Time-Dependent Changes in Malondialdehyde and Free-Hemoglobin in Leukoreduced and Non-Leukoreduced Canine Packed Red Blood Cells Units During Storage
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Selection
2.2. Blood Collection, Processing and Storage
2.3. Samples Collection
2.4. Malondialdehyde (MDA) Method
2.5. Harboe Direct Spectrophotometric Method
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MDA | Malondialdehyde |
| fHb | free hemoglobin |
| LR | leukoreduced |
| NLR | Non-leukoreduced |
| pRBC | Packed red blood cells |
| CPD-SAGM | Citrate-phosphate-dextrose saline-adenine-glucose-mannitol additive solution |
| CPD | Citrate-phosphate-dextrose |
| SAGM | Saline-adenine-glucose-mannitol |
| AVHTM | Association of Veterinary Hematology and Transfusion Medicine |
| TRACS | Transfusion Reaction Small Animal Consensus Statement |
| ROS | Reactive oxygen species |
| 2,3-DPG | 2,3-diphosphoglycerate |
| CPDA-1 | Citrate-phosphate-dextrose-adenine-1 |
References
- Divers, T.J. Blood Component Transfusions. Vet. Clin. N. Am. Food Anim. Pract. 2005, 21, 615–622. [Google Scholar] [CrossRef]
- Kisielewicz, C.; Self, I.A. Canine and Feline Blood Transfusions: Controversies and Recent Advances in Administration Practices. Vet. Anaesth. Analg. 2014, 41, 233–242. [Google Scholar] [CrossRef]
- Mangiaterra, S.; Rossi, G.; Antognoni, M.T.; Cerquetella, M.; Marchegiani, A.; Miglio, A.; Gavazza, A. Canine Blood Group Prevalence and Geographical Distribution around the World: An Updated Systematic Review. Animals 2021, 11, 342. [Google Scholar] [CrossRef]
- Vossoughi, S.; Perez, G.; Whitaker, B.I.; Fung, M.K.; Stotler, B. Analysis of Pediatric Adverse Reactions to Transfusions. Transfusion 2018, 58, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Spinella, P.C.; Carroll, C.L.; Staff, I.; Gross, R.; Mc Quay, J.; Keibel, L.; Wade, C.E.; Holcomb, J.B. Duration of Red Blood Cell Storage Is Associated with Increased Incidence of Deep Vein Thrombosis and in Hospital Mortality in Patients with Traumatic Injuries. Crit. Care 2009, 13, R151. [Google Scholar] [CrossRef] [PubMed]
- Tocci, L.J.; Ewing, P.J. Increasing Patient Safety in Veterinary Transfusion Medicine: An Overview of Pretransfusion Testing. J. Vet. Emerg. Crit. Care 2009, 19, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Maglaras, C.H.; Koenig, A.; Bedard, D.L.; Brainard, B.M. Retrospective Evaluation of the Effect of Red Blood Cell Product Age on Occurrence of Acute Transfusion-related Complications in Dogs: 210 Cases (2010–2012). J. Vet. Emerg. Crit. Care 2017, 27, 108–120. [Google Scholar] [CrossRef]
