut of adult zebrafish. Environ. Pollut. 2018, 235, 322–329. [Google Scholar] [CrossRef]
- Lu, L.; Wan, Z.; Luo, T.; Fu, Z.; Jin, Y. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci. Total Environ. 2018, 631–632, 449–458. [Google Scholar] [CrossRef]
- Dong, X.; Liu, X.; Hou, Q.; Wang, Z. From natural environment to animal tissues: A review of microplastics (nanoplastics) translocation and hazards studies. Sci. Total Environ. 2023, 855, 158686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, L.; Kannan, K. Polyethylene Terephthalate and Polycarbonate Microplastics in Pet Food and Feces from the United States. Environ. Sci. Technol. 2019, 53, 12035–12042. [Google Scholar] [CrossRef]
- Wagner, M.; Scherer, C.; Alvarez-Munoz, D.; Brennholt, N.; Bourrain, X.; Buchinger, S.; Fries, E.; Grosbois, C.; Klasmeier, J.; Marti, T.; et al. Microplastics in freshwater ecosystems: What we know and what we need to know. Environ. Sci. Eur. 2014, 26, 12. [Google Scholar] [CrossRef]
- Brugman, S. The zebrafish as a model to study intestinal inflammation. Dev. Comp. Immunol. 2016, 64, 82–92. [Google Scholar] [CrossRef]
- Qiao, R.; Sheng, C.; Lu, Y.; Zhang, Y.; Ren, H.; Lemos, B. Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Sci. Total Environ. 2019, 662, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, H.; Chen, L.; Wang, X. Ecological interception effect of mangroves on microplastics. J. Hazard. Mater. 2022, 423, 127231. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Baalkhuyur, F.; Valluzzi, L.; Saderne, V.; Cusack, M.; Almahasheer, H.; Krishnakumar, P.K.; Rabaoui, L.; Qurban, M.A.; Arias-Ortiz, A.; et al. Exponential increase of plastic burial in mangrove sediments as a major plastic sink. Sci. Adv. 2020, 6, eaaz5593. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; He, J.; Feng, D.; Zhao, Y.; Sun, W.; Yu, H.; Ge, C. Microplastics pollution in mangrove ecosystems: A critical review of current knowledge and future directions. Sci. Total Environ. 2021, 753, 142041. [Google Scholar] [CrossRef]
- Duan, J.; Han, J.; Cheung, S.G.; Chong, R.K.Y.; Lo, C.M.; Lee, F.W.; Xu, S.J.; Yang, Y.; Tam, N.F.; Zhou, H.C. How mangrove plants affect microplastic distribution in sediments of coastal wetlands: Case study in Shenzhen Bay, South China. Sci. Total Environ. 2021, 767, 144695. [Google Scholar] [CrossRef]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Screening of Bacillus strains isolated from mangrove ecosystems in Peninsular Malaysia for microplastic degradation. Environ. Pollut. 2017, 231, 1552–1559. [Google Scholar] [CrossRef]
- Auta, H.S.; Emenike, C.U.; Jayanthi, B.; Fauziah, S.H. Growth kinetics and biodeterioration of polypropylene microplastics by Bacillus sp. and Rhodococcus sp. isolated from mangrove sediment. Mar. Pollut. Bull. 2018, 127, 15–21. [Google Scholar] [CrossRef]
- Yuan, J.; Ma, J.; Sun, Y.; Zhou, T.; Zhao, Y.; Yu, F. Microbial degradation and other environmental aspects of microplastics/plastics. Sci. Total Environ. 2020, 715, 136968. [Google Scholar] [CrossRef]
- Kesavan, S.; Martin Xavier, K.A.; Nair, M.M.; Gurjar, U.R.; Sukla, S.P.; Jaiswar, A.K.; Bhusan, S.; Abdul Azeez, S.; Deshmukhe, G. Assessment of secondary microplastics trapped in mangrove ecosystem of a highly populated tropical megacity, India. J. Hazard. Mater. Adv. 2025, 17, 100587. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiao, R.; Zhang, S.; Wang, G. Metabolomic profiling reveals the intestinal toxicity of different length of microplastic fibers on zebrafish (Danio rerio). J. Hazard. Mater. 2021, 403, 123663. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, E.G.; Li, J.; Chen, Q.; Ma, L.; Zeng, E.Y.; Shi, H. A Review of Microplastics in Table Salt, Drinking Water, and Air: Direct Human Exposure. Environ. Sci. Technol. 2020, 54, 3740–3751. [Google Scholar] [CrossRef]
- Arpia, A.A.; Chen, W.H.; Ubando, A.T.; Naqvi, S.R.; Culaba, A.B. Microplastic degradation as a sustainable concurrent approach for producing biofuel and obliterating hazardous environmental effects: A state-of-the-art review. J. Hazard. Mater. 2021, 418, 126381. [Google Scholar] [CrossRef]
- Umamaheswari, S.; Priyadarshinee, S.; Kadirvelu, K.; Ramesh, M. Polystyrene microplastics induce apoptosis via ROS-mediated p53 signaling pathway in zebrafish. Chem. Biol. Interact. 2021, 345, 109550. [Google Scholar] [CrossRef]
- Ighodaro, O.; Akinloye, O. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio rerio) and Toxic Effects in Liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef]
