Sarcocystosis in Farm Animals in Brazil: A One-Health Approach
Simple Summary
Abstract
1. Introduction
2. Material and Methods
3. Results
3.1. History
3.2. Etiology
3.3. Biological Cycle
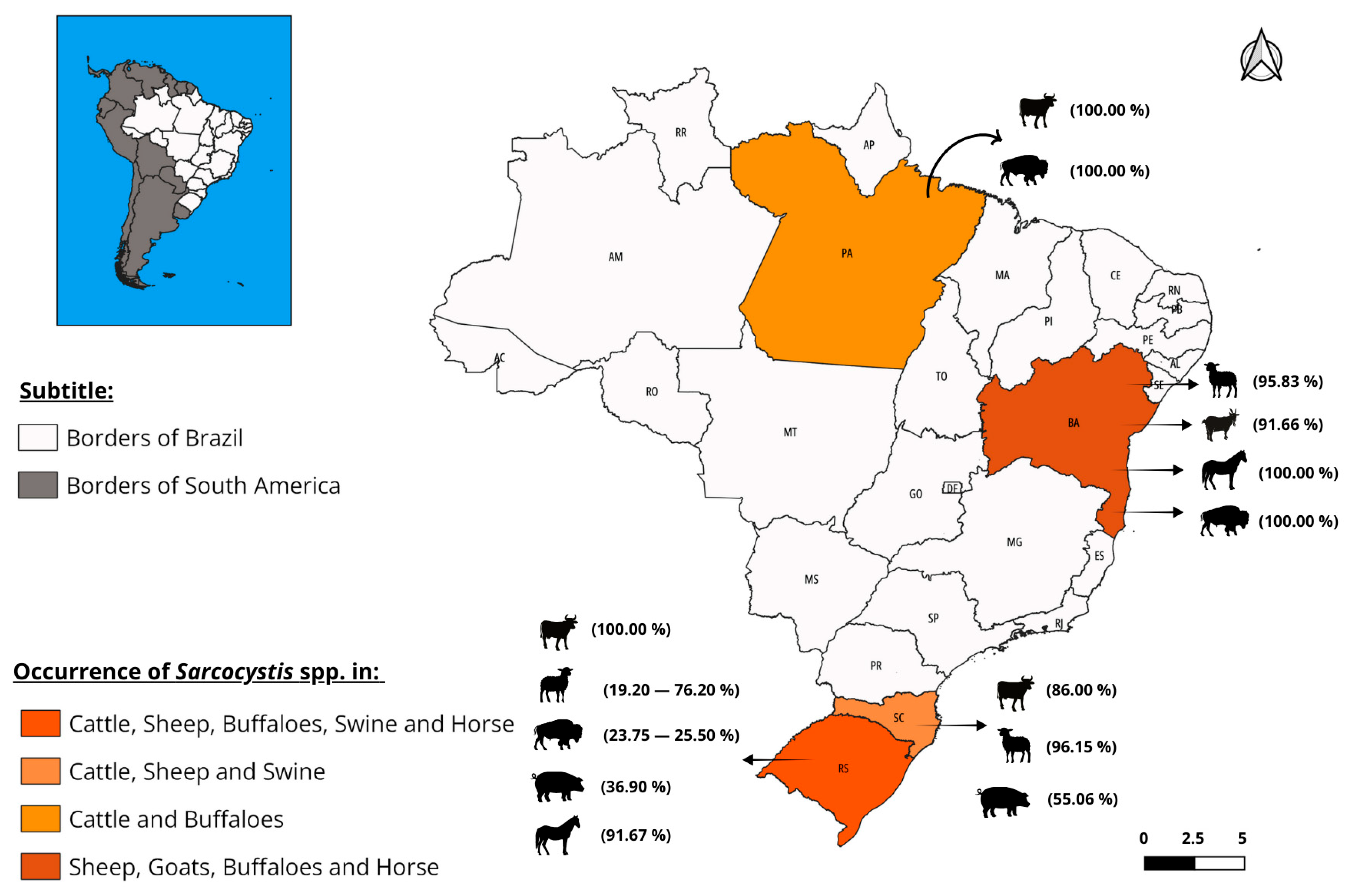
3.4. Epidemiology
| Year | Species | State | Nº Examined Animals | Diagnostic Method | Nº Positive Animals | Prevalence (%) | Authors |
|---|---|---|---|---|---|---|---|
| 2015 | Cattle | Pará | 200 | Peptide digestion and Scarification | 200 | 100 | Mangas et al., [6] |
| 2018 | Cattle | Rio Grande do Sul | 314 | Scarification | 314 | 100 | Ferreira et al., [26] |
| 2019 | Cattle | Santa Catarina | 146 | Histopathology | 122 | 86.00 | Quadros et al., [2] |
| 2016 | Sheep | Rio Grande do Sul | 80 | Scarification | 61 | 76.20 | Portella et al., [27] |
| 2016 | Sheep | Bahia | 120 | Tissue squash and Tissue grinding | 115 | 95.83 | Bittencourt et al., [28] |
| 2018 | Sheep | Rio Grande do Sul | 161 | Macroscopic and Histopathology | 31 | 19.20 | Panziera et al., [29] |
| 2019 | Sheep | Santa Catarina | 130 | Tissue grinding | 125 | 96.15 | Minuzzi et al., [9] |
| 2016 | Goats | Bahia | 120 | Tissue squash and Tissue grinding | 110 | 91.66 | Bittencourt et al., [28] |
| 1996 | Buffaloes | Bahia | 43 | Scarification | 33 | 76.74 | Rebouças et al., [30] |
| 2016 | Buffaloes | Rio Grande do Sul | 220 | IFAT | 56 | 25.50% | Portella et al., [31] |
| 2016 | Buffaloes | Pará | 100 | Peptide digestion | 100 | 100 | Rabello, [32] |
| 2021 | Buffaloes | Rio Grande do Sul | 80 | Tissue grinding | 19 | 23.75 | Portella et al., [33] |
| 2019 | Swine | Santa Catarina | 296 | Histopathology | 163 | 55.06 | Morés et al., [22] |
| 2022 | Swine | Rio Grande do Sul | 84 | IFAT | 31 | 36.90 | Espindola et al., [23] |
| 2022 | Horse | Bahia | 51 | Tissue grinding | 51 | 100 | Marques et al., [20] |
| 2024 | Horse | Rio Grande do Sul | 24 | PCR | 22 | 91.67 | Rosa et al., [21] |
| Year | Species | State | Nº Examined Animals | Diagnostic Method | Nº Positive Animals | Prevalence (%) | Authors |
|---|---|---|---|---|---|---|---|
