Molecular Detection and Prevalence of Equine Piroplasmosis and Other Blood Parasites in Equids of Western Aegean Türkiye
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Study Area
2.2. Detection of T. equi, B. caballi, A. phagocytophilum, Trypanosoma spp., and Leishmania spp.
2.3. Sequencing of Amplified PCR Products
2.4. Statistical Analysis
2.5. Ethics Statement
3. Results
3.1. Prevalence of T. equi, B. caballi, A. phagocytophilum, Trypanosoma spp. and Leishmania spp.
3.2. Sequence Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EP | Equine piroplasmosis |
| EGA | Equine granulocytic anaplasmosis |
| ECL | Equine cutaneous leishmaniasis |
| PCR | Polymerase Chain Reaction |
| n-PCR | Nested Polymerase Chain Reaction |
| DNA | Deoxyribonucleic acid |
References
- Ramadan, R.M.; Taha, N.M.; Auda, H.M.; Elsamman, E.M.; El-Bahy, M.M.; Salem, M.A. Molecular and immunological studies on Theileria equi and its vector in Egypt. Exp. Appl. Acarol. 2024, 93, 439–458. [Google Scholar] [CrossRef]
- Yilmaz, O.; Wilson, R.T. The domestic livestock resources of Turkey: Occurrence and control of diseases of horses, donkeys and mules. J. Equine Vet. Sci. 2013, 33, 1021–1030. [Google Scholar] [CrossRef]
- Çırak, V.Y.; Girişgin, A.O. Parasites of horses, donkeys and mules in Turkey. Türkiye Parazitol. Derg. 2021, 45, 56–75. [Google Scholar] [CrossRef]
- Brun, R.; Hecker, H.; Lun, Z.-R. Trypanosoma evansi and T. equiperdum: Distribution, biology, treatment and phylogenetic relationship (a review). Vet. Parasitol. 1998, 79, 95–107. [Google Scholar] [CrossRef]
- Limeira, C.H.; Alves, C.J.; Azevedo, S.S.; Santos, C.S.A.B.; Melo, M.A.; Soares, R.R.; Barnabé, N.N.D.C.; Rodrigues, G.Q. Clinical aspects and diagnosis of leishmaniasis in equids: A systematic review and meta-analysis. Rev. Bras. Parasitol. Vet. 2019, 28, 574–581. [Google Scholar] [CrossRef]
- Sazmand, A.; Bahari, A.; Papi, S.; Otranto, D. Parasitic diseases of equids in Iran (1931–2020): A literature review. Parasites Vectors 2020, 13, 568. [Google Scholar] [CrossRef]
- Carbonara, M.; Mendoza-Roldan, J.A.; Bezerra-Santos, M.A.; de Abreu Teles, P.P.; Lia, R.P.; Locantore, F.; Iatta, R.; Volf, P.; Otranto, D. Leishmania spp. in equids and their potential vectors in endemic areas of canine leishmaniasis. PLoS Neglected Trop. Dis. 2024, 18, e0012290. [Google Scholar] [CrossRef]
- Tirosh-Levy, S.; Gottlieb, Y.; Fry, L.M.; Knowles, D.P.; Steinman, A. Twenty years of equine piroplasmosis research: Global distribution, molecular diagnosis, and phylogeny. Pathogens 2020, 9, 926. [Google Scholar] [CrossRef]
- Wise, L.N.; Kappmeyer, L.S.; Mealey, R.H.; Knowles, D.P. Review of equine piroplasmosis. J. Vet. Intern. Med. 2013, 27, 1334–1346. [Google Scholar] [CrossRef]
- Scoles, G.A.; Ueti, M.W. Vector ecology of equine piroplasmosis. Annu. Rev. Entomol. 2015, 60, 561–580. [Google Scholar] [CrossRef]
- Atabek, B.; Zhyldyz, A.; Aitakin, K.; Rysbek, N.; Jailobek, O.; Ahedor, B.; Mumbi, N.N.M.; Ma, Y.; Otgonsuren, D.; Perera, W.P.P.S.I.; et al. Molecular prevalence and genotypic diversity of Theileria equi and Babesia caballi infecting horses in Kyrgyzstan. Parasitol. Int. 2024, 102, 102915. [Google Scholar] [CrossRef]
- Ahedor, B.; Sivakumar, T.; Valinotti, M.F.R.; Otgonsuren, D.; Yokoyama, N.; Acosta, T.J. PCR detection of Theileria equi and Babesia caballi in apparently healthy horses in Paraguay. Vet. Parasitol. Reg. Stud. Rep. 2023, 39, 100835. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Moumouni, P.F.A.; Lee, S.H.; Galon, E.M.; Tumwebaze, M.A.; Yang, H.; Huercha; Liu, M.; Guo, H.; et al. First description of Coxiella burnetii and Rickettsia spp. infection and molecular detection of piroplasma co-infecting horses in Xinjiang Uygur Autonomous Region, China. Parasitol. Int. 2020, 76, 102028. [Google Scholar] [CrossRef]
- Otgonsuren, D.; Amgalanbaatar, T.; Narantsatsral, S.; Enkhtaivan, B.; Munkhgerel, D.; Zoljargal, M.; Davkharbayar, B.; Myagmarsuren, P.; Battur, B.; Battsetseg, B.; et al. Epidemiology and genetic diversity of Theileria equi and Babesia caballi in Mongolian horses. Infect. Genet. Evol. 2024, 119, 105571. [Google Scholar] [CrossRef]
- Saleem, S.; Ijaz, M.; Farooqi, S.H.; Ghaffar, A.; Ali, A.; Iqbal, K.; Mehmood, K.; Zhang, H. Equine granulocytic anaplasmosis 28 years later. Microb. Pathog. 2018, 119, 1–8. [Google Scholar] [CrossRef]
