Exploratory, Randomized, Dose-Response Study of the Anti-PD-L1 Antibody HFC-L1/c4G12 in Dogs with Pulmonary Metastatic Oral Malignant Melanoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Clinical Study
2.2. Assessment of Safety
2.3. Evaluation of Tumor Response
2.4. Evaluation of Survival
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population
3.2. Safety of HFC-L1 Therapy
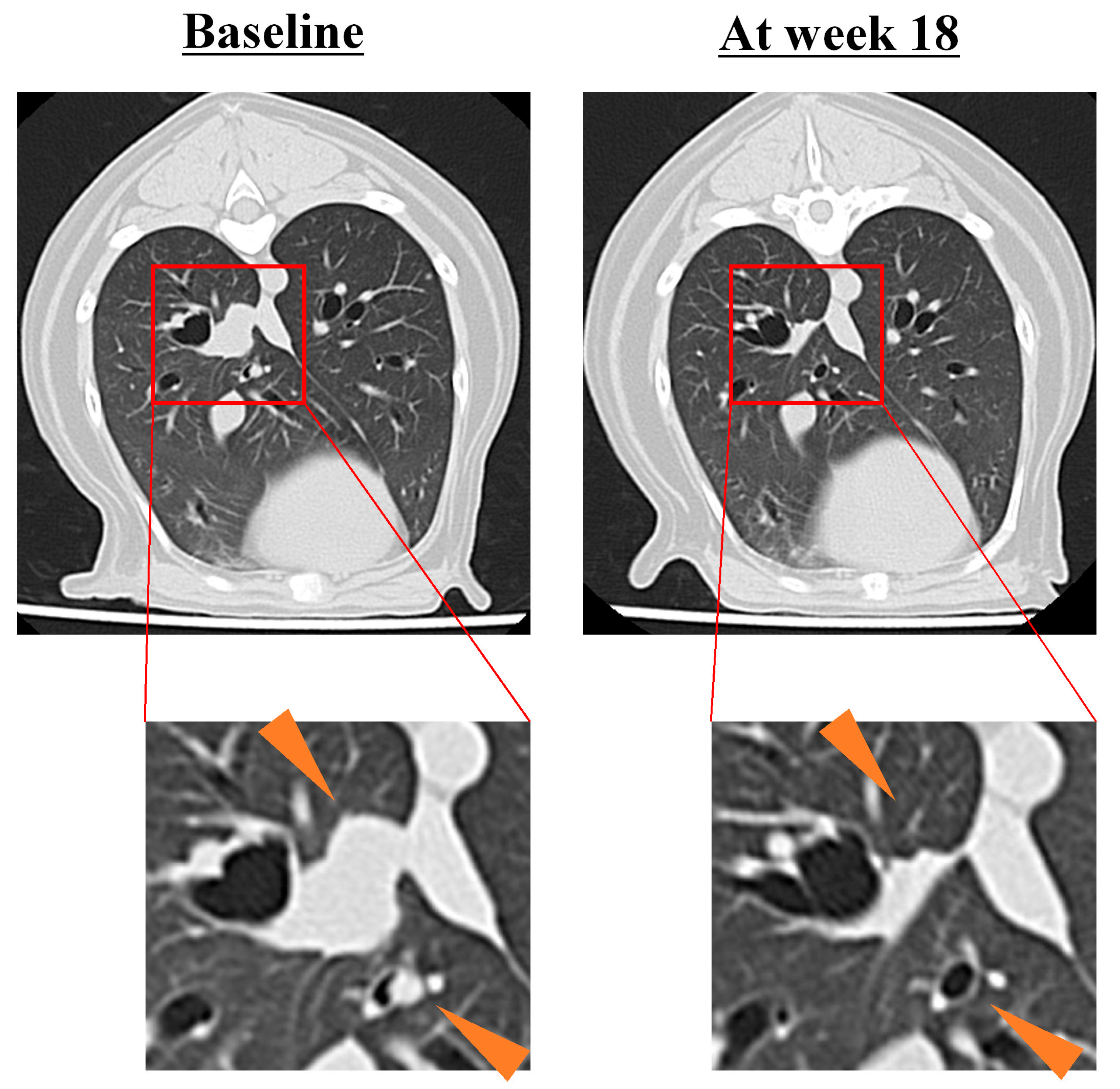
3.3. Tumor Response to HFC-L1 Therapy
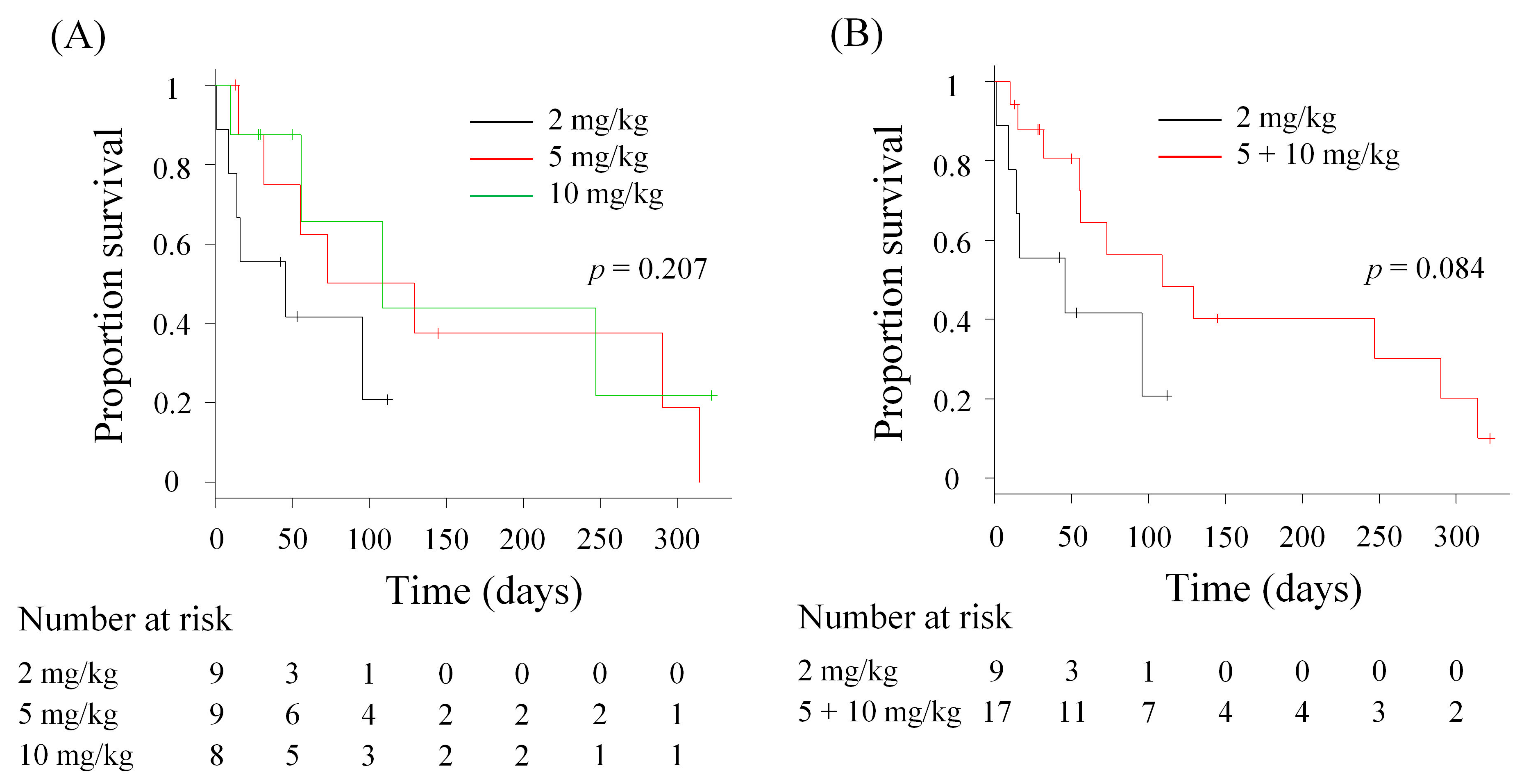
3.4. Comparison of OS
3.5. Comparison of Survival After the Diagnosis of PM
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AU | Azabu University |
| BUN | Blood Urea Nitrogen |
| CR | Complete Response |
| CT | Computed Tomography |
| CI | Confidence Interval |
| HU | Hokkaido University |
| ICI | Immune Checkpoint Inhibitor |
| irAE | Immune-Related Adverse Event |
| NE | Not Evaluable |
| OUAVM | Obihiro University of Agriculture and Veterinary Medicine |
| OMM | Oral Malignant Melanoma |
| OS | Overall Survival |
| PR | Partial Response |
| PD-1 | Programmed Death 1 |
| PD-L1 | Programmed Death Ligand 1 |
| PD | Progressive Disease |
| PM | Pulmonary Metastasis |
| cRECIST | Response Evaluation Criteria for Solid Tumours in Dogs |
| SD | Stable Disease |
| TRAE | Treatment-Related Adverse Event |
| VCOG-CTCAE | Veterinary Cooperative Oncology Group–Common Terminology Criteria for Adverse Events |
References
- Bergman, P.J. Canine oral melanoma. Clin. Tech. Small Anim. Pract. 2007, 22, 55–60. [Google Scholar] [CrossRef]
- Smith, S.H.; Goldschmidt, M.H.; McManus, P.M. A comparative review of melanocytic neoplasms. Vet. Pathol. 2002, 39, 651–678. [Google Scholar] [CrossRef]
- MacEwen, E.G.; Patnaik, A.K.; Harvey, H.J.; Hayes, A.A.; Matus, R. Canine oral melanoma: Comparison of surgery versus surgery plus Corynebacterium parvum. Cancer Investig. 1986, 4, 397–402. [Google Scholar] [CrossRef]