| 2002 | Dogs | São Paulo | 271 | FSSCS and CFSZSS | 6 | 2.20 | Oliveira et al., [34] |
| 2014 | Dogs | Sergipe | 93 | FSSCS and SS | 4 | 4.30 | Lima et al., [35] |
| 2015 | Dogs | Rio de Janeiro | 221 | CFSS and Direct examination | 1 | 0.45 | Leal et al., [36] |
| 2015 | Dogs | Paraná | 123 | FSSCS and SS | 4 | 3.25 | Ribeiro et al., [37] |
| 2016 | Dogs | São Paulo | 3099 | CSWE, CFSS, and FSSCS | 16 | 0.50 | Ferreira et al., [38] |
| 2016 | Dogs | São Paulo | 1000 | FSSCS, CFSS, and CSFES | 37 | 3.70 | Lallo et al., [39] |
| 2017 | Dogs | Mato Grosso | 120 | CFSZSS and SS | 2 | 1.60 | Lima & Malheiros, [40] |
| 2017 | Dogs | Paraná | 120 | FSSCS, SS, and CFSS | 1 | 0.80 | Snak, [41] |
| 2018 | Dogs | São Paulo | 22 | CFSS | 5 | 22.70 | Sevá et al., [42] |
| 2016 | Cats | São Paulo | 502 | CSWE, CFSS and FSSCS | 7 | 1.30 | Ferreira et al., [38] |
| 2016 | Cats | Mato Grosso | 210 | CSWE and SS | 1 | 0.47 | Lins, [43] |
| 2014 | Cats | Bahia | 272 | IFAT | 11 | 4.00 | Meneses et al., [44] |
3.5. Pathogenesis and Clinical Signs
3.6. Zoonotic Aspects
3.7. Diagnosis
3.8. Treatment
3.9. Prevention and Control
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gonçalves, S.P.; Aragão, K.C.G.; Takeda, G.K.F. Pesquisa de sarcocistos de Sarcocystis spp na musculatura bovina. Ciên Saúde 2016, 4, 39–49. [Google Scholar]
- Quadros, R.M.D.; Barbosa, J.A.; Marques, S.M.T.; Pilati, C. Sarcocistose em bovinos abatidos em frigorífico com inspeção federal em Santa Catarina. Pubvet 2019, 13, 1–5. [Google Scholar] [CrossRef]
- Dubey, J.P.; Calero-Bernal, R.; Rosenthal, B.M.; Speer, C.A.; Fayer, R. Sarcocystis of Aniamls and Humans, 2nd ed.; Taylor & Francis: Boca Raton, FL, USA; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Dubey, J.P.; Lindsay, D.S. Neosporosis, Toxoplasmosis, and Sarcocystosis in Ruminants. Vet. Clin. Food Anim. 2006, 22, 645–671. [Google Scholar] [CrossRef]
- Lopes, C.W.G.; de Sá, W.F.; Botelho, G.G. Lesões em vacas mestiças gestantes, infectadas experimentalmente com Sarcocystis cruzi (Hasselmann, 1923) Wenyon, 1926 (Apicomplexa: Sarcocystidae). Rev. Bras. de Parasitol. Vet. 2005, 14, 79–83. [Google Scholar]
