Epidemiological Investigation of Infectious Diseases at the Domestic–Synanthropic–Wild Animal Interface Reveals Threats to Endangered Species Reintroduction in AlUla, Saudi Arabia
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
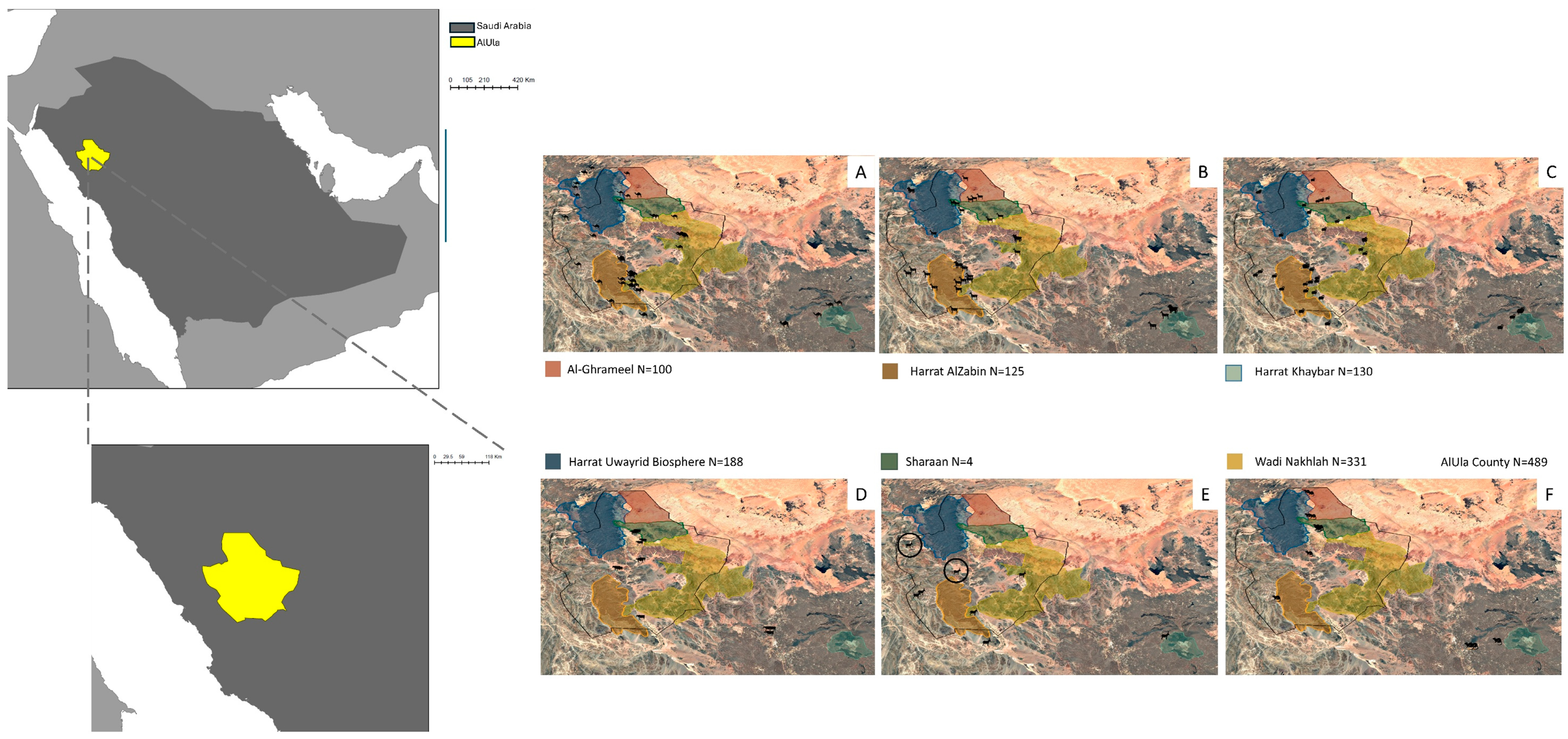
2.1. Study Settings
2.2. Expert Review and Disease Prioritization
2.3. Sample Collection
2.4. Molecular and Serological Analyses
2.5. Statistical Analysis
3. Results
3.1. Animal Populations Management and Nomadic Nature
3.2. Prioritization of Infectious Diseases
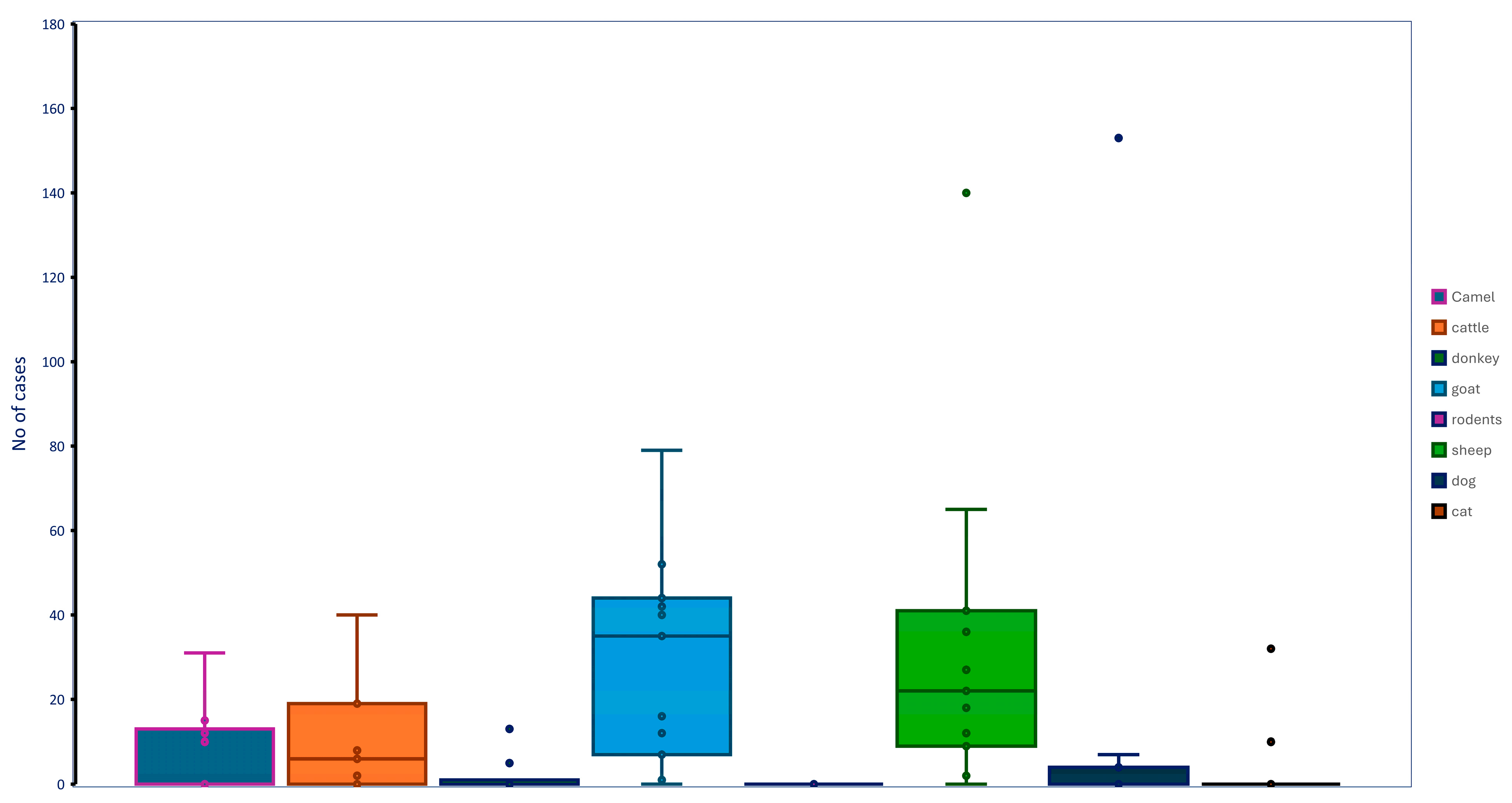
3.3. Prevalence of Infectious Diseases
3.4. Determinants of Prioritized Diseases
3.5. Spatial Distribution of Prioritized Diseases
3.6. Univariate Space–Time Scan-Significant Clusters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Garine-Wichatitsky, M.; Miguel, E.; Kock, R.; Valls-Fox, H.; Caron, A. The ecology of pathogens transmission at the wildlife-livestock interface: Beyond disease ecology, towards socio-ecological system health. In Diseases at the Wildlife-Livestock Interface: Research and Perspectives in a Changing World; Springer International Publishing: Cham, Germany, 2021; pp. 91–119. [Google Scholar]
- Fong, I.W. Animals and mechanisms of disease transmission. In Emerging Zoonoses: A Worldwide Perspective; Springer: Cham, Germany, 2017; pp. 15–38. [Google Scholar]
- Cook, R.A.; Karesh, W.B. Emerging diseases at the interface of people, domestic animals, and wildlife. Fowler’s Zoo Wild Ani Med. 2011, 22, 136. [Google Scholar]
- Mohamed, W.F. Environmental effects of the feral donkey Equus asinus (Linnaeus, 1758) in Al-Ula governorate, western Saudi Arabia. KUWAIT J. SCI. 2022, 2, 49. [Google Scholar]
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulsen, J.R.; Stevens, M.H.; White, J.S. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 2009, 24, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Soultan, A.; Darwish, M.; Al-Johani, N.; Abdulkareem, A.; Alfaifi, Y.; Assaeed, A.M.; El-Bana, M.; Browne, S. Feral Donkey Distribution and Ecological Impacts in a Hyper-Arid Region. Animals 2023, 13, 2885. [Google Scholar] [CrossRef]