- Tuohy, J.L.; Selmic, L.E.; Worley, D.R.; Ehrhart, N.P.; Withrow, S.J. Outcome following curative-intent surgery for oral melanoma in dogs: 70 cases (1998–2011). J. Am. Vet. Med. Assoc. 2014, 245, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Bateman, K.E.; Catton, P.A.; Pennock, P.W.; Kruth, S.A. 0-7-21 radiation therapy for the treatment of canine oral melanoma. J. Vet. Intern. Med. 1994, 8, 267–272. [Google Scholar] [CrossRef]
- Blackwood, L.; Dobson, J.M. Radiotherapy of oral malignant melanomas in dogs. J. Am. Vet. Med. Assoc. 1996, 209, 98–102. [Google Scholar] [CrossRef]
- Cancedda, S.; Rohrer Bley, C.; Aresu, L.; Dacasto, M.; Leone, V.F.; Pizzoni, S.; Gracis, M.; Marconato, L. Efficacy and side effects of radiation therapy in comparison with radiation therapy and temozolomide in the treatment of measurable canine malignant melanoma. Vet. Comp. Oncol. 2016, 14, e146–e157. [Google Scholar] [CrossRef]
- Kawabe, M.; Mori, T.; Ito, Y.; Murakami, M.; Sakai, H.; Yanai, T.; Maruo, K. Outcomes of dogs undergoing radiotherapy for treatment of oral malignant melanoma: 111 cases (2006–2012). J. Am. Vet. Med. Assoc. 2015, 247, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Boria, P.A.; Murry, D.J.; Bennett, P.F.; Glickman, N.W.; Snyder, P.W.; Merkel, B.L.; Schlittler, D.L.; Mutsaers, A.J.; Thomas, R.M.; Knapp, D.W. Evaluation of cisplatin combined with piroxicam for the treatment of oral malignant melanoma and oral squamous cell carcinoma in dogs. J. Am. Vet. Med. Assoc. 2004, 224, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Brockley, L.K.; Cooper, M.A.; Bennett, P.F. Malignant melanoma in 63 dogs (2001–2011): The effect of carboplatin chemotherapy on survival. N. Z. Vet. J. 2013, 61, 25–31. [Google Scholar] [CrossRef]
- Murphy, S.; Hayes, A.M.; Blackwood, L.; Maglennon, G.; Pattinson, H.; Sparkes, A.H. Oral malignant melanoma—The effect of coarse fractionation radiotherapy alone or with adjuvant carboplatin therapy. Vet. Comp. Oncol. 2005, 3, 222–229. [Google Scholar] [CrossRef]