- Mangas, T.P.; do Couto Rocha, H.P.; da Silva Filho, E.; Serra-Freire, N.M.; Benigno, R.N.M. Efficiency of peptide digestion and scarification techniques for detecting Sarcocystis spp. in beefcattle. Coccidia 2014, 2, 52–57. [Google Scholar]
- Fayer, R.; Heydorn, A.O.; Johnson, A.J.; Leek, R.G. Transmission of Sarcocystis suihominis from humans to swine to nonhuman primates (Pan troglodytes Macaca mulatta Macaca irus). Z Parasitenkd 1979, 59, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Fayer, R. Sarcocystis spp. in human infections. Clin. Microbiol. Rev. 2004, 17, 894–902. [Google Scholar] [CrossRef]
- Minuzzi, C.E.; Cezar, A.S.; Bräunig, P.; Portella, L.P.; Rodrigues, F.D.S.; Sangioni, L.A.; Vogel, F.S. Occurrence of Sarcocystis gigantea macrocysts and high frequency of S. tenella microcysts in sheep from southern Brazil. Vet. Parasitol. Reg. Stud. Rep. 2019, 15, 100256. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Speer, C.A.; Charleston, W.A.G. Ultrastructural differenciation between sarcocysts of Sarcocystis hirsuta and Sarcocystis hominis. Vet. Parasitol. 1989, 34, 153–157. [Google Scholar] [CrossRef]
- Senaud, J. Contribution a l’etude des sarcosporidies et des toxoplasmes Toxoplasma. Protistologica 1967, 3, 169–232. [Google Scholar]
- Odening, K. The present state of espécies-systematics in Sarcocystis Lankaster, 1882 (Protista, Sporozoa, Coccidia). Syst. Parasitol. 1998, 41, 209–233. [Google Scholar] [CrossRef]
- Ruggiero, M.A.; Gordon, D.P.; Orrell, T.M.; Bailly, N.; Bourgoin, T.; Brusca, R.C.; Cavalier-Smith, T.; Guiry, M.D.; Kirk, P.M. A Higher Level Classification of All Living Organisms. PLoS ONE 2015, 10, e0119248. [Google Scholar] [CrossRef]
- Vargas, C.A. Sarcocistiosis (Arrocillo, Falsa triquina, Falso cisticercos, Sarcosporidiosis);: Revisión literaria. Rev. Investig. Innov. Agropecu. Recur. Nat. 2018, 5, 193–206. [Google Scholar]
- Reys, L. Parasitologia, 4th ed.; Guanabara Koogan: Rio de Janeiro, Brazil, 2008. [Google Scholar]