- Jansen, J.; McGregor, H.; Axford, G.; Dean, A.T.; Comte, S.; Johnson, C.N.; Moseby, K.E.; Brandle, R.; Peacock, D.E.; Jones, M.E. Long-distance movements of feral cats in semi-arid South Australia and implications for conservation management. Animals 2021, 11, 3125. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Best Practices in Ranking Emerging Infectious Disease Threats; ECDC: Stockholm, Sweden, 2015; Available online: https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/emerging-infectious-disease-threats-best-practices-ranking.pdf (accessed on 7 July 2025).
- Wang, X.; Ji, X. Sample size estimation in clinical research: From randomized controlled trials to observational studies. Chest 2020, 158, 12–20. [Google Scholar] [CrossRef]
- Dal, T.; Kara, S.S.; Cikman, A.; Balkan, C.E.; Acıkgoz, Z.C.; Zeybek, H.; Uslu, H.; Durmaz, R. Comparison of multiplex real-time polymerase chain reaction with serological tests and culture for diagnosing human brucellosis. J. Infect. Public Health 2019, 12, 337–342. [Google Scholar] [CrossRef]
- Albini, S.; Brodard, I.; Jaussi, A.; Wollschlaeger, N.; Frey, J.; Miserez, R.; Abril, C. Real-time multiplex PCR assays for reliable detection of Clostridium perfringens toxin genes in animal isolates. Vet. Microbiol. 2008, 127, 179–185. [Google Scholar] [CrossRef]
- Settypalli, T.B.; Lamien, C.E.; Spergser, J.; Lelenta, M.; Wade, A.; Gelaye, E.; Loitsch, A.; Minoungou, G.; Thiaucourt, F.; Diallo, A. One-step multiplex RT-qPCR assay for the detection of Peste des petits ruminant’s virus, Capripoxvirus, Pasteurella multocida and Mycoplasma capricolum subspecies (ssp.) capripneumoniae. PLoS ONE 2016, 11, e0153688. [Google Scholar] [CrossRef]
- Dykema, P.E.; Stokes, K.D.; Beckwith, N.R.; Mungin, J.W.; Xu, L.; Vickers, D.J.; Reising, M.M.; Bravo, D.M.; Thomsen, B.V.; Robbe-Austerman, S. Development and validation of a direct real-time PCR assay for Mycobacterium bovis and implementation into the United States national surveillance program. PeerJ/PrePrints 2016, 4, e1703v1. [Google Scholar]
- Klee, S.R.; Tyczka, J.; Ellerbrok, H.; Franz, T.; Linke, S.; Baljer, G.; Appel, B. Highly sensitive real-time PCR for specific detection and quantification of Coxiella burnetii. BMC Microbiol. 2006, 6, 2. [Google Scholar] [CrossRef]