- Dzięgiel, B.; Adaszek, Ł.; Kalinowski, M.; Winiarczyk, S. Equine granulocytic anaplasmosis. Res. Vet. Sci. 2013, 95, 316–320. [Google Scholar] [CrossRef]
- Praskova, I.; Bezdekova, B.; Zeman, P.; Jahn, P. Seroprevalence of Anaplasma phagocytophilum in horses in the Czech Republic. Ticks Tick-Borne Dis. 2011, 2, 111–115. [Google Scholar] [CrossRef]
- Madigan, J.E.; Barlough, J.E.; Dumler, J.S.; Schankman, N.S.; DeRock, E. Equine granulocytic ehrlichiosis in Connecticut caused by an agent resembling the human granulocytotropic ehrlichia. J. Clin. Microbiol. 1996, 34, 434–435. [Google Scholar] [CrossRef]
- Aguilar, C.M.; Fernández, E.S.; Fernandez, R.; Deane, L.M. Study of an outbreak of cutaneous leishmaniasis in Venezuela: The role of domestic animals. Mem. Do Inst. Oswaldo Cruz 1984, 79, 181–195. [Google Scholar] [CrossRef]
- Truppel, J.H.; Otomura, F.; Teodoro, U.; Massafera, R.; da Costa-Ribeiro, M.C.V.; Catarino, C.M.; Dalagrana, L.; Costa Ferreira, M.E.M.; Thomaz-Soccol, V.; Schallig, H.D.F.H. Can equids be a reservoir of Leishmania braziliensis in endemic areas? PLoS ONE 2014, 9, e93731. [Google Scholar] [CrossRef] [PubMed]
- Mhadhbi, M.; Sassi, A. Infection of the equine population by Leishmania parasites. Equine Vet. J. 2020, 52, 28–33. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Fernández-Bellon, H.; Serra, P.; Gállego, M.; Ramis, A.; Fondevila, D.; Ferrer, L. Cutaneous leishmaniosis in three horses in Spain. Equine Vet. J. 2003, 35, 320–323. [Google Scholar] [CrossRef]
- Gama, A.; Elias, J.; Ribeiro, A.J.; Alegria, N.; Schallig, H.D.; Silva, F.; Santarém, N.; Cardoso, L.; Cotovio, M. Cutaneous leishmaniosis in a horse from northern Portugal. Vet. Parasitol. 2014, 200, 189–192. [Google Scholar] [CrossRef]
- Gazzonis, A.L.; Morganti, G.; Porcellato, I.; Roccabianca, P.; Avallone, G.; Gavaudan, S.; Canonico, C.; Rigamonti, G.; Brachelente, C.; Veronesi, F. Detection of Leishmania spp. in chronic dermatitis: Retrospective study in exposed horse populations. Pathogens 2022, 11, 634. [Google Scholar] [CrossRef]
- Pala, S.; Martínez-Sáez, L.; Llobat, L.; Marín-García, P.J. Prevalence and factors associated with Leishmania spp. and Toxoplasma gondii infections in apparently healthy horses in Eastern Spain. Res. Vet. Sci. 2024, 171, 105236. [Google Scholar] [CrossRef]
- Cardoso, L.; Schallig, H.; Persichetti, M.F.; Pennisi, M.G. New epidemiological aspects of animal leishmaniosis in Europe: The role of vertebrate hosts other than dogs. Pathogens 2021, 10, 307. [Google Scholar] [CrossRef]
- Müller, N.; Welle, M.; Lobsiger, L.; Stoffel, M.H.; Boghenbor, K.K.; Hilbe, M.; Gottstein, B.; Frey, C.F.; Geyer, C.; von Bomhard, W. Occurrence of Leishmania sp. in cutaneous lesions of horses in Central Europe. Vet. Parasitol. 2009, 166, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Berlin, D.; Nasereddin, A.; Azmi, K.; Ereqat, S.; Abdeen, Z.; Baneth, G. Longitudinal study of an outbreak of Trypanosoma evansi infection in equids and dromedary camels in Israel. Vet. Parasitol. 2010, 174, 317–322. [Google Scholar] [CrossRef]
- Desquesnes, M.; Holzmuller, P.; Lai, D.H.; Dargantes, A.; Lun, Z.R.; Jittaplapong, S. Trypanosoma evansi and surra: A review and perspectives on origin, history, distribution, taxonomy, morphology, hosts, and pathogenic effects. BioMed Res. Int. 2013, 2013, 194176. [Google Scholar] [CrossRef] [PubMed]
- Desquesnes, M.; Dargantes, A.; Lai, D.-H.; Lun, Z.-R.; Holzmuller, P.; Jittapalapong, S. Trypanosoma evansi and surra: A review and perspectives on transmission, epidemiology and control, impact, and zoonotic aspects. BioMed Res. Int. 2013, 2013, 321237. [Google Scholar] [CrossRef] [PubMed]
- Jaimes-Dueñez, J.; Jiménez-Leaño, Á.; Enrique-Niño, S.; Arias-Landazábal, N.; Bedoya-Ríos, M.; Rangel-Pachón, D. Clinical and epidemiological aspects of the infection by Babesia, Theileria and Trypanosoma species in horses from northeastern Colombia. Ticks Tick-Borne Dis. 2023, 14, 102208. [Google Scholar] [CrossRef]
- Desquesnes, M.; Gonzatti, M.; Sazmand, A.; Thévenon, S.; Bossard, G.; Boulangé, A.; Gimonneau, G.; Truc, P.; Herder, S.; Ravel, S.; et al. A review on the diagnosis of animal trypanosomoses. Parasites Vectors 2022, 15, 64. [Google Scholar] [CrossRef]