- Domenis, L.; Peletto, S.; Sacchi, L.; Clementi, E.; Genchi, M.; Felisari, L.; Felisari, C.; Mo, P.; Modesto, P.; Zuccon, F.; et al. Detection of a morphogenetically novel Sarcocystis hominis-like in the context of a prevalence study in semi-intensively bred cattle in Italy. Parasitol. Res. 2011, 109, 1677–1687. [Google Scholar] [CrossRef]
- Ferreira, M.S.; Fernandes, F.D.A.; Bräunig, P.; Guerra, R.R.; Sangioni, L.A.; Vogel, F.S. Sarcocystis spp. detection in cattle using different diagnostic methods. Pesqui. Vet. Bras. 2023, 43, e07206. [Google Scholar] [CrossRef]
- De Assis Santana, V.L.; Alves, L.C.; Souto-Maior, M.P.; da Gloria Faustino, M.A.; de Lima, M.M. Ocorrência de Sarcocystis (Lankester, 1882) na musculatura cardíaca de bovinos comercializados em feiras livres do município de São Lourenço da Mata-Pernambuco-Brasil. Rev. Bras. Cienc. Vet. 2003, 10, 39–41. [Google Scholar] [CrossRef]
- Metwally, A.M.; Abd Ellah, M.R.; Al-Hosary, A.A.; Omar, M.A. Microscopical and serological studies on Sarcocystis infection with first report of S. cruzi in buffaloes (Bubalus bubalis) in Assiut, Egypt. J. Parasitol. Dis. 2014, 38, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.; da Silva, B.; Nogueira, Y.; Bezerra, T.; Tavares, A.; Borges-Silva, W.; Gondim, L. Brazilian horses from Bahia state are highly infected with Sarcocystis bertrami. Animals 2022, 12, 3491. [Google Scholar] [CrossRef]
- Rosa, G.; de Freitas Daudt, G.; Roman, I.J.; Cargnelutti, J.F.; Sangioni, L.A.; Flores, M.M.; Vogel, F.S.F. Sarcocystis in horses from Rio Grande do Sul, Brazil: Molecular identification of Sarcocystis bertrami and Sarcocystis neurona in muscle tissues. Vet. Parasitol. Reg. Stud. Rep. 2024, 47, 100973. [Google Scholar] [CrossRef] [PubMed]
- Morés, M.A.Z.; Morés, N.; Albuquerque, E.R.; Kich, J.D. Pathologic Diagnosis of Zoonotic Parasitosis in Slaughter Pigs in Brazil. In Proceedings of the 13th SafePork 2019: One Health Tear Down Interdisciplinary Walls, Berlin, Germany, 26–29 August 2019; Available online: https://www.alice.cnptia.embrapa.br/alice/bitstream/doc/1124979/1/final9435.pdf (accessed on 13 June 2025).