- Pantchev, A.; Sting, R.; Bauerfeind, R.; Tyczka, J.; Sachse, K. Detection of all Chlamydophila and Chlamydia spp. of veterinary interest using species-specific real-time PCR assays. Comp. Immunol. Microbiol. Infect. Dis. 2010, 33, 473–484. [Google Scholar] [CrossRef]
- Callahan, J.D.; Brown, F.; Osorio, F.A.; Sur, J.H.; Kramer, E.; Long, G.W.; Lubroth, J.; Ellis, S.J.; Shoulars, K.S.; Gaffney, K.L.; et al. Use of a portable real-time reverse transcriptase polymerase chain reaction assay for rapid detection of foot-and-mouth disease virus. J. Am. Vet. Med. Assoc. 2002, 220, 1636–1642. [Google Scholar] [CrossRef]
- Lin, M.H.; Chen, T.C.; Kuo, T.T.; Tseng, C.C.; Tseng, C.P. Real-time PCR for quantitative detection of Toxoplasma gondii. J. Clin. Microbiol. 2000, 38, 4121–4125. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Behera, B.K.; Padhi, S.K.; Sahoo, S.; Sahoo, N.; Biswal, S.; Sahoo, P. Comparative evaluation of diagnostic methods for detection of Theileria spp. in cows. Anim. Biotechnol. 2023, 34, 3514–3518. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Delmelle, E. Space-time cluster detection techniques for infectious diseases: A systematic review. Spat. Spatio-Temporal 2023, 44, 100563. [Google Scholar] [CrossRef]
- Alhumaid, N.K.; Alajmi, A.M.; Alosaimi, N.F.; Alotaibi, M.; Almangour, T.A.; Nassar, M.S.; Memish, Z.A.; Binjomah, A.Z.; Al-Jedai, A.; Almutairi, A.S.; et al. Epidemiology of reportable bacterial infectious diseases in Saudi Arabia. Infect. Dis. Ther. 2024, 13, 667–684. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. ECDC Tool for the Prioritization of Infectious Disease Threats; ECDC: Stockholm, Sweden, 2017; Available online: https://ecdc.europa.eu/en/publications-data/ecdc-tool-prioritisation-infectious-disease-threats (accessed on 7 July 2025).
- Devanathan, N.; Mukhopadhyay, H.K.; Sihag, K.K.; Nathan, A.T.; Chakkaravarthi, A.; Srinivasan, L.; Srinivas, M.V.; Vasu, J.; Shanmugam, V.P.; Rahi, M.; et al. Synanthropic rodents and shrews are reservoirs of zoonotic bacterial pathogens and act as sentinels for antimicrobial resistance spillover in the environment: A study from Puducherry, India. One Health 2024, 18, 100759. [Google Scholar] [CrossRef]