- Davidow, E.B.; Blois, S.L.; Goy-Thollot, I.; Harris, L.; Humm, K.; Musulin, S.; Nash, K.J.; Odunayo, A.; Sharp, C.R.; Spada, E.; et al. Association of Veterinary Hematology and Transfusion Medicine (AVHTM) Transfusion Reaction Small Animal Consensus Statement (TRACS). Part 1: Definitions and Clinical Signs. J. Vet. Emerg. Crit. Care 2021, 31, 141–166. [Google Scholar] [CrossRef]
- Davidow, B. Transfusion Medicine in Small Animals. Vet. Clin. North Am. Small Anim. Pract. 2013, 43, 735–756. [Google Scholar] [CrossRef]
- Miglio, A.; Rocconi, F.; Cremoni, V.; D’Alessandro, A.; Reisz, J.A.; Maslanka, M.; Lacroix, I.S.; Di Francesco, D.; Antognoni, M.T.; Di Tommaso, M. Effect of Leukoreduction on the Omics Phenotypes of Canine Packed Red Blood Cells during Refrigerated Storage. Vet. Intern. Medicne 2024, 38, 1498–1511. [Google Scholar] [CrossRef]
- Can, O.M.; Ülgen, Y. Estimation of Free Hemoglobin Concentrations in Blood Bags by Diffuse Reflectance Spectroscopy. J. Biomed. Opt. 2018, 23, 1. [Google Scholar] [CrossRef]
- Antognoni, M.T.; Marenzoni, M.L.; Misia, A.L.; Avellini, L.; Chiaradia, E.; Gavazza, A.; Miglio, A. Effect of Leukoreduction on Hematobiochemical Parameters and Storage Hemolysis in Canine Whole Blood Units. Animals 2021, 11, 925. [Google Scholar] [CrossRef]
- Bujok, J.; Wajman, E.; Trochanowska-Pauk, N.; Walski, T. Evaluation of Selected Hematological, Biochemical and Oxidative Stress Parameters in Stored Canine CPDA-1 Whole Blood. BMC Vet. Res. 2022, 18, 255. [Google Scholar] [CrossRef]
- Stefani, A.; Capello, K.; Carminato, A.; Wurzburger, W.; Furlanello, T.; Bertazzo, V.; Marsilio, E.; Albertin, E.; La Pietra, G.; Bozzato, E.; et al. Effects of Leukoreduction on Storage Lesions in Whole Blood and Blood Components of Dogs. Vet. Intern. Medicne 2021, 35, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Miglio, A.; Cremonini, V.; Leonardi, L.; Manuali, E.; Coliolo, P.; Barbato, O.; Dall’Aglioa, C.; Antognoni, M.T. Omics Technologies in Veterinary Medicine: Literature Review and Perspectives in Transfusion Medicine. Transfus. Med. Hemotherapy 2023, 50, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Miglio, A.; Di Tommaso, M.; Rocconi, F.; Reisz, J.H.; D’Alessandro, A. Impact of Leukoreduction on the Metabolome of Ovine Packed Red Blood Cells during Refrigerated Storage: Metabolomics of Ovine Stored RBC. Blood Transfus. 