- Espindola, B.D.; Fernandes, F.D.; Roman, I.J.; Samoel, G.V.A.; Barcelos, R.A.D.; Döhler, A.R.; Botton, S.A.; Vogel, F.S.F.; Sangioni, L.A. Detection of Sarcocystis spp. and Toxoplasma gondii in swine and detection of DNA of these protozoa in tissues and sausages. Rev. Bras. Parasitol. Vet. 2022, 31, e009322. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.M.; Sousa, S.K.; Paludo, G.R.; de Melo, C.B.; Llano, H.A.; Soares, R.M.; Castro, M.B. An undescribed species of Sarcocystis associated with necrotizing meningoencephalitis in naturally infected backyard chickens in the Midwest of Brazil. Parasitol. Int. 2020, 76, 102098. [Google Scholar] [CrossRef]
- Prakas, P.; Calero-Bernal, R.; Dubey, J.P. Sarcocystis infection in domestic and wild avian hosts: Inseparable flight partners. Ve. Parasitol. 2025, 335, 110413. [Google Scholar] [CrossRef]
- Ferreira, M.S.; Vogel, F.S.F.; Sangioni, L.A.; Cezar, A.S.; Braunig, P.; de Avilla Botton, S.; Camillo, G.; Portella, L.P. Sarcocystis species identification in cattle hearts destined to human consumption in southern Brazil. Vet. Parasitol. Reg. Stud. Rep. 2018, 14, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Portella, L.P.; Cadore, G.C.; Sangioni, L.A.; Alves, M.E.M.; Chemeris, R.; Brum, L.P.; Vogel, F.S.F. Molecular detection of protozoa of the Sarcocystidae family in sheep from the State of Rio Grande do Sul, Brazil. Cienc. Rural. 2016, 46, 1613–1617. [Google Scholar] [CrossRef]
- Bittencourt, M.V.; Meneses, I.D.S.; Ribeiro-Andrade, M.; de Jesus, R.F.; de Araújo, F.R.; Gondim, L.F.P. Sarcocystis spp. in sheep and goats: Frequency of infection and species identification by morphological, ultrastructural, and molecular tests in Bahia, Brazil. Parasitol. Res. 2016, 115, 1683–1689. [Google Scholar] [CrossRef]
- Panziera, W.; Vielmo, A.; Lorenzo, C.D.; Heck, L.C.; Pavarini, S.P.; Sonne, L.; Soares, J.F.; Driemeier, D. Caracterização das lesões parasitárias de ovinos observadas na linha de abate. Pesq. Vet. Bras. 2018, 38, 1491–1504. [Google Scholar] [CrossRef]
- Rebouças, M.M.; Barci, L.A.G.; Fujii, T.U.; Martins, A.M.C.R.P.F.; Filha, E.S.; Oliveira, S.M. Sarcocystis spp. (Apicomplexa Sarcocystidae) em búfalos (Bubalus Bubalisl.) do Vale do Ribelra, São Paulo, Brasil1. Arq. Inst. Biol. 1996, 63, 65–67. [Google Scholar] [CrossRef]
- Portella, L.P.; Cadore, G.C.; Lima, M.D.; Sangioni, L.A.; Fischer, G.; Vogel, F.S. Antibodies against Neospora caninum, Sarcocystis spp. and Toxoplasma gondii detected in buffaloes from Rio Grande do Sul, Brazil. Pesq. Vet. Bras. 2016, 36, 947–950. [Google Scholar] [CrossRef][Green Version]
- Rabello, L.D.A. Ocorrência de Sarcocystis (Apicomplexa: Sarcocystidae) em Búfalos da Ilha de Marajó, Estado do Pará, Brasil. Master’s Thesis, Universidade Federal Rural da Amazônia, Belém, Brazil, 2016. Available online: http://repositorio.ufra.edu.br/jspui/handle/123456789/774 (accessed on 23 April 2024).