- Perera, J.M.; Gurtler, C.; Barnes, A.N. A Systematic Review of Zoonotic Enteric Parasites in Synanthropic Mammalian Species in Florida. Pathogens 2024, 13, 1065. [Google Scholar] [CrossRef]
- McFarlane, R.O.; Sleigh, A.; McMichael, T. Synanthropy of wild mammals as a determinant of emerging infectious diseases in the Asian–Australasian region. EcoHealth 2012, 9, 24–35. [Google Scholar] [CrossRef]
- Omer, S.A.; Babiker, S.E.; Aljulaifi, M.Z.; Al-Olayan, E.M.; Alagaili, A.N.; Mohammed, O.B. Epidemiology of Enterotoxemia in livestock in the Kingdom of Saudi Arabia. J. King Saud Univ. Sci. 2020, 32, 2662–2668. [Google Scholar] [CrossRef]
- Mohamed, K. Toxoplasmosis in humans and animals in Saudi Arabia: A systematic review. J. Infect. Dev. Ctries. 2020, 14, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Hemida, M.G.; Alghadeer, H.M.; Alhammadi, M.; Ali, S. Prevalence and molecular characterization of some circulating strains of the peste-des-petits-ruminants virus in Saudi Arabia between 2014–2016. PeerJ 2020, 27, e9035. [Google Scholar] [CrossRef] [PubMed]
- Dhar, P.; Sreenivasa, B.P.; Barrett, T.; Corteyn, M.; Singh, R.P.; Bandyopadhyay, S.K. Recent epidemiology of peste des petits ruminant’s virus (PPRV). Vet. Microbiol. 2002, 88, 153–159. [Google Scholar] [CrossRef]
- Elsohaby, I.; Elmoslemany, A.; El-Sharnouby, M.; Alkafafy, M.; Alorabi, M.; El-Deeb, W.M.; Al-Marri, T.; Qasim, I.; Alaql, F.A.; Fayez, M. Flock management risk factors associated with Q fever infection in sheep in Saudi Arabia. Animals 2021, 11, 1948. [Google Scholar] [CrossRef]
- Baroom, H.M.; Alkenani, N.A.; Al-Johny, B.O.; Almohimeed, A.A.; Mohammed, M.S.; Alshehri, L.A.; Althobaiti, S.S.; Omar, R.I.; Alshaeri, M.A.; Al-Mmaqar, S.M. Molecular detection of Coxiella burnetii infection (Q fever) in livestock in Makkah Province, Saudi Arabia. Z. Naturforsch. C 2024, 24, 275–284. [Google Scholar] [CrossRef]
- Rogers, D.J.; Randolph, S.E. Studying the global distribution of infectious diseases using GIS and RS. Nat. Rev. Microbiol. 2003, 1, 231–237. [Google Scholar] [CrossRef]
- Hohl, A.; Delmelle, E.M.; Desjardins, M.R.; Lan, Y. Daily surveillance of COVID-19 using the prospective space-time scan statistic in the United States. Spat. Spatio-Temporal 2020, 34, 100354. [Google Scholar] [CrossRef]