2025, 23, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Antonelou, M.H.; Tzounakas, V.L.; Velentzas, A.D.; Stamoulis, K.E.; Kriebardis, A.G.; Papassideri, I.S. Effects of Pre-Storage Leukoreduction on Stored Red Blood Cells Signaling: A Time-Course Evaluation from Shape to Proteome. J. Proteom. 2012, 76, 220–238. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.R.; Sutton, J.M.; Hexley, P.; Johannigman, T.A.; Lentsch, A.B.; Pritts, T.A. Leukoreduction of Packed Red Blood Cells Attenuates Proinflammatory Properties of Storage-Derived Microvesicles. J. Surg. Res. 2018, 223, 128–135. [Google Scholar] [CrossRef]
- Miglio, A.; Maslanka, M.; Di Tommaso, M.; Rocconi, F.; Nemkov, T.; Buehler, P.W.; Antognoni, M.T.; Spitalnik, S.L.; D’Alessandro, A. ZOOMICS: Comparative Metabolomics of Red Blood Cells from Dogs, Cows, Horses and Donkeys during Refrigerated Storage for up to 42 Days. Blood Transfus. 2022, 21, 314–326. [Google Scholar] [CrossRef]
- Carl, H.; Soumya, R.; Srinivas, P.; Vani, R. Oxidative Stress in Erythrocytes of Banked ABO Blood. Hematology 2016, 21, 630–634. [Google Scholar] [CrossRef][Green Version]
- Mustafa, I.; Al Marwani, A.; Mamdouh Nasr, K.; Abdulla Kano, N.; Hadwan, T. Time Dependent Assessment of Morphological Changes: Leukodepleted Packed Red Blood Cells Stored in SAGM. BioMed Res. Int. 2016, 2016, 4529434. [Google Scholar] [CrossRef]
- Tan, H.; Bi, J.; Wang, Y.; Zhang, J.; Zuo, Z. Transfusion of Old RBCs Induces Neuroinflammation and Cognitive Impairment. Crit. Care Med. 2015, 43, e276–e286. [Google Scholar] [CrossRef] [PubMed]
- Papac-Milicevic, N.; Busch, C.J.-L.; Binder, C.J. Malondialdehyde Epitopes as Targets of Immunity and the Implications for Atherosclerosis. Adv. Immunol. 2016, 131, 1–59. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.; Prabhu, N.C.S.; Rajashekaraiah, V. Age-Related Modulations in Erythrocytes under Blood Bank Conditions. Transfus. Med. Hemother 2019, 46, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Nędzi, M.; Chabowska, A.M.; Rogowska, A.; Boczkowska-Radziwon, B.; Nędzi, A.; Radziwon, P. Leucoreduction Helps to Preserve Activity of Antioxidant Barrier Enzymes in Stored Red Blood Cell Concentrates. Vox Sang. 2016, 110, 126–133. [Google Scholar] [CrossRef]