- Portella, L.P.; Fernandes, F.D.A.; Minuzzi, C.E.; De Pelegrini, L.F.V.; Sangioni, L.A.; Cargnelutti, J.F.; Vogel, F.S.F. Molecular detection and characterization of Sarcocystis infection in naturally infected buffaloes, Brazil. J. Food Prot. 2021, 84, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.C.G.S.; Amarante, A.F.T.; Ferrari, T.B.; Nunes, L.C. Prevalence of intestinal parasites in dogs from São Paulo State, Brazil. Vet. Parasitol. 2002, 103, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Lima, V.F.S.; Santos, T.D.J.; Bezerra, T.L.; Santos, M.S.; Santos, P.O.M. Helmintozoonoses e protozoonoses caninas no bairro Rosa Elze, São Cristóvão/Sergipe–Brasil. Encicl. Biosfera 2014, 10, 1133–1145. [Google Scholar]
- Leal, P.D.S.; Figueiredo, L.P.; Moraes, M.I.M.R.; de Oliveira Barbosa, L.L.; Lima, S.; Lopes, C.W.G. Parasitos gastrintestinais em cães domiciliados atendidos em serviço de saúde animal, Rio de Janeiro, Brasil. Rev. Bras. Med. Vet. 2015, 37, 37–44. [Google Scholar]
- Ribeiro, C.M.; Lima, D.E.; Katagiri, S. Infecções por parasitos gastrintestinais em cães domiciliados e suas implicações na transmissão zoonótica. Vet. Zootec. 2015, 22, 238–244. [Google Scholar]
- Ferreira, J.I.G.D.S.; Pena, H.F.J.; Azevedo, S.S.; Labruna, M.B.; Gennari, S.M. Occurrences of gastrointestinal parasites in fecal samples from domestic dogs in São Paulo, SP, Brazil. Rev. Bras. de Parasitol. Vet. 2016, 25, 435–440. [Google Scholar] [CrossRef]
- Lallo, M.A.; Spadacci-Morena, D.D.; Dall, S.; Coutinho, A. Comportamento humano na criação de cães e a prevalência de parasitos intestinais com potencial zoonótico. Rev. Acadêm Ciênc Animal 2016, 14, 119–128. [Google Scholar] [CrossRef]
- Lima Rosales, T.F.; Malheiros, A.F. Contaminação Ambiental por enteroparasitas presentes em fezes de cães em uma região do Pantanal. Mundo Saúde 2017, 4, 368–377. [Google Scholar] [CrossRef]
- Snak, A. Prevalência e Fatores de Risco Associados a Infecção por Neospora Caninum e Trypanosoma Vivax em Bovinos Leiteiros e Ocorrência de N. caninum e Parasitos Gastrointestinais em Cães de Propriedades Rurais do Oeste do Paraná, Brasil; Universidade Federal do Paraná: Palotina, Brazil, 2017; Available online: https://hdl.handle.net/1884/46069 (accessed on 11 November 2024).
- Sevá, A.D.P.; Pena, H.F.D.J.; Nava, A.; Sousa, A.O.D.; Holsback, L.; Soares, R.M. Endoparasites in domestic animals surrounding an Atlantic Forest remnant, in São Paulo State, Brazil. Rev. Bras. de Parasitol. Vet. 2018, 27, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Lins, S.B.H. Parasitos de Interesse Zoonótico em Felinos (Felis catus Domesticus), Campo Grande, Mato Grosso do Sul; Universidade Federal do Mato Grosso do Sul: Campo Grande, Brazil, 2016; Available online: https://repositorio.ufms.br/handle/123456789/2920 (accessed on 11 November 2024).
- Meneses, I.D.S.D.; Andrade, M.R.; Uzêda, R.S.; Bittencourt, M.V.; Lindsay, D.S.; Gondim, L.F.P. Frequency of antibodies against Sarcocystis neurona and Neospora caninum in domestic cats in the state of Bahia, Brazil. Rev. Bras. de Parasitol. Vet. 2014, 23, 526–529. [Google Scholar] [CrossRef]
- Alves, M.E.; Cadore, G.C.; Oliveira, C.S.; Portella, L.P.; Sangioni, L.A.; Vogel, F.S. Caracterização molecular de Sarcocystis spp. em amostras de carne. Pesqui. Veterinária Bras. 2018, 38, 425–429. [Google Scholar] [CrossRef]
- Meireles, G.S.; Paes-De-Almeida, E.C.; Carvalho Filho, P.R.; Flausino, W.; Rodrigues, J.D.S.; Ferreira, A.M.R.; Lopes, C.W.G. Avaliação do intestino delgado e linfonodos mesentéricos de cães (Canis familiaris) infectados experimentalmente com Sarcocystis cruzi (Hasselman, 1923) Wenyon, 1926 (Apicomplexa: Sarcocystidae). Rev. Bras. de Parasitol. Vet. 2008, 17, 331–334. [Google Scholar]
- Ydrogo, E.M. Frecuencia de Sarcocystis spp. en Perros (Canis lupus Familiaris) Criados en Tres Empresas Alpaqueras de Cajamarca, 2017; Universidad Nacional de Cajamarca: Cajamarca, Brazil, 2018; Available online: http://hdl.handle.net/20.500.14074/2966 (accessed on 17 November 2024).