- Whiteman, A.; Desjardins, M.R.; Eskildsen, G.A.; Loaiza, J.R. Detecting space-time clusters of dengue fever in Panama after adjusting for vector surveillance data. PLoS Negl. Trop. Dis. 2019, 13, e0007266. [Google Scholar] [CrossRef]


| Disease | Primer and Probe Sequences | Annealing Temperature (°C) | qPCR Confirmation | Reference |
|---|---|---|---|---|
| Brucellosis (Brucella spp.) | F: GCTCGGTTGCCAATATCAATGC R: GGGTAAAGCGTCGCCAGAAG P: FAM-AAATCTTCCACCTTGCCCTTGCCATCA-TAMRA | 60 | Yes | [10] |
| Enterotoxemia (Clostridium perfringens) | F: AAGAACTAGTAGCTTACATATCAACTAGTGGTG R: TTTCCTGGGTTGTC-CATTTCC P: VIC-TTGGAATCAAAACAAAGGATGGAAAAACTCAAG-TAMRA | 54 | Yes | [11] |
| Hemorrhagic septicemia (Pasteurella multocida) | F: CAGAGTTTGGTGTGTTGA R: CAGACTGACAAGGAAATATAAAC P: FAM-AATCTGCTTCCTTGAC-BHQ1 | 52 | Yes | [12] |
| Tuberculosis (Mycobacterium bovis) | F: GGCTGCTCTCGACGTTCATC R: CGCTGATTGGACCGCTCAT P: FAM5′-CTGAAGCCGACGCCCTGTGC-BHQ | 55 | No | [13] |
| Contagious Caprine Pleuropneumonia (Mycoplasma capricolum subsp. Capripneumoniae) | F: TTTTTCAAGTGCAAACGACTATG R: TGACTTGGGTGTTAGGACCA P: Cy5-CGGATAGAACAATAGCTTTTACAGA-BHQ | 52 | No | [11] |
| Q fever (Coxiella burnetii) | F: CGTTATTTTACGGGTGTGCCA R: CAGAATTTTCGCGGAAAATCA P: FAM-CATATTCACCTTTTCAGGCGTTTTGACCGT-TAMRA | 52 | Yes | [14] |
| Chlamydiosis (Chlamydia psittaci) | F: CACTATGTGGGAAGGTGCTTCA R: CTGCGCGGATGCTAATGG P: FAM-CGCTACTTGGTGTGAC–TAMRA 1 | 50 | Yes | [15] |
| Chlamydiosis (Chlamydia Abortus) | F: GCAACTGACACTAAGTCGGCTACA R: ACAAGCATGTTCAATCGATAAGAGA P: FAM-TAAATACCACGAATGGCAAGTTGGTTTAGCG–TAMRA 1 | 50 | Yes | [15] |
| Peste des petits ruminants | F: CCATCAYTACCCGTTCAAG R: ATYCGCTGKATCARTTGC P: HEX-GIGACTCYACGAACA-BHQ1 | 47 | No | [12] |
| Foot and Mouth Disease | F: ACTGGGTTTTACAAACCTGTGA R: GCGAGTCCTGCCACGGA P: FAM-TCCTTTGCACGCCGTGGGAC-TAMRA | 60 | No | [16] |
| Toxoplasmosis (Toxoplasma gondii) | F: TCCCCTCTGCTGGCGAAAAGT R: AGCGTTCGTGGTCAACTATCGATTG P: FAM-TCTGTGCAACTTTGGTGTATTCGCAG-TAMRA | 50 | Yes | [17] |
| Theileriosis (Theileria annulata) | F: AGACCTTAACCTGCTAAATAGG R: CATCACAGACCTGTTATTGC P: FAM-AAGTTTCTACTGTCCCGTT-BHQ 2 | 50 | Yes | [18] |