- Nnamdi, O.H.; Ijeoma, U.R.; Gilbert, N.L.; Toochukwu, E.H.; Ositadinma, U.S. In Vitro Assessment of Time-Dependent Changes in Red Cell Cytoplasmic Antioxidants of Donkey Blood Preserved in Citrate Phosphate Dextrose Adenine 1 Anticoagulant. Vet. World 2020, 13, 726–730. [Google Scholar] [CrossRef]
- Chaudhary, R.; Katharia, R. Oxidative Injury as Contributory Factor for Red Cells Storage Lesion during Twenty Eight Days of Storage. Blood Transfus. 2012, 10, 59–62. [Google Scholar] [CrossRef]
- Mustafa, I.; Hadwan, T.A.Q. Hemoglobin Oxidation in Stored Blood Accelerates Hemolysis and Oxidative Injury to Red Blood Cells. J. Lab. Physicians 2020, 12, 244–249. [Google Scholar] [CrossRef]
- Arif, S.H.; Yadav, N.; Rehman, S.; Mehdi, G. Study of Hemolysis During Storage of Blood in the Blood Bank of a Tertiary Health Care Centre. Indian J. Hematol. Blood Transfus. 2017, 33, 598–602. [Google Scholar] [CrossRef]
- Oh, J.-Y.; Marques, M.B.; Xu, X.; Li, J.; Genschmer, K.; Gaggar, A.; Jansen, J.O.; Holcomb, J.B.; Pittet, J.-F.; Patel, R.P. Damage to Red Blood Cells during Whole Blood Storage. J. Trauma Acute Care Surg. 2020, 89, 344–350. [Google Scholar] [CrossRef]
- Pulliam, K.E.; Joseph, B.; Veile, R.A.; Friend, L.A.; Makley, A.T.; Caldwell, C.C.; Lentsch, A.B.; Goodman, M.D.; Pritts, T.A. Expired But Not Yet Dead: Examining the Red Blood Cell Storage Lesion in Extended-Storage Whole Blood. Shock 2021, 55, 526–535. [Google Scholar] [CrossRef]
- Tzounakas, V.L.; Anastasiadi, A.T.; Lekka, M.E.; Papageorgiou, E.G.; Stamoulis, K.; Papassideri, I.S.; Kriebardis, A.G.; Antonelou, M.H. Deciphering the Relationship Between Free and Vesicular Hemoglobin in Stored Red Blood Cell Units. Front. Physiol. 2022, 13, 840995. [Google Scholar] [CrossRef]
- Spada, E.; Proverbio, D.; Baggiani, L.; Bagnagatti De Giorgi, G.; Ferro, E.; Perego, R. Change in Haematological and Selected Biochemical Parameters Measured in Feline Blood Donors and Feline Whole Blood Donated Units. J. Feline Med. Surg. 2017, 19, 375–381. [Google Scholar] [CrossRef]