- Golombieski, L.; Bassi das Neves, G.; da Silva Casa, M.; Campos de Souza Costa, G.; Miletti, L.C.; Saito, M.E.; Fonteque, J.H. Prevalence and risk factors associated with natural infection by Trypanosoma evansi in Campeiro horses. J. Equine Vet. Sci. 2023, 126, 104300. [Google Scholar] [CrossRef] [PubMed]
- Shoraba, M.; Shoulah, S.A.; Arnaout, F.; Selim, A.; El-Magd, M. Equine trypanosomiasis: Molecular detection, hematological, and oxidative stress profiling. Vet. Med. Int. 2024, 2024, 6550276. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, O.; Benedicto, B.; Ceylan, C.; Tumwebaze, M.; Galon, E.M.; Liu, M.; Xuan, X.; Sevinc, F. A survey on equine tick-borne diseases: The molecular detection of Babesia ovis DNA in Turkish racehorses. Ticks Tick-Borne Dis. 2021, 12, 101784. [Google Scholar] [CrossRef]
- Gün Aydin, E.; Pekkaya, S.; Kuzugüden, F.; Zeybek, M.; Güven Gökmen, T.; Ütük, A.E. The first detection of anti-Anaplasma phagocytophilum antibodies in horses in Turkey. Kafkas Univ. Vet. Fak. Derg. 2018, 24, 867–871. [Google Scholar] [CrossRef]
- Oğuz, B. First molecular detection and phylogenetic analysis of Anaplasma phagocytophilum in horses in Muş Province of Turkey. Kocaeli Univ. Sağlık Bilim. Derg. 2021, 7, 312–318. [Google Scholar] [CrossRef]
- Dean, A.; Sullivan, K.; Soe, M. OpenEpi: Open-source epidemiologic statistics for public health. 2013. Available online: https://www.openepi.com/Menu/OE_Menu.htm (accessed on 11 August 2025).
- Alhassan, A.; Pumidonming, W.; Okamura, M.; Hirata, H.; Battsetseg, B.; Fujisaki, K.; Yokoyama, N.; Igarashi, I. Development of a single-round and multiplex PCR method for the simultaneous detection of Babesia caballi and Babesia equi in horse blood. Vet. Parasitol. 2005, 129, 43–49. [Google Scholar] [CrossRef]
- Schwint, O.N.; Knowles, D.P.; Ueti, M.W.; Kappmeyer, L.S.; Scoles, G.A. Transmission of Babesia caballi by Dermacentor nitens (Acari: Ixodidae) is restricted to one generation in the absence of alimentary reinfection on a susceptible equine host. J. Med. Entomol. 2008, 45, 1152–1155. [Google Scholar] [CrossRef] [PubMed]
- Massung, R.F.; Slater, K.; Owens, J.H.; Nicholson, W.L.; Mather, T.N.; Solberg, V.B.; Olson, J.G. Nested PCR assay for detection of granulocytic ehrlichiae. J. Clin. Microbiol. 1998, 36, 1090–1095. [Google Scholar] [CrossRef]
- Desquesnes, M.; McLaughlin, G.; Zoungrana, A.; Dávila, A.M. Detection and identification of Trypanosoma of African livestock through a single PCR based on internal transcribed spacer 1 of rDNA. Int. J. Parasitol. 2001, 31, 610–614. [Google Scholar] [CrossRef]
- Le Fichoux, Y.; Schwenkenbecher, J.; Goralczyk, K.; Marty, P.; Boni, M.; Rioux, J.A.; Pratlong, F. Occurrence of Leishmania infantum parasitemia in asymptomatic blood donors living in an area of endemicity in southern France. J. Clin. Microbiol. 1999, 37, 1953–1957. [Google Scholar] [CrossRef]
- Divan Baldani, C.; Cristina, A.; Nakaghi, H.O.; Rosangela, S.; Machado, Z. Occurrence of Theileria equi in horses raised in the Jaboticabal microregion, São Paulo State, Brazil. Rev. Bras. Parasitol. Vet. 2011, 20, 44–47. [Google Scholar] [CrossRef]
- Sevinc, F.; Maden, M.; Kumas, C.; Sevinc, M.; Ekici, O.D. A comparative study on the prevalence of Theileria equi and Babesia caballi infections in horse sub-populations in Turkey. Vet. Parasitol. 2008, 156, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Friedhoff, K. Transmission of Babesia. In Babesiosis of Domestic Animals and Man; Ristic, M., Ed.; CRC Press: Boca Raton, FL, USA, 1988; Chapter 2; pp. 23–52. [Google Scholar]
- Schein, E. Equine babesiosis. In Babesiosis of Domestic Animals and Man; Ristic, M., Ed.; CRC Press: Boca Raton, FL, USA, 1988; Chapter 12; pp. 197–208. [Google Scholar]
- Nardini, R.; Trotta, M.; Mannelli, A.; Ebani, V.V.; Rocchigiani, G.; Papini, R.; Poli, A.; Perego, R. Comparison of direct and indirect methods to maximise the detection of Babesia caballi and Theileria equi infections in Central Southern Italy. Ticks Tick Borne Dis. 2022, 13, 101939. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Agriculture and Forestry, Türkiye. Available online: https://www.tarimorman.gov.tr/GKGM/Belgeler/Veteriner%20Hizmetleri/SaglikSertifikasi/ithalat/01_ab_disi_gecici_at_sertifikasi.pdf (accessed on 10 July 2025).