| Theileriosis (Theileria orientalis) | F: GGAAACCAAGGATCTCGATG R: GAATGGTCCGACGAAGTCAT P: JOE-TTGCAGAGGCAGGTCTTTTT-BHQ 2 | 50 | Yes | [18] |
| Animal Diseases and Number of Samples Investigated | qPCR | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Animals Detected Positive | |||||||||||
| Diseases | Processed Samples | Camels N = 254 | Cattle N = 90 | Donkey N = 111 | Goat N = 290 | Rodents N = 123 | Sheep N = 294 | Dog N = 168 | Cat N = 37 | Overall % Prevalence | p-Value |
| Theileriosis | 934 | 0 1 | 40 | 0 | 0 | 0 | 27 | 4 | 0 | 7.59 | ≤0.01 |
| Enterotoxemia | 1032 | 15 | 8 | 13 | 40 | 0 | 22 | 153 | 32 | 27.42 | ≤0.01 |
| Hemorrhagic septicemia | 876 | 31 | 6 | 1 | 79 | 1 | 140 | – 5 | – | 29.45 | ≤0.01 |
| Chlamydiosis | 609 | 8 | 0 | 0 | 11 | 0 | 11 | 0 | 0 | 4.9 | 0.119 |
| Brucellosis | 605 | 0 | 0 | 0 | 9 | 0 | 4 | 0 | – | 2.11 | 0.06 |
| Q fever | 656 | 1 | 1 | 0 | 15 | 0 | 7 | 0 | 0 | 3.66 | 0.016 |
| ELISA | |||||||||||
| PPR 2 | 364 | 0 | – | – | 44 | – | 65 | 4 | – | 31.04 | ≤0.01 |
| Chlamydiosis | 360 | 4 | 19 | – | 5 | – | 1 | – | – | 8.06 | ≤0.01 |
| CCPP 3 | 352 | 0 | – | – | 12 | – | 0 | – | – | 3.41 | ≤0.01 |
| Brucellosis | 460 | – | 2 | – | 43 | – | 37 | – | – | 17.83 | ≤0.01 |
| FMD 4 | 360 | – | 19 | – | 35 | – | 36 | – | – | 25.00 | 0.99 |
| Q fever | 184 | 9 | 2 | – | 27 | – | 11 | – | – | 26.63 | ≤0.01 |
| Toxoplasmosis | 484 | 13 | 7 | 5 | 1 | 0 | 2 | 7 | 10 | 9.30 | 0.003 |
| Tuberculosis | 484 | 0 | 0 | 0 | 7 | 0 | 9 | 0 | 0 | 3.31 | ≤0.01 |
| Diseases | Sex | Husbandry | |||||||
|---|---|---|---|---|---|---|---|---|---|
| qPCR | |||||||||
| Female | Male | p Value | Farms | Free Grazing | Grazing in Defined Areas | In Pen | ND * | p Value | |
| Theileriosis | 61 | 10 | 0.327 | 40 | 0 | 21 | 3 | 7 | ≤0.01 |
| Enterotoxemia | 188 | 95 | 0.030 | 10 | 0 | 52 | 18 | 203 | 0.44 |
| Hemorrhagic septicemia | 239 | 19 | <0.01 | 24 | 0 | 220 | 11 | 3 | ≤0.01 |
| Chlamydiosis | 30 | 0 | 1 | 0 | 0 | 30 | 0 | 0 | 0.001 |
| Brucellosis | 13 | - | 1 | 1 | 0 | 12 | 0 | 0 | 0.59 |
| Q fever | 24 | 0 | 1 | 1 | 0 | 23 | 0 | 0 | 0.06 |
| ELISA | |||||||||
| PPR 1 | 98 | 15 | 0.499 | 5 | 0 | 88 | 8 | 12 | 0.93 |
| Chlamydiosis | 27 | 2 | 0.281 | 22 | 0 | 6 | 0 | 1 | ≤0.01 |