- Blasi Brugué, C.; Ferreira, R.R.F.; Mesa Sanchez, I.; Graça, R.M.C.; Cardoso, I.M.; De Matos, A.J.F.; Ruiz De Gopegui, R. In Vitro Quality Control Analysis After Processing and during Storage of Feline Packed Red Blood Cells Units. BMC Vet. Res. 2018, 14, 141. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wei, H.-W.; Shen, H.-C.; Li, Z.-Z.; Cheng, Y.; Duan, L.-S.; Yin, L.; Yu, J.; Guo, J.-R. To Study the Effect of Oxygen Carrying Capacity on Expressed Changes of Erythrocyte Membrane Protein in Different Storage Times. Biosci. Rep. 2020, 40, BSR20200799. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Sun, J.; Solomon, S.B.; Klein, H.G.; Natanson, C. Transfusion of Older Stored Blood and Risk of Death: A Meta-analysis. Transfusion 2012, 52, 1184–1195. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Sibbald, W.J.; Chin-Yee, I.H. Effects of Storage on Efficacy of Red Cell Transfusion: When Is It Not Safe? Crit. Care Med. 2003, 31, S687–S697. [Google Scholar] [CrossRef]
- Adamzik, M.; Hamburger, T.; Petrat, F.; Peters, J.; De Groot, H.; Hartmann, M. Free Hemoglobin Concentration in Severe Sepsis: Methods of Measurement and Prediction of Outcome. Crit. Care 2012, 16, R125. [Google Scholar] [CrossRef]
- Akhter, F.; Womack, E.; Vidal, J.E.; Le Breton, Y.; McIver, K.S.; Pawar, S.; Eichenbaum, Z. Hemoglobin Stimulates Vigorous Growth of Streptococcus Pneumoniae and Shapes the Pathogen’s Global Transcriptome. Sci. Rep. 2020, 10, 15202. [Google Scholar] [CrossRef]
- Greite, R.; Wang, L.; Gohlke, L.; Schott, S.; Kreimann, K.; Doricic, J.; Leffler, A.; Tudorache, I.; Salman, J.; Natanov, R.; et al. Cell-Free Hemoglobin in Acute Kidney Injury after Lung Transplantation and Experimental Renal Ischemia/Reperfusion. IJMS 2022, 23, 13272. [Google Scholar] [CrossRef]
- Pettilä, V.; Westbrook, A.J.; Nichol, A.D.; Bailey, M.J.; Wood, E.M.; Syres, G.; Phillips, L.E.; Street, A.; French, C.; Murray, L.; et al. Age of Red Blood Cells and Mortality in the Critically Ill. Crit. Care 2011, 15, R116. [Google Scholar] [CrossRef]
- Rifkind, J.M.; Mohanty, J.G.; Nagababu, E. The Pathophysiology of Extracellular Hemoglobin Associated with Enhanced Oxidative Reactions. Front. Physiol. 2015, 5, 500. [Google Scholar] [CrossRef]
- Ross, J.T.; Robles, A.J.; Mazer, M.B.; Studer, A.C.; Remy, K.E.; Callcut, R.A. Cell-Free Hemoglobin in the Pathophysiology of Trauma: A Scoping Review. Crit. Care Explor. 2024, 6, e1052. [Google Scholar] [CrossRef]