- Sam, R.; Mansilla, I.; Morales, C.; Ramírez, A. Efecto tóxico de macroquistes de Sarcocystis aucheniae en ratones, cobayos y conejos. Rev. Investig. Pecu. 1998, 9, 11–18. [Google Scholar]
- Italiano, C.M.; Wong, K.T.; AbuBakar, S.; Lau, Y.L.; Ramli, N.; Syed Omar, S.F.; Bador, M.K.; Tan, C.T. Sarcocystis nesbitti causes acute, relapsing febrile myositis with a high attack rate: Description of a large outbreak of muscular sarcocystosis in Pangkor Island, Malaysia, 2012. PLoS Negl. Trop. Dis. 2014, 8, e2876. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.L.; Chang, P.Y.; Tan, C.T.; Fong, M.Y.; Mahmud, R.; Wong, K.T. Sarcocystis nesbitti infection in human skeletal muscle: Possible transmission from snakes. Am. J. Trop. Med. Hyg. 2014, 90, 361. [Google Scholar] [CrossRef]
- Harada, S.; Furukawa, M.; Tokuoka, E.; Matsumoto, K.; Yahiro, S.; Miyasaka, J.; Saito, M.; Kamata, Y.; Watanabe, M.; Irikura, D.; et al. Control of toxicity of Sarcocystis fayeri in horsemeat by freezing treatment and prevention of food poisoning caused by raw consumption of horsemeat. Shokuhin eiseigaku zasshi. J. Food Hyg. Soc. Jpn. 2013, 54, 198–203. [Google Scholar] [CrossRef]
- Kamata, Y.; Saito, M.; Irikura, D.; Yahata, Y.; Ohnishi, T.; Bessho, T.; Inui, T.; Watanabe, M.; Sugita-Konishi, Y. A toxin isolated from Sarcocystis fayeri in raw horse meat may be responsible for food poisoning. J. Food Prot. 2014, 77, 814–819. [Google Scholar] [CrossRef]
- FAOSTAT—Food and Agriculture Data. Meat, Horse. 2022. Available online: http://data.un.org/Data.aspx?q=meat&d=FAO&f=itemCode%3A1097 (accessed on 13 June 2025).
- Brazil 2023 Sistema de Informações Gerenciais do SIF; Relatório de Abates por Ano e UF. Ministério da Agricultura, Pecuária e Abastecimento (MAPA). Available online: https://sistemas.agricultura.gov.br/pga_sigsif/pages/view/sigsif/abateporano/indexAbatePorAno.xhtml (accessed on 10 June 2025).
- Menezes, R.C.A.A.; Lopes, C.W.G. Epizootiologia da Eimeria arloingi em caprinos na microrregião serrana-fluminense, Rio de Janeiro, Brasil. Rev. Cienc. Vida 2013, 17, 5–12. [Google Scholar]
- Brazil, 2020. Regulamento da Inspeção Industrial e Sanitária de Produtos de Origem Animal. Decreto Nº 10.468. 18 August 2020. Available online: https://www.gov.br/agricultura/pt-br/assuntos/inspecao/produtos-animal/arquivos-publicacoes-dipoa/decreto-revisao-riispoa-decreto-10-468-2020.pdf/view (accessed on 7 June 2025).