- Rothschild, C.M. Equine piroplasmosis. J. Equine Vet. Sci. 2013, 33, 497–508. [Google Scholar] [CrossRef]
- Onyiche, T.E.; Suganuma, K.; Igarashi, I.; Yokoyama, N.; Xuan, X.; Thekisoe, O. A Review on equine piroplasmosis: Epidemiology, vector ecology, risk factors, host immunity, diagnosis and control. Int. J. Environ. Res. Public Health 2019, 16, 1736. [Google Scholar] [CrossRef] [PubMed]
- Ogunremi, O.; Halbert, G.; Mainar-Jaime, R.; Benjamin, J.; Pfister, K.; Lopez-Rebollar, L.; Georgiadis, M.P. Accuracy of an indirect fluorescent-antibody test and of a complement-fixation test for the diagnosis of Babesia caballi in field samples from horses. Prev. Vet. Med. 2008, 83, 41–51. [Google Scholar] [CrossRef]
- Montes Cortés, M.G.; Fernández–García, J.L.; Habela Martínez–Estéllez, M.Á. A multinested PCR for detection of the equine piroplasmids Babesia caballi and Theileria equi. Ticks Tick-Borne Dis. 2019, 10, 305–313. [Google Scholar] [CrossRef]
- Braga, M.d.S.C.d.O.; Costa, F.N.; Gomes, D.R.M.; Xavier, D.R.; André, M.R.; Gonçalves, L.R.; Freschi, C.R.; Machado, R.Z. Genetic diversity of piroplasmids species in equids from island of São Luís, northeastern Brazil. Rev. Bras. Parasitol. Vet. 2017, 26, 331–339. [Google Scholar] [CrossRef]
- Coultous, R.M.; Racz, C.; Wakeel, R.A.; Sutton, D.G.M.; Coumbe, K.; Wood, J.L.N.; Newton, J.R. Equine piroplasmosis status in the UK: An assessment of laboratory diagnostic submissions and techniques. Vet. Rec. 2019, 184, 95. [Google Scholar] [CrossRef]
- Güçlü, H.Z.; Karaer, K.Z. Ankara yöresinde sportif ve gösteri amaçlı yetiştirilen atlarda Babesia caballi (Nuttall, 1910) ve Theileria equi polimeraz zincir reaksiyonu ile araştırılması. Türkiye Parazitol. Derg. 2007, 31, 89–93. [Google Scholar]
- Kizilarslan, F.; Yildirim, A.; Duzlu, O.; Inci, A.; Onder, Z.; Ciloglu, A. Molecular detection and characterization of Theileria equi and Babesia caballi in horses (Equus ferus caballus) in Turkey. J. Equine Veter Sci. 2015, 35, 830–835. [Google Scholar] [CrossRef]
- Guven, E.; Avcioglu, H.; Deniz, A.; Balkaya, I.; Abay, U.; Yavuz, Ş.; Akyüz, M. Prevalence and molecular characterization of Theileria equi and Babesia caballi in jereed horses in Erzurum, Turkey. Acta Parasitol. 2017, 62, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Knowles, D.P.; Kappmeyer, L.S.; Haney, D.; Herndon, D.R.; Fry, L.M.; Munro, J.B.; Sears, K.; Ueti, M.W.; Wise, L.N.; Silva, M.; et al. Discovery of a novel species, Theileria haneyi n. sp., infective to equids, highlights exceptional genomic diversity within the genus Theileria: Implications for apicomplexan parasite surveillance. Int. J. Parasitol. 2018, 48, 679–690. [Google Scholar] [CrossRef]
- Bhoora, R.V.; Collins, N.E.; Schnittger, L.; Troskie, C.; Marumo, R.; Labuschagne, K.; Smith, R.M.; Dalton, D.L.; Mbizeni, S. Molecular genotyping and epidemiology of equine piroplasmids in South Africa. Ticks Tick-Borne Dis. 2020, 11, 101358. [Google Scholar] [CrossRef]
- Bhoora, R.V.; Mbaba, T.V.; Troskie, M.; Ackermann, R.E.; Collins, N.E. Quantitative detection of Theileria haneyi in South African horses. Ticks Tick-Borne Dis. 2025, 16, 102487. [Google Scholar] [CrossRef]
- Bakirci, S.; Sarali, H.; Aydin, L.; Eren, H.; Karagenc, T. Distribution and seasonal activity of tick species on cattle in the West Aegean Region of Turkey. Exp. Appl. Acarol. 2012, 56, 165–178. [Google Scholar] [CrossRef]