- Rassnick, K.M.; Ruslander, D.M.; Cotter, S.M.; Al-Sarraf, R.; Bruyette, D.S.; Gamblin, R.M.; Meleo, K.A.; Moore, A.S. Use of carboplatin for treatment of dogs with malignant melanoma: 27 cases (1989–2000). J. Am. Vet. Med. Assoc. 2001, 218, 1444–1448. [Google Scholar] [CrossRef]
- Knight, A.; Karapetyan, L.; Kirkwood, J.M. Immunotherapy in Melanoma: Recent Advances and Future Directions. Cancers 2023, 15, 1106. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.M.; Hwu, W.J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, N.; Konnai, S.; Ikebuchi, R.; Okagawa, T.; Adachi, M.; Takagi, S.; Kagawa, Y.; Nakajima, C.; Suzuki, Y.; Murata, S.; et al. Expression of PD-L1 on canine tumor cells and enhancement of IFN-γ production from tumor-infiltrating cells by PD-L1 blockade. PLoS ONE 2014, 9, e98415. [Google Scholar] [CrossRef]
- Maekawa, N.; Konnai, S.; Nishimura, M.; Kagawa, Y.; Takagi, S.; Hosoya, K.; Ohta, H.; Kim, S.; Okagawa, T.; Izumi, Y.; et al. PD-L1 immunohistochemistry for canine cancers and clinical benefit of anti-PD-L1 antibody in dogs with pulmonary metastatic oral malignant melanoma. NPJ Precis. Oncol. 2021, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, N.; Konnai, S.; Okagawa, T.; Nishimori, A.; Ikebuchi, R.; Izumi, Y.; Takagi, S.; Kagawa, Y.; Nakajima, C.; Suzuki, Y.; et al. Immunohistochemical Analysis of PD-L1 Expression in Canine Malignant Cancers and PD-1 Expression on Lymphocytes in Canine Oral Melanoma. PLoS ONE 2016, 11, e0157176. [Google Scholar] [CrossRef]
- Shosu, K.; Sakurai, M.; Inoue, K.; Nakagawa, T.; Sakai, H.; Morimoto, M.; Okuda, M.; Noguchi, S.; Mizuno, T. Programmed Cell Death Ligand 1 Expression in Canine Cancer. In Vivo 2016, 30, 195–204. [Google Scholar]
- Igase, M.; Nemoto, Y.; Itamoto, K.; Tani, K.; Nakaichi, M.; Sakurai, M.; Sakai, Y.; Noguchi, S.; Kato, M.; Tsukui, T.; et al. A pilot clinical study of the therapeutic antibody against canine PD-1 for advanced spontaneous cancers in dogs. Sci. Rep. 2020, 10, 18311. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, N.; Konnai, S.; Takagi, S.; Kagawa, Y.; Okagawa, T.; Nishimori, A.; Ikebuchi, R.; Izumi, Y.; Deguchi, T.; Nakajima, C.; et al. A canine chimeric monoclonal antibody targeting PD-L1 and its clinical efficacy in canine oral malignant melanoma or undifferentiated sarcoma. Sci. Rep. 2017, 7, 8951. [Google Scholar] [CrossRef]