| CCPP 2 | 12 | 0 | 0.225 | 0 | 0 | 12 | 0 | 0 | 0.68 |
| Brucellosis | 74 | 8 | 1 | 6 | 0 | 61 | 0 | 15 | ≤0.01 |
| FMD 3 | 86 | 4 | 0.029 | 23 | 0 | 58 | 9 | 0 | 0.07 |
| Q fever | 42 | 7 | 0.186 | 5 | 0 | 37 | 3 | 4 | ≤0.01 |
| Toxoplasmosis | 34 | 11 | 0.822 | 7 | 0 | 15 | 6 | 17 | 0.62 |
| Tuberculosis | 13 | 3 | 0.532 | 1 | 0 | 11 | 2 | 2 | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljasir, S.F.; Draz, A.A.; Aslam, B.; Aljohani, A.S.M.; Sadan, M.; Al-Johani, N.; Elbehiry, A.; Al Abdulmonem, W.; Aldubaib, M.; Aldurubi, B.; et al. Epidemiological Investigation of Infectious Diseases at the Domestic–Synanthropic–Wild Animal Interface Reveals Threats to Endangered Species Reintroduction in AlUla, Saudi Arabia. Vet. Sci. 2025, 12, 836. https://doi.org/10.3390/vetsci12090836
Aljasir SF, Draz AA, Aslam B, Aljohani ASM, Sadan M, Al-Johani N, Elbehiry A, Al Abdulmonem W, Aldubaib M, Aldurubi B, et al. Epidemiological Investigation of Infectious Diseases at the Domestic–Synanthropic–Wild Animal Interface Reveals Threats to Endangered Species Reintroduction in AlUla, Saudi Arabia. Veterinary Sciences. 2025; 12(9):836. https://doi.org/10.3390/vetsci12090836
Chicago/Turabian StyleAljasir, Sulaiman F., Abdelmaged A. Draz, Bilal Aslam, Abdullah S. M. Aljohani, Madeh Sadan, Nawaf Al-Johani, Ayman Elbehiry, Waleed Al Abdulmonem, Musaad Aldubaib, Basheer Aldurubi, and et al. 2025. "Epidemiological Investigation of Infectious Diseases at the Domestic–Synanthropic–Wild Animal Interface Reveals Threats to Endangered Species Reintroduction in AlUla, Saudi Arabia" Veterinary Sciences 12, no. 9: 836. https://doi.org/10.3390/vetsci12090836
APA StyleAljasir, S. F., Draz, A. A., Aslam, B., Aljohani, A. S. M., Sadan, M., Al-Johani, N., Elbehiry, A., Al Abdulmonem, W., Aldubaib, M., Aldurubi, B., Alyahya, A. M., Alduhami, A., Aljaralh, A., Alkhamis, M. A., Chandler, J. C., Bisha, B., & Mohammed, O. B. (2025). Epidemiological Investigation of Infectious Diseases at the Domestic–Synanthropic–Wild Animal Interface Reveals Threats to Endangered Species Reintroduction in AlUla, Saudi Arabia. Veterinary Sciences, 12(9), 836. https://doi.org/10.3390/vetsci12090836