- Rüegg, S.R.; Torgerson, P.; Deplazes, P.; Mathis, A. Age-dependent dynamics of Theileria equi and Babesia caballi infections in southwest Mongolia based on IFAT and/or PCR prevalence data from domestic horses and ticks. Parasitology 2007, 134, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Kouam, M.K.; Kantzoura, V.; Gajadhar, A.A.; Theis, J.H.; Papadopoulos, E.; Theodoropoulos, G. Seroprevalence of equine piroplasms and host-related factors associated with infection in Greece. Vet. Parasitol. 2010, 169, 273–278. [Google Scholar] [CrossRef]
- Roberts, C.W.; Walker, W.; Alexander, J. Sex-associated hormones and immunity to protozoan parasites. Clin. Microbiol. Rev. 2001, 14, 476–488. [Google Scholar] [CrossRef]
- Sellau, J.; Hansen, C.S.; Gálvez, R.I.; Linnemann, L.; Honecker, B.; Lotter, H. Immunological clues to sex differences in parasitic diseases. Trends Parasitol. 2024, 40, 1029–1041. [Google Scholar] [CrossRef]
- Moretti, A.; Trotta, M.; D’Alterio, N.; Di Palo, M.; Greco, A.; Fioretti, D. Prevalence and diagnosis of Babesia and Theileria infections in horses in Italy: A preliminary study. Vet. J. 2010, 184, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Li, Q.; Chen, J.; Liu, H.; Zhao, Y.; Ma, R. Prevalence and genetic diversity of Theileria equi from horses in Xinjiang Uygur Autonomous region, China. Ticks Tick Borne Dis. 2023, 14, 102193. [Google Scholar] [CrossRef]
- Giubega, S.; Vlad, A.; Pet, M.; Ioniță, M.; Popescu, M.; Munteanu, A.; Mihalca, A.D. Molecular investigations of Babesia caballi from clinically healthy horses in southwestern Romania. Vet. Sci. 2024, 11, 600. [Google Scholar] [CrossRef]
- Sumbria, D.; Das Singla, L.; Sharma, A. Theileria equi and Babesia caballi infection of equids in Punjab, India: A serological and molecular survey. Trop. Anim. Health Prod. 2016, 48, 45–52. [Google Scholar] [CrossRef]
- Afridi, M.J.K.; Khan, M.N.; Ahmad, M.; Hussain, S.; Khan, A.; Muhammad, G.; Ali, Q.; Ullah, Z. Seroprevalence and risk factors for Theileria equi infection in equines from Khyber Pakhtunkhwa province, Pakistan. Iran. J. Parasitol. 2017, 12, 597–605. [Google Scholar]
- Costa, S.C.L.; Santos, H.F.R.; Costa, F.B.; Oliveira, A.M.; Barros, M.R.; Menezes, R.C.; Silva, A.G. Frequency and factors associated with Theileria equi, Babesia caballi and Trypanosoma evansi in equids from Bahia (Northeast Brazil). Rev. Bras. Parasitol. Vet. 2019, 28, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Minervino, A.H.H.; Marques, M.M.; Carvalho, W.S.; Barbosa, J.D.; Moura, J.R.; Soares, H.S.; Silva, F.M. Factors associated with the prevalence of antibodies against Theileria equi in equids of Western Pará, Brazil. Transbound. Emerg. Dis. 2020, 67 (Suppl. S2), 100–105. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar, R.; Sugimoto, C. A perspective on Theileria equi infections in donkeys. Jpn. J. Vet. Res. 2009, 56, 171–180. [Google Scholar]
- Aktas, M.; Altay, K.; Dumanli, N. Molecular detection and identification of Anaplasma and Ehrlichia species in cattle from Turkey. Ticks Tick Borne Dis. 2011, 2, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Aktas, M.; Özübek, S. Bovine anaplasmosis in Turkey: First laboratory confirmed clinical cases caused by Anaplasma phagocytophilum. Vet. Microbiol. 2015, 178, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Hoşgör, M.; Bilgiç, H.B.; Bakırcı, S.; Ünlü, A.H.; Karagenç, T.; Eren, H. Detection of Anaplasma/Ehrlichia species of cattle and ticks in Aydin Region. Türkiye Parazitol. Derg. 2015, 39, 291–298. [Google Scholar] [CrossRef]
- Altay, K.; Dumanlı, N.; Aktaş, M.; Özübek, S. Doğu Anadolu Bölgesinde koyun ve keçilerde Anaplasma enfeksiyonlarının araştırılması. Kafkas Univ. Vet. Fak. Derg. 2014, 20, 1–4. [Google Scholar] [CrossRef]