- Ministero della Salute Dipartimento della Sanità Pubblica e dell’Innovazione Linea Guida per l’esercizio Delle Attività Sanitarie Veterinarie Riguardanti La Produzione Di Sangue Intero e Di Emocomponenti Ad Uso Trasfusionale Nel Cane e Nel Gatto 2025. Available online: https://www.regioni.it/news/2025/05/14/linee-guida-attivita-produzione-sangue-intero-ed-emocomponenti-ad-uso-trasfusionale-nel-cane-e-nel-gatto-accordo-17-4-2025-gazzetta-ufficiale-n-109-del-13-5-2025-661359/ (accessed on 18 July 2025).
- Malinauskas, R.A. Plasma Hemoglobin Measurement Techniques for the In Vitro Evaluation of Blood Damage Caused by Medical Devices. Artif. Organs 1997, 21, 1255–1267. [Google Scholar] [CrossRef]
- Harboe, M. A Method for Determination of Hemoglobin in Plasma by Near-Ultraviolet Spectrophotometry. Scand. J. Clin. Lab. Investig. 1959, 11, 66–70. [Google Scholar] [CrossRef]
- Cookson, P.; Sutherland, J.; Cardigan, R. A Simple Spectrophotometric Method for the Quantification of Residual Haemoglobin in Platelet Concentrates. Vox Sang. 2004, 87, 264–271. [Google Scholar] [CrossRef]
- Racek, J.; Herynková, R.; Holeček, V.; Faltysová, J.; Krejčová, I. What Is the Source of Free Radicals Causing Hemolysis in Stored Blood? Physiol. Res. 2001, 50, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Nagura, Y.; Tsuno, N.H.; Tanaka, M.; Matsuhashi, M.; Takahashi, K. The Effect of Pre-Storage Whole-Blood Leukocyte Reduction on Cytokines/Chemokines Levels in Autologous CPDA-1 Whole Blood. Transfus. Apher. Sci. 2013, 49, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Silliman, C.C.; Ambruso, D.R.; Boshkov, L.K. Transfusion-Related Acute Lung Injury. Blood 2005, 105, 2266–2273. [Google Scholar] [CrossRef] [PubMed]
- Nunns, G.R.; Vigneshwar, N.; Kelher, M.R.; Stettler, G.R.; Gera, L.; Reisz, J.A.; D’Alessandro, A.; Ryon, J.; Hansen, K.C.; Gamboni, F.; et al. Succinate Activation of SUCNR1 Predisposes Severely Injured Patients to Neutrophil-Mediated ARDS. Ann. Surg. 2022, 276, e944–e954. [Google Scholar] [CrossRef]
- Avenick, D.; Kidd, L.; Istvan, S.; Dong, F.; Richter, K.; Edwards, N.; Hisada, Y.; Posma, J.J.N.; Massih, C.A.; Mackman, N. Effects of Storage and Leukocyte Reduction on the Concentration and Procoagulant Activity of Extracellular Vesicles in Canine Packed Red Cells. J. Vet. Emergen Crit. Care 2021, 31, 221–230. [Google Scholar] [CrossRef]