- Deguchi, T.; Maekawa, N.; Konnai, S.; Owaki, R.; Hosoya, K.; Morishita, K.; Nakamura, M.; Okagawa, T.; Takeuchi, H.; Kim, S.; et al. Enhanced Systemic Antitumour Immunity by Hypofractionated Radiotherapy and Anti-PD-L1 Therapy in Dogs with Pulmonary Metastatic Oral Malignant Melanoma. Cancers 2023, 15, 3013. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, N.; Konnai, S.; Hosoya, K.; Kim, S.; Kinoshita, R.; Deguchi, T.; Owaki, R.; Tachibana, Y.; Yokokawa, M.; Takeuchi, H.; et al. Safety and clinical efficacy of an anti-PD-L1 antibody (c4G12) in dogs with advanced malignant tumours. PLoS ONE 2023, 18, e0291727. [Google Scholar] [CrossRef] [PubMed]
- Polton, G.; Borrego, J.F.; Clemente-Vicario, F.; Clifford, C.A.; Jagielski, D.; Kessler, M.; Kobayashi, T.; Lanore, D.; Queiroga, F.L.; Rowe, A.T.; et al. Melanoma of the dog and cat: Consensus and guidelines. Front. Vet. Sci. 2024, 11, 1359426. [Google Scholar] [CrossRef]
- Veterinary Cooperative Oncology Group (VCOG). Veterinary cooperative oncology group—Common terminology criteria for adverse events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.1. Vet. Comp. Oncol. 2016, 14, 417–446. [Google Scholar] [CrossRef]
- Nguyen, S.M.; Thamm, D.H.; Vail, D.M.; London, C.A. Response evaluation criteria for solid tumours in dogs (v1.0): A Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet. Comp. Oncol. 2015, 13, 176–183. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef]
- Schneider, B.J.; Lacchetti, C.; Bollin, K. Management of the Top 10 Most Common Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy. JCO Oncol. Pract. 2022, 18, 431–444. [Google Scholar] [CrossRef]