- Öter, K.; Çetinkaya, H.; Vuruşaner, C.; Toparlak, M.; Ergünay, K. Türkiye’nin Trakya bölgesindeki küçük ruminantlarda görülen Anaplasma türlerinin moleküler yöntemlerle tespiti ve tiplendirmesi. Kafkas Univ. Vet. Fak. Derg. 2016, 22, 133–138. [Google Scholar] [CrossRef]
- Zhou, M.; Cao, S.; Sevinc, F.; Sevinc, M.; Ceylan, O.; Ekici, S.; Jirapattharasate, C.; Moumouni, P.F.A.; Liu, M.; Wang, G.; et al. Molecular detection and genetic characterization of Babesia, Theileria and Anaplasma amongst apparently healthy sheep and goats in the central region of Turkey. Ticks Tick Borne Dis. 2017, 8, 246–252. [Google Scholar] [CrossRef]
- Bilgic, H.B.; Bakırcı, S.; Kose, O.; Unlu, A.H.; Hacılarlıoglu, S.; Eren, H.; Weir, W.; Karagenc, T. Prevalence of tick-borne haemoparasites in small ruminants in Turkey and diagnostic sensitivity of single-PCR and RLB. Parasites Vectors 2017, 10, 211. [Google Scholar] [CrossRef]
- Çetinkaya, H.; Matur, E.; Akyazi, İ.; Ekiz, E.E.; Aydin, L.; Toparlak, M. Serological and molecular investigation of Ehrlichia spp. and Anaplasma spp. in ticks and blood of dogs, in the Thrace Region of Turkey. Ticks Tick Borne Dis. 2016, 7, 706–714. [Google Scholar] [CrossRef]
- Yamazaki, A.; Suganuma, K.; Kayano, M.; Acosta, T.J.; Saitoh, T.; Inoue, N. Risk factors for equine trypanosomosis and hematological analysis of horses in Paraguay. Acta Tropica 2022, 233, 106543. [Google Scholar] [CrossRef]
- Ereqat, S.; Nasereddin, A.; Al-Jawabreh, A.; Al-Jawabreh, H.; Al-Laham, N.; Abdeen, Z. Prevalence of Trypanosoma evansi in livestock in Palestine. Parasit Vectors 2020, 13, 21. [Google Scholar] [CrossRef]
- Javanshir, A.; Tavassoli, M.; Esmaeilnejad, B. Morphological, serological, molecular detection, and phylogenetic analysis of Trypanosoma evansi in horses of different regions in Iran. Parasitol. Res. 2023, 122, 1873–1881. [Google Scholar] [CrossRef]
- Akhoundi, M.; Kuhls, K.; Cannet, A.; Votýpka, J.; Marty, P.; Delaunay, P.; Sereno, D. A historical overview of the classification, evolution, and dispersion of Leishmania parasites and sandflies. PLoS Negl. Trop. Dis. 2016, 10, e0004349. [Google Scholar] [CrossRef]
- Koehler, K.; Stechele, M.; Hetzel, U.; Domingo, M.; Schönian, G.; Zahner, H.; Burkhardt, E. Cutaneous leishmaniosis in a horse in southern Germany caused by Leishmania infantum. Vet. Parasitol. 2002, 109, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Karakuş, M.; Arserim, S.K.; Kasap, Ö.E.; Pekağırbaş, M.; Aküzüm, D.; Alten, B.; Toz, S.; Özbel, Y. Vector and reservoir surveillance study in a canine and human leishmaniasis endemic area in most western part of Turkey, Karaburun. Acta Trop. 2019, 190, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Bakırcı, S.; Bilgiç, H.B.; Köse, O.; Aksulu, A.; Hacılarlıoğlu, S.; Erdoğan, H.; Karagenç, T. Molecular and seroprevalence of canine visceral leishmaniasis in West Anatolia, Turkey. Turk. J. Vet. Anim. Sci. 2016, 40, 637–644. [Google Scholar] [CrossRef]
- Schäfer, I.; Silaghi, C.; Fischer, S.; Marsboom, C.; Hendrickx, G.; Gehlen, H.; Müller, E. Detection of Anaplasma phagocytophilum in horses from Germany by molecular and serological testing (2008–2021). Vet. Parasitol. 2022, 312, 109840. [Google Scholar] [CrossRef] [PubMed]
- WOAH. Terrestrial Manual, Chapter 3.1.21. Surra in All Species (Trypanosoma evansi Infection). Report of the Meeting of the WOAH Terrestrial Animal Health Standards Commission. 2021, pp. 4–5. Available online: https://www.woah.org/fileadmin/Home/fr/Health_standards/tahm/3.01.21_SURRA_TRYPANO.pdf (accessed on 15 August 2025).