- Kusaba, A.; Tago, E.; Kusaba, H.; Kawasumi, K. Study of Age-Related Changes in Plasma Metabolites and Enzyme Activity of Healthy Small Dogs That Underwent Medical Checkups. Front. Vet. Sci. 2024, 11, 1437805. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Kawasumi, K.; Okada, Y.; Ishikawa, S.; Yamamoto, I.; Arai, T.; Mori, N. Comparison of Plasma Lipoprotein Profiles and Malondialdehyde Between Hyperlipidemia Dogs with/Without Treatment. BMC Vet. Res. 2014, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Perez-Montero, B.; Fermin-Rodriguez, M.L.; Portero-Fuentes, M.; Sarquis, J.; Caceres, S.; Portal, J.C.I.D.; Juan, L.D.; Miro, G.; Cruz-Lopez, F. Malondialdehyde (MDA) and 8-Hydroxy-2’-Deoxyguanosine (8-OHdG) Levels in Canine Serum: Establishing Reference Intervals and Influencing Factors. BMC Vet. Res. 2025, 21, 161. [Google Scholar] [CrossRef]
- Nazzal, A.R.; Al-Magsoosi, H.H.E.; Alwan, W.N. Serosurvey and Enzymatic Evaluation of Canine Hepatitis B in Herding Dogs. Ann. Rom. Soc. Cell Biol. 2021, 25, 13996–14005. [Google Scholar]
- Tran, L.N.T.; González-Fernández, C.; Gomez-Pastora, J. Impact of Different Red Blood Cell Storage Solutions and Conditions on Cell Function and Viability: A Systematic Review. Biomolecules 2024, 14, 813. [Google Scholar] [CrossRef]
- Kamel, N.; Goubran, F.; Ramsis, N.; Ahmed, A.S. Effects of Storage Time and Leucocyte Burden of Packed and Buffy-Coat Depleted Red Blood Cell Units on Red Cell Storage Lesion. Blood Transfus. 2010, 8, 260–266. [Google Scholar] [CrossRef]
- Gammon, R.R.; Strayer, S.A.; Avery, N.L.; Mintz, P.D. Hemolysis during Leukocyte-Reduction Filtration of Stored Red Blood Cells. Ann. Clin. Lab. Sci. 2000, 30, 195–199. [Google Scholar]
- Ferreira, R.R.F.; Graça, R.M.C.; Cardoso, I.M.; Gopegui, R.R.; de Matos, A.J.F. In Vitro Hemolysis of Stored Units of Canine Packed Red Blood Cells: Hemolysis in Stored Canine pRBC. J. Vet. Emerg. Crit. Care 2018, 28, 512–517. [Google Scholar] [CrossRef]
- Lacerda, L.A.; Hlavac, N.R.C.; Terra, S.R.; Back, F.P.; Jane Wardrop, K.; González, F.H.D. Effects of Four Additive Solutions on Canine Leukoreduced Red Cell Concentrate Quality during Storage. Vet. Clin. Pathol. 2014, 43, 362–370. [Google Scholar] [CrossRef]
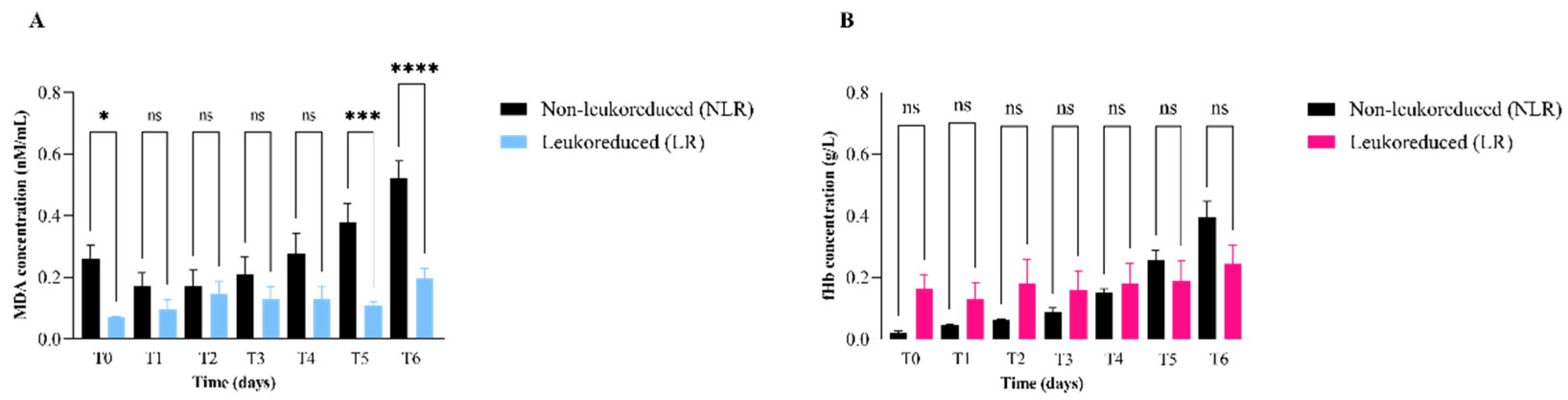

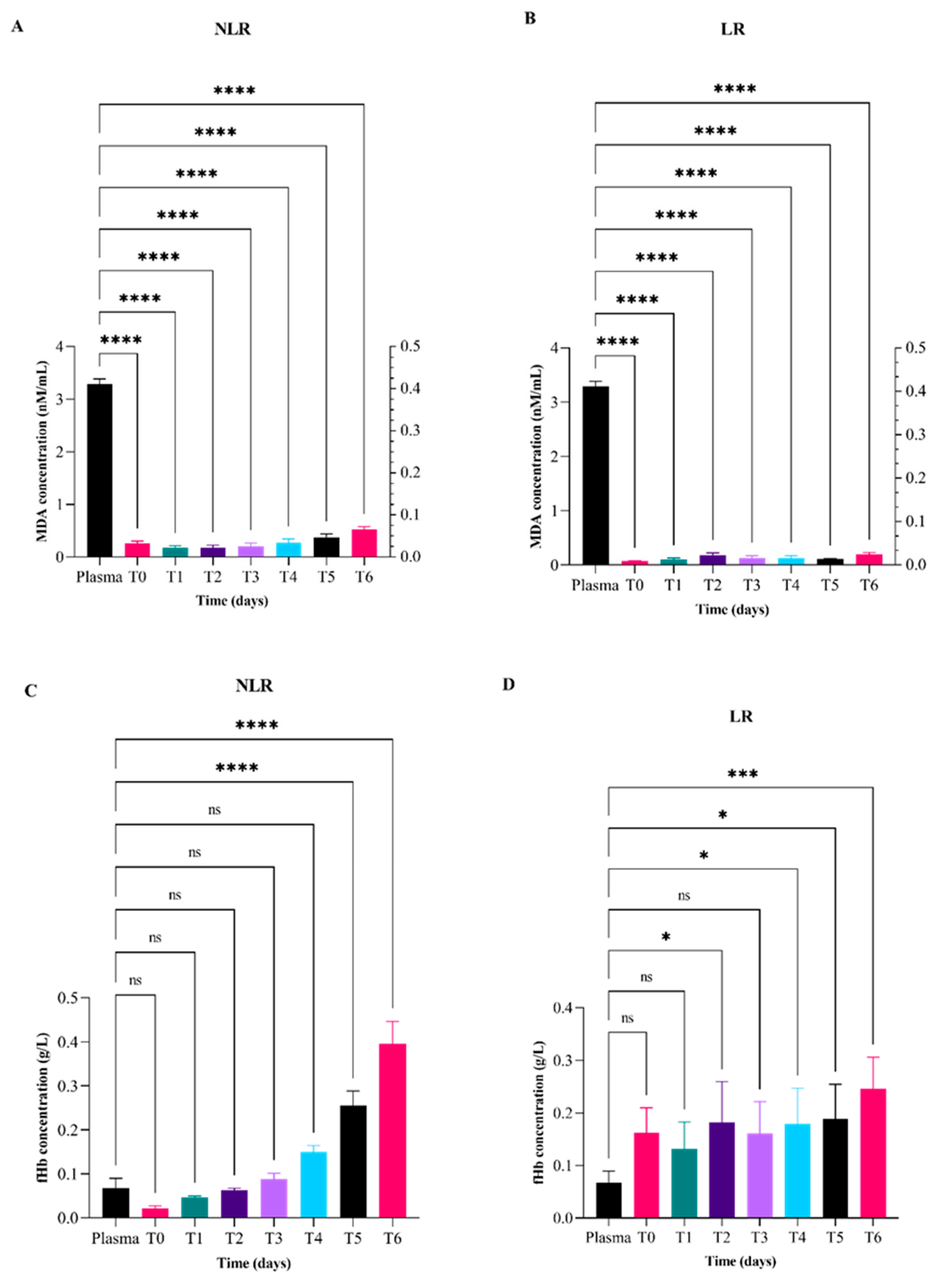
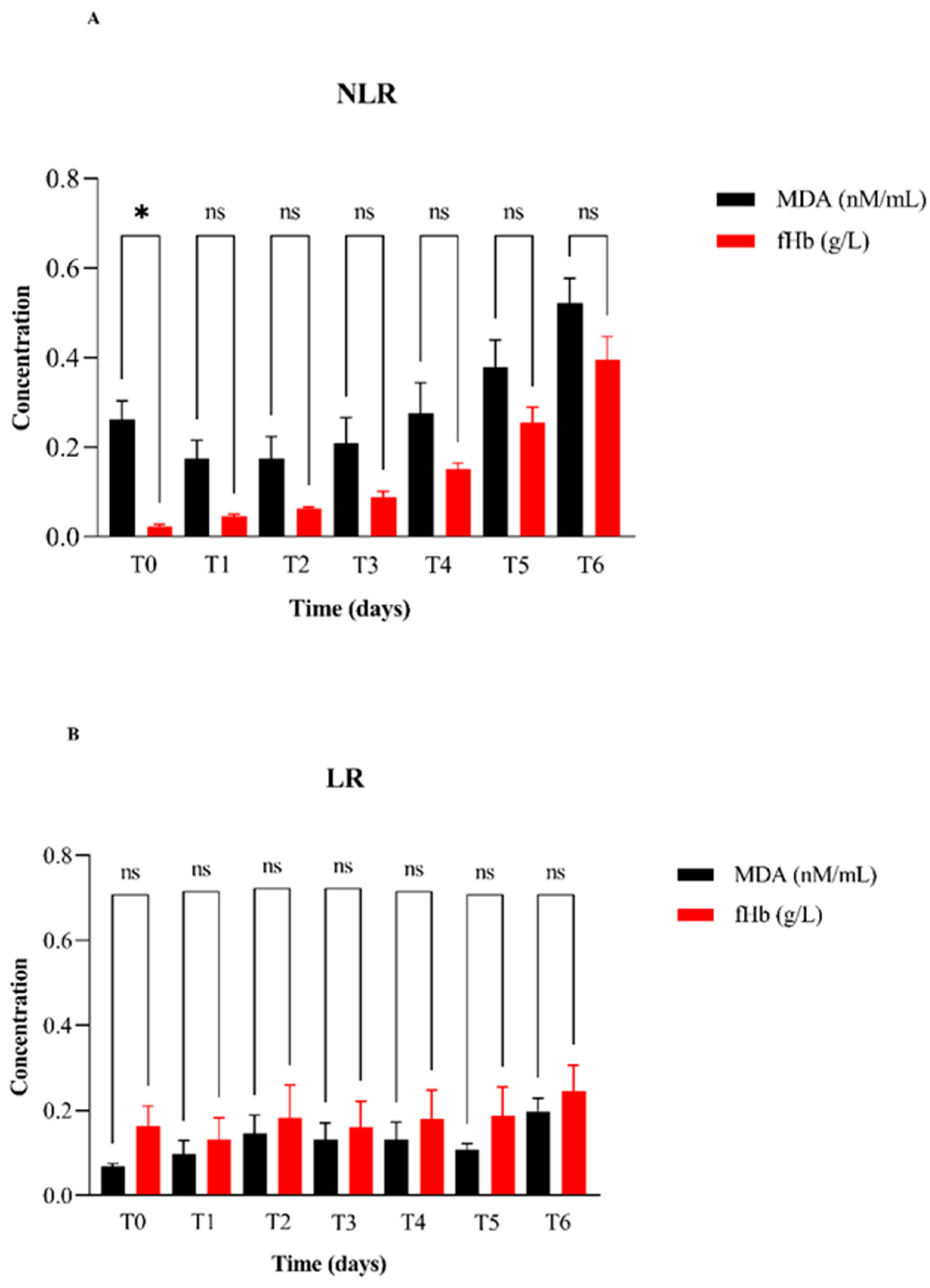