- Oryan, A.; Sharifiyazdi, H.; Khordadmehr, M.; Larki, S. Characterization of Sarcocystis fusiformis based on sequencing and PCR-RFLP in water buffalo (Bubalus bubalis) in Iran. Parasitol. Res. 2011, 109, 1563–1570. [Google Scholar] [CrossRef]
- El-Kady, A.M.; Hussein, N.M.; Hassan, A.A. First molecular characterization of Sarcocystis spp. in cattle in Qena Governorate, Upper Egypt. J. Parasitic Dis. 2018, 42, 114–121. [Google Scholar] [CrossRef]
- Rosa, G.; Roman, I.J.; Gressler, L.T.; Cargnelutti, J.F.; Vogel, F.S.F. Molecular identification of Sarcocystis species in wild boar (Sus. scrofa) and pigs (Sus. scrofa domesticus) in Brazil. Vet. Parasitol. Reg. Stud. Rep. 2024, 50, 101020. [Google Scholar] [CrossRef] [PubMed]
- Rosa, G.; Roman, I.J.; Gressler, L.T.; Cargnelutti, J.F.; Vogel, F.S.F. Molecular identification of Sarcocystis neurona in tissues of wild boars (Sus. scrofa) in the border region between Brazil and Uruguay. J. Parasitic Dis. 2024, 48, 74–80. [Google Scholar] [CrossRef]
- Roman, I.J.; Tagarra, L.G.; Rodrigues, F.S.; Cargnelutti, J.F.; Sangioni, L.A.; Vogel, F.S. Sarcocystis neurona, Toxoplasma gondii, and Neospora caninum infection in bovine fetuses from a slaughterhouse in southern Brazil. Pesqui. Vet. Bras. 2025, 45, e07504. [Google Scholar] [CrossRef]
- Croft, J.C. Nonamebic Protozoal Enteridities. Infectious Processes, 5th ed.; Lippincott: Philadelphia, PA, USA, 1994; pp. 769–774. [Google Scholar]
- Mensa, J.; Gatell, J.M.; de Anta, J.; Prats, G. Guia e Terapeutica Antimicrobiana, 9th ed.; Masson, S.A.: Barcelona, Spain, 1999. [Google Scholar]


| Definitive Hosts | Intermediate Hosts | ||||||
|---|---|---|---|---|---|---|---|
| Cattle | Buffaloes | Sheep | Goat | Horse | Swines | Chickens | |
| Dogs | S. cruzi * | S. levini * | S. tenella * S. arienticanis * | S. capracanis * S. hircicanis | S. bertrami * | S. miescheriana * | S. wenzeli |
| Cats | S. hirsuta S. bovifelis S. rommeli | S. fusiformis S. buffalonis | S.gigantea * S. medusiformis | S. moulei | S. porcifelis | S. wenzeli | |
| Humans | S. hominis * S. heydorni | S. suihominis * | |||||
| Unknown | S. bovini | S. dubeyi | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, M.d.S.; Camargo Neto, J.A.B.; Lopes, C.W.G.; Paiva, F.; Barros, L.D.d.; Felippelli, G.; Rodrigues, F.d.S.; Widmer, G.; Bresciani, K.D.S. Sarcocystosis in Farm Animals in Brazil: A One-Health Approach. Vet. Sci. 2025, 12, 842. https://doi.org/10.3390/vetsci12090842
Pinto MdS, Camargo Neto JAB, Lopes CWG, Paiva F, Barros LDd, Felippelli G, Rodrigues FdS, Widmer G, Bresciani KDS. Sarcocystosis in Farm Animals in Brazil: A One-Health Approach. Veterinary Sciences. 2025; 12(9):842. https://doi.org/10.3390/vetsci12090842
Chicago/Turabian StylePinto, Michel dos Santos, João Alfredo Biagi Camargo Neto, Carlos Wilson Gomes Lopes, Fernando Paiva, Luiz Daniel de Barros, Gustavo Felippelli, Fernando de Souza Rodrigues, Giovanni Widmer, and Katia Denise Saraiva Bresciani. 2025. "Sarcocystosis in Farm Animals in Brazil: A One-Health Approach" Veterinary Sciences 12, no. 9: 842. https://doi.org/10.3390/vetsci12090842
APA StylePinto, M. d. S., Camargo Neto, J. A. B., Lopes, C. W. G., Paiva, F., Barros, L. D. d., Felippelli, G., Rodrigues, F. d. S., Widmer, G., & Bresciani, K. D. S. (2025). Sarcocystosis in Farm Animals in Brazil: A One-Health Approach. Veterinary Sciences, 12(9), 842. https://doi.org/10.3390/vetsci12090842