- Maekawa, N.; Konnai, S.; Watari, K.; Takeuchi, H.; Nakanishi, T.; Tachibana, T.; Hosoya, K.; Kim, S.; Kinoshita, R.; Owaki, R.; et al. Development of caninized anti-CTLA-4 antibody as salvage combination therapy for anti-PD-L1 refractory tumors in dogs. Front. Immunol. 2025, 16, 1570717. [Google Scholar] [CrossRef] [PubMed]
| 2 mg/kg (n = 9) | 5 mg/kg (n = 9) | 10 mg/kg (n = 8) | |
|---|---|---|---|
| Breed―No. (%) | |||
| American Cocker Spaniel | 0 | 1 (11.1) | 0 |
| Chihuahua | 0 | 1 (11.1) | 1 (12.5) |
| Miniature Dachshund | 4 (44.4) | 1 (11.1) | 3 (37.5) |
| Miniature Schnauzer | 1 (11.1) | 0 | 0 |
| Norfolk Terrier | 0 | 1 (11.1) | 0 |
| Shetland Sheepdog | 0 | 1 (11.1) | 0 |
| Shiba | 0 | 1 (11.1) | 0 |
| Tosa | 1 (11.1) | 0 | 0 |
| Toy Poodle | 1 (11.1) | 1 (11.1) | 2 (25.0) |
| Welsh Corgi | 0 | 0 | 1 (12.5) |
| Yorkshire Terrier | 0 | 0 | 1 (12.5) |
| Mix | 2 (22.2) | 2 (22.2) | 0 |
| Age―year | |||
| Median | 12 | 12 | 15 |
| Range | 6–16 | 9–15 | 13–19 |
| Sex―No. (%) | |||
| Male, intact | 2 (22.2) | 0 | 2 (25.0) |
| Male, castrated | 0 | 5 (55.6) | 3 (37.5) |
| Female, intact | 1 (11.1) | 0 | 0 |
| Female, spayed | 6 (66.7) | 4 (44.4) | 3 (37.5) |
| PD-L1 expression―No. (%) | |||
| Positive | 5 (55.6) | 5 (55.6) | 6 (75.0) |
| Negative | 1 (11.1) | 0 | 0 |
| Not determined | 3 (33.3) | 4 (44.4) | 2 (25.0) |
| Measurable lesion(s)―No. (%) | |||
| Present | 2 (22.2) | 1 (11.1) | 1 (12.5) |
| Absent | 7 (77.8) | 8 (88.9) | 7 (87.5) |
| TRAEs―No. (%) | 2 mg/kg (n = 9) | 5 mg/kg (n = 9) | 10 mg/kg (n = 8) | |||
|---|---|---|---|---|---|---|
| Any Grade | Grade 3 | Any Grade | Grade 3 | Any Grade | Grade 3 | |
| Any TRAEs | 3 (33.3) | 1 (11.1) | 5 (55.6) | 1 (11.1) | 3 (37.5) | 1 (12.5) |
| ALP, high | 0 | 0 | 0 | 0 | 1 (12.5) | 0 |
| ALT, high | 1 (11.1) | 0 | 0 | 0 | 1 (12.5) | 0 |
| AST, high | 1 (11.1) | 0 | 0 | 0 | 0 | 0 |
| Creatinine, high | 0 | 0 | 2 (22.2) | 0 | 2 (25.0) | 0 |
| BUN, high | 0 | 0 | 2 (22.2) | 1 (11.1) | 0 | 0 |
| Anorexia | 1 (11.1) | 0 | 0 | 0 | 1 (12.5) | 1 (12.5) |
| Allergic reaction | 0 | 0 | 0 | 0 | 1 (12.5) | 0 |
| Anaphylaxis | 1 (11.1) | 1 (11.1) | 0 | 0 | 0 | 0 |