- Hamed, M.; Soliman, H.A.M.; Osman, A.G.M.; Sayed, A.E.H. Antioxidants and molecular damage in Nile Tilapia (Oreochromis niloticus) after exposure to microplastics. Environ. Sci. Pollut. Res. Int. 2020, 27, 14581–14588. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Wang, C.; Zhou, J.; Shen, M.; Wang, X.; Fu, Z.; Jin, Y. Effects of polystyrene microplastics on the composition of the microbiome and metabolism in larval zebrafish. Chemosphere 2019, 217, 646–658. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Prasad, N.; Agarwal, V.; Rai, M. # 5108 sustained IL-6 secretion can lead to an amplified inflammatory response, mediated by the activation of stat3 and nfkb via the IL-6 amplifier loop. Nephrol. Dial. Transplant. 2023, 38, gfad063c_5108. [Google Scholar]
- Huang, J.N.; Wen, B.; Zhu, J.G.; Zhang, Y.S.; Gao, J.Z.; Chen, Z.Z. Exposure to microplastics impairs digestive performance, stimulates immune response and induces microbiota dysbiosis in the gut of juvenile guppy (Poecilia reticulata). Sci. Total Environ. 2020, 733, 138929. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.W.; Chen, Y.H.; Lin, S.J. Long-term exposure to oxidized low-density lipoprotein enhances tumor necrosis factor-alpha-stimulated endothelial adhesiveness of monocytes by activating superoxide generation and redox-sensitive pathways. Free Radic. Biol. Med. 2006, 40, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Choi, C.Y.; Lee, K.J.; Shin, D.W.; Jung, K.S.; Chung, Y.C.; Lee, S.S.; Shin, J.G.; Jeong, H.G. Induction of inducible nitric oxide synthase and proinflammatory cytokines expression by o,p’-DDT in macrophages. Toxicol. Lett. 2004, 147, 261–269. [Google Scholar] [CrossRef]
- Yang, H.; Lai, H.; Huang, J.; Sun, L.; Mennigen, J.A.; Wang, Q.; Liu, Y.; Jin, Y.; Tu, W. Polystyrene microplastics decrease F-53B bioaccumulation but induce inflammatory stress in larval zebrafish. Chemosphere 2020, 255, 127040. [Google Scholar] [CrossRef] [PubMed]
- Zitouni, N.; Bousserrhine, N.; Missawi, O.; Boughattas, I.; Chevre, N.; Santos, R.; Belbekhouche, S.; Alphonse, V.; Tisserand, F.; Balmassiere, L.; et al. Uptake, tissue distribution and toxicological effects of environmental microplastics in early juvenile fish Dicentrarchus labrax. J. Hazard. Mater. 2021, 403, 124055. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, S.; Razanajatovo, R.M.; Zou, H.; Zhu, W. Accumulation, tissue distribution, and biochemical effects of polystyrene microplastics in the freshwater fish red tilapia (Oreochromis niloticus). Environ. Pollut. 2018, 238, 1–9. [Google Scholar] [CrossRef]
| Gene | Forward (5′–3′) | Reverse (3′–5′) |
|---|---|---|
| IL-1β | GATTCGCAGATGGTGGAGATGGAC | TCGTCTTTGGATGGAAGCACAGC |
| IL-6 | TCAACTTCTCCAGCGTGATG | TCTTTCCCTCTTTTCCTCCTG |
| IL-8 | AACATGGAGGTCATTGCCACTGTG | GAGGTAGAATTTGGAGGGAGGGTA |
| TNF-α | GTCGGGTGTATGGAGGGTGTTTG | CTGGTCTTATGGAGCGTGAAGC |
| SOD | GTCCGCACTTCAACCCTCA | TCCTCATTGCCACCCTTCC |
| CAT | AGGGCAACTGGGATCTTACA | TTTATGGGACCAGACCTTGG |
| β-actin | GTGATGGACTCTGGTGATGGTGTG | CACGCTCGGTCAGGATCTTCATC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.-Y.; Wei, W.; Muhammad, A.; Zhang, M.; Chen, Y.-J.; Xie, J.-H.; Kang, D.-J.; Chen, J.-J. Influence of Untreated and Microbially Degraded Mangrove Sediment Microplastics on Zebrafish (Danio rerio) Intestinal Histology and Immune and Antioxidant Biomarkers. Vet. Sci. 2025, 12, 854. https://doi.org/10.3390/vetsci12090854
Zheng X-Y, Wei W, Muhammad A, Zhang M, Chen Y-J, Xie J-H, Kang D-J, Chen J-J. Influence of Untreated and Microbially Degraded Mangrove Sediment Microplastics on Zebrafish (Danio rerio) Intestinal Histology and Immune and Antioxidant Biomarkers. Veterinary Sciences. 2025; 12(9):854. https://doi.org/10.3390/vetsci12090854
Chicago/Turabian StyleZheng, Xin-Yu, Wan Wei, Asim Muhammad, Min Zhang, Yan-Jun Chen, Jia-Hong Xie, Dan-Ju Kang, and Jin-Jun Chen. 2025. "Influence of Untreated and Microbially Degraded Mangrove Sediment Microplastics on Zebrafish (Danio rerio) Intestinal Histology and Immune and Antioxidant Biomarkers" Veterinary Sciences 12, no. 9: 854. https://doi.org/10.3390/vetsci12090854
APA StyleZheng, X.-Y., Wei, W., Muhammad, A., Zhang, M., Chen, Y.-J., Xie, J.-H., Kang, D.-J., & Chen, J.-J. (2025). Influence of Untreated and Microbially Degraded Mangrove Sediment Microplastics on Zebrafish (Danio rerio) Intestinal Histology and Immune and Antioxidant Biomarkers. Veterinary Sciences, 12(9), 854. https://doi.org/10.3390/vetsci12090854