- Kocan, K.M.; Blouin, E.F.; Barbet, A.F. Anaplasmosis control. Past, present, and future. Ann. N. Y. Acad. Sci. 2000, 916, 501–509. [Google Scholar] [CrossRef]
- Rubel, W.; Ganter, M.; Bauer, B.U. Detection of Anaplasma phagocytophilum in ovine serum samples—A retrospective study. Ruminants 2022, 2, 351–359. [Google Scholar] [CrossRef]
| Organisms | Genes | Primer Names | Sequences, 5′-3′ | Amplicons Size (Base Pairs) | PCR Conditions | References |
|---|---|---|---|---|---|---|
| Theileria equi | 18S rRNA | BEC-UF2 | TCG AAG ACG A TC AGA TAC CGT CG | 435 | 94 °C 50 s 55 °C 50 s 35 cycle 72 °C 1 m | [39] |
| EQUI-R | TGC CTT AAA CTT CCT TGC GAT | |||||
| Babesia caballi * | rap-1 | Bca-F | GAT TAC TTG TCG GCT GTG TCT | 222 (nested product) | 94 °C 30 s 59 °C 30 s 35 cycle 72 °C 1 m | [40] |
| Bca-R | CGC AAG TTC TCA ATG TCA G | |||||
| BcaN-F b | GCT AAG TAC CAA CCG CTG A | |||||
| BcaN-R b | CGC AAG TTC TCA ATG TCA G | |||||
| Anaplasma phagocytophilum * | 16S rRNA | ge3a | CACATGCAAGTCGAACGGATTATTC | 546 (nested product) | 94 °C 50 s 55 °C 50 s 35 cycle 72 °C 1 m | [41] |
| ge10r | TTCCGTTAAGAAGGATCTAATCTCC | |||||
| ge9f b | AACGGATTATTCTTTATAGCTTGCT | |||||
| ge2 b | GGCAGTATTAAAAGCAGCTCCAGG | |||||
| Trypanosoma spp. | ITS1 | Kin1 Kin2 | GCG TTC AAA GAT TGG GCA AT CGC CCG AAA GTT CAC C | 540 (Try. evansi) 300 (Try. vivax) | 94 °C 1 m 58 °C 1 m 4 cycle 72 °C 1 m 94 °C 1 m 56 °C 1 m 8 cycle 72 °C 1 m 94 °C 1 m 54 °C 1 m 23 cycle 72 °C 1 m | [42] |
| Leishmania spp | LT1 | RV1 | CTT TTC TGG TCC CGC GGG TAG G | 145 | 94 °C 55 s 55 °C 45 s 35 cycle 72 °C 1 m | [43] |
| RV2 | CCA CCT GGC CTA TTT TAC ACC A | |||||
| Factors | Positive Samples (No (%)/(Mean ± Standard Error of The Mean) | ||||
|---|---|---|---|---|---|
| T. equi | p Value * | B. caballi | p Value * | ||
| Age | ≥3 years | 54 (25.11%) (1.74 ± 0.43) | 19 (8.83%) (1.91 ± 0.28) | ||
| <3 years | 9 (21.43%) (1.78 ± 0.41) | 3 (7.14%) (1.92 ± 0.26) | |||
| Unknown | 33 (25.19%) (1.74 ± 0.43) | 28 (21.37%) (1.78 ± 0.41) | |||
| 0.871 *a | 0.002 * | ||||
| Sex | Female | 24 (27.27%) (1.72 ± 0.04) | 8 (9.09%) (1.90 ± 0.03) | ||
| Male | 35 (25.93%) (1.74 ± 0.03) | 10 (7.41%) (1.92 ± 0.02) | |||
| Unknown | 37 (22.42%) (1.77 ± 0.03) | 32 (19.39%) (1.80 ± 0.03) | |||
| 0.645 *b | 0.004 * | ||||
| Breed | Ambler | 27 (35.52%) (1.64 ± 0.05) | 0 (0%) (2.00 ± 0.00) | ||
| Arabian | 8 (11.43%) (1.88 ± 0.03) | 12 (17.14%) (1.82 ± 0.04) | |||