| Diarrhea | 1 (11.1) | 0 | 2 (22.2) | 0 | 1 (12.5) | 0 |
| Vomiting | 1 (11.1) | 0 | 3 (33.3) | 0 | 0 | 0 |
| Weight loss | 0 | 0 | 0 | 0 | 1 (12.5) | 1 (12.5) |
| Best Overall Response―No. (%) | 2 mg/kg (n = 2) | 5 mg/kg (n = 1) | 10 mg/kg (n = 1) |
|---|---|---|---|
| CR | 0 | 0 | 0 |
| PR | 0 | 1 (100.0) | 0 |
| SD | 0 | 0 | 0 |
| PD | 1 (50.0) | 0 | 0 |
| NE | 1 (50.0) | 0 | 1 (100.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosoya, K.; Kim, S.; Kinoshita, R.; Maekawa, N.; Konnai, S.; Takagi, S.; Tagawa, M.; Kagawa, Y.; Deguchi, T.; Owaki, R.; et al. Exploratory, Randomized, Dose-Response Study of the Anti-PD-L1 Antibody HFC-L1/c4G12 in Dogs with Pulmonary Metastatic Oral Malignant Melanoma. Vet. Sci. 2025, 12, 850. https://doi.org/10.3390/vetsci12090850
Hosoya K, Kim S, Kinoshita R, Maekawa N, Konnai S, Takagi S, Tagawa M, Kagawa Y, Deguchi T, Owaki R, et al. Exploratory, Randomized, Dose-Response Study of the Anti-PD-L1 Antibody HFC-L1/c4G12 in Dogs with Pulmonary Metastatic Oral Malignant Melanoma. Veterinary Sciences. 2025; 12(9):850. https://doi.org/10.3390/vetsci12090850
Chicago/Turabian StyleHosoya, Kenji, Sangho Kim, Ryohei Kinoshita, Naoya Maekawa, Satoru Konnai, Satoshi Takagi, Michihito Tagawa, Yumiko Kagawa, Tatsuya Deguchi, Ryo Owaki, and et al. 2025. "Exploratory, Randomized, Dose-Response Study of the Anti-PD-L1 Antibody HFC-L1/c4G12 in Dogs with Pulmonary Metastatic Oral Malignant Melanoma" Veterinary Sciences 12, no. 9: 850. https://doi.org/10.3390/vetsci12090850
APA StyleHosoya, K., Kim, S., Kinoshita, R., Maekawa, N., Konnai, S., Takagi, S., Tagawa, M., Kagawa, Y., Deguchi, T., Owaki, R., Tachibana, Y., Yokokawa, M., Takeuchi, H., Nakamura, H., Yamauchi, A., Kudo, A., Kamo, S., Kato, Y., Kanazawa, S., ... Ohashi, K. (2025). Exploratory, Randomized, Dose-Response Study of the Anti-PD-L1 Antibody HFC-L1/c4G12 in Dogs with Pulmonary Metastatic Oral Malignant Melanoma. Veterinary Sciences, 12(9), 850. https://doi.org/10.3390/vetsci12090850