| Measurand | Plasma | T0 2 | T1 3 | T2 4 | T3 5 | T4 6 | T5 7 | T6 8 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MDA 1a (nMN/mL) | NLR 9 | LR 10 | NLR | LR | NLR | LR | NLR | LR | NLR | LR | NLR | LR | NLR | LR | ||
| Media | 3.29 | 0.26 | 0.10 | 0.20 | 0.10 | 0.17 | 0.17 | 0.21 | 0.10 | 0.30 | 0.10 | 0.38 | 0.11 | 0.52 | 0.20 | |
| Min | 3.02 | 0.06 | 0.10 | 0.10 | 0.00 | 0.04 | 0.02 | 0.04 | 0.00 | 0.00 | 0.00 | 0.11 | 0.06 | 0.34 | 0.09 | |
| Max | 3.61 | 0.33 | 0.10 | 0.30 | 0.20 | 0.33 | 0.33 | 0.41 | 0.20 | 0.40 | 0.30 | 0.53 | 0.16 | 0.75 | 0.30 | |
| fHb 1b (g/L) | ||||||||||||||||
| Media | 0.16 | 0.02 | 0.20 | 0.00 | 0.10 | 0.06 | 0.18 | 0.09 | 0.20 | 0.20 | 0.20 | 0.26 | 0.19 | 0.45 | 0.25 | |
| Min | 0.08 | 0.01 | 0.00 | 0.00 | 0.00 | 0.04 | 0.04 | 0.06 | 0.10 | 0.10 | 0.10 | 0.17 | 0.07 | 0.30 | 0.10 | |
| Max | 0.24 | 0.05 | 0.40 | 0.10 | 0.40 | 0.07 | 0.53 | 0.14 | 0.40 | 0.20 | 0.50 | 0.36 | 0.50 | 0.50 | 0.50 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miglio, A.; Barbetta, A.; Cremonini, V.; Barbato, O.; Ricci, G.; Toppi, V.; Avellini, L.; Cavani, V.; Antognoni, M.T. Time-Dependent Changes in Malondialdehyde and Free-Hemoglobin in Leukoreduced and Non-Leukoreduced Canine Packed Red Blood Cells Units During Storage. Vet. Sci. 2025, 12, 838. https://doi.org/10.3390/vetsci12090838
Miglio A, Barbetta A, Cremonini V, Barbato O, Ricci G, Toppi V, Avellini L, Cavani V, Antognoni MT. Time-Dependent Changes in Malondialdehyde and Free-Hemoglobin in Leukoreduced and Non-Leukoreduced Canine Packed Red Blood Cells Units During Storage. Veterinary Sciences. 2025; 12(9):838. https://doi.org/10.3390/vetsci12090838
Chicago/Turabian StyleMiglio, Arianna, Aurora Barbetta, Valentina Cremonini, Olimpia Barbato, Giovanni Ricci, Valeria Toppi, Luca Avellini, Valentina Cavani, and Maria Teresa Antognoni. 2025. "Time-Dependent Changes in Malondialdehyde and Free-Hemoglobin in Leukoreduced and Non-Leukoreduced Canine Packed Red Blood Cells Units During Storage" Veterinary Sciences 12, no. 9: 838. https://doi.org/10.3390/vetsci12090838
APA StyleMiglio, A., Barbetta, A., Cremonini, V., Barbato, O., Ricci, G., Toppi, V., Avellini, L., Cavani, V., & Antognoni, M. T. (2025). Time-Dependent Changes in Malondialdehyde and Free-Hemoglobin in Leukoreduced and Non-Leukoreduced Canine Packed Red Blood Cells Units During Storage. Veterinary Sciences, 12(9), 838. https://doi.org/10.3390/vetsci12090838