| English | 1 (2.86%) (1.97 ± 0.02) | 3 (8.57%) (1.91 ± 0,04) | |||
| Local breed | 12 (60.0%) (1.40 ± 0.11) | 4 (20.0%) (1.80 ± 0.09) | |||
| Haflinger | 0 (0%) (2.00 ± 0.00) | 0 (0%) (2.00 ± 0.00) | |||
| Pony | 2 (50%) (1.50 ± 0.28) | 1 (25%) (1.75 ± 0.25) | |||
| Donkey/Local | 9 (42.86%) (1.57 ± 0.11) | 0 (0%) (2.00 ± 0.00) | |||
| Mule/Local | 2 (11.76%) (1.88 ± 0.08) | 0 (0%) (2.00 ± 0.00) | |||
| Unknown | 35 (24.65%) (1.75 ± 0.03) | 30 (21.13%) (1.78 ± 0.03) | |||
| 0.000 * | 0.000 * | ||||
| Factors | Positive Samples (No (%)/(Mean ± Standard Error of The Mean) | ||||
|---|---|---|---|---|---|
| T. equi | p Value * | B. caballi | p Value * | ||
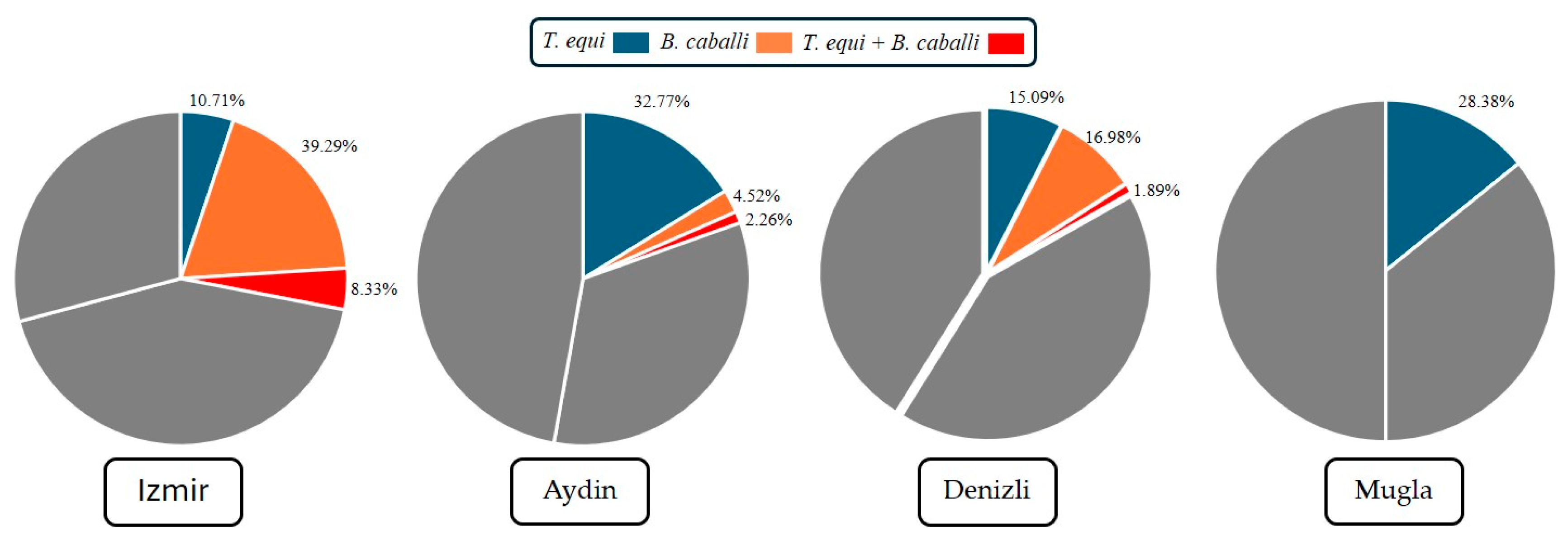
| Province | Aydın | 58 (32.77%) (1.67 ± 0.03) | 8 (4.52%) (1.95 ± 0.01) | ||
| Denizli | 8 (15.09%) (1.84 ± 0.04) | 9 (16.98%) (1.83 ± 0.05) | |||
| İzmir | 9 (10.71%) (1.89 ± 0.03) | 33 (39.29%) (1.60 ± 0.05) | |||
| Muğla | 21 (28.38%) (1.71 ± 0.05) | 0 (0%) (2.00 ± 0.00) | |||
| 0.000 * | 0.000 * | ||||
| Overall | 96 (24.74%) | 50 (12.89%) | 0.896 *c | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hacilarlioglu, S.; Bilgic, H.B.; Karagenc, T.; Aydin, H.B.; Toker, H.; Kanlioglu, H.; Pekagirbas, M.; Bakirci, S. Molecular Detection and Prevalence of Equine Piroplasmosis and Other Blood Parasites in Equids of Western Aegean Türkiye. Vet. Sci. 2025, 12, 826. https://doi.org/10.3390/vetsci12090826
Hacilarlioglu S, Bilgic HB, Karagenc T, Aydin HB, Toker H, Kanlioglu H, Pekagirbas M, Bakirci S. Molecular Detection and Prevalence of Equine Piroplasmosis and Other Blood Parasites in Equids of Western Aegean Türkiye. Veterinary Sciences. 2025; 12(9):826. https://doi.org/10.3390/vetsci12090826
Chicago/Turabian StyleHacilarlioglu, Selin, Huseyin Bilgin Bilgic, Tulin Karagenc, Heycan Berk Aydin, Hasan Toker, Hakan Kanlioglu, Metin Pekagirbas, and Serkan Bakirci. 2025. "Molecular Detection and Prevalence of Equine Piroplasmosis and Other Blood Parasites in Equids of Western Aegean Türkiye" Veterinary Sciences 12, no. 9: 826. https://doi.org/10.3390/vetsci12090826
APA StyleHacilarlioglu, S., Bilgic, H. B., Karagenc, T., Aydin, H. B., Toker, H., Kanlioglu, H., Pekagirbas, M., & Bakirci, S. (2025). Molecular Detection and Prevalence of Equine Piroplasmosis and Other Blood Parasites in Equids of Western Aegean Türkiye. Veterinary Sciences, 12(9), 826. https://doi.org/10.3390/vetsci12090826








