RNA Sequencing-Based Transcriptome Analysis of Liver in Laying Hens Supplemented with Dietary Probiotic Bacillus Species and Prebiotic Yeast (Saccharomyces cerevisiae) Cell Walls
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Birds and Dietary Treatments
2.2. Sample Collection and RNA-Seq-Based Transcriptome Analysis
2.3. Statistical Analysis
3. Results
3.1. Identification of Differentially Expressed Genes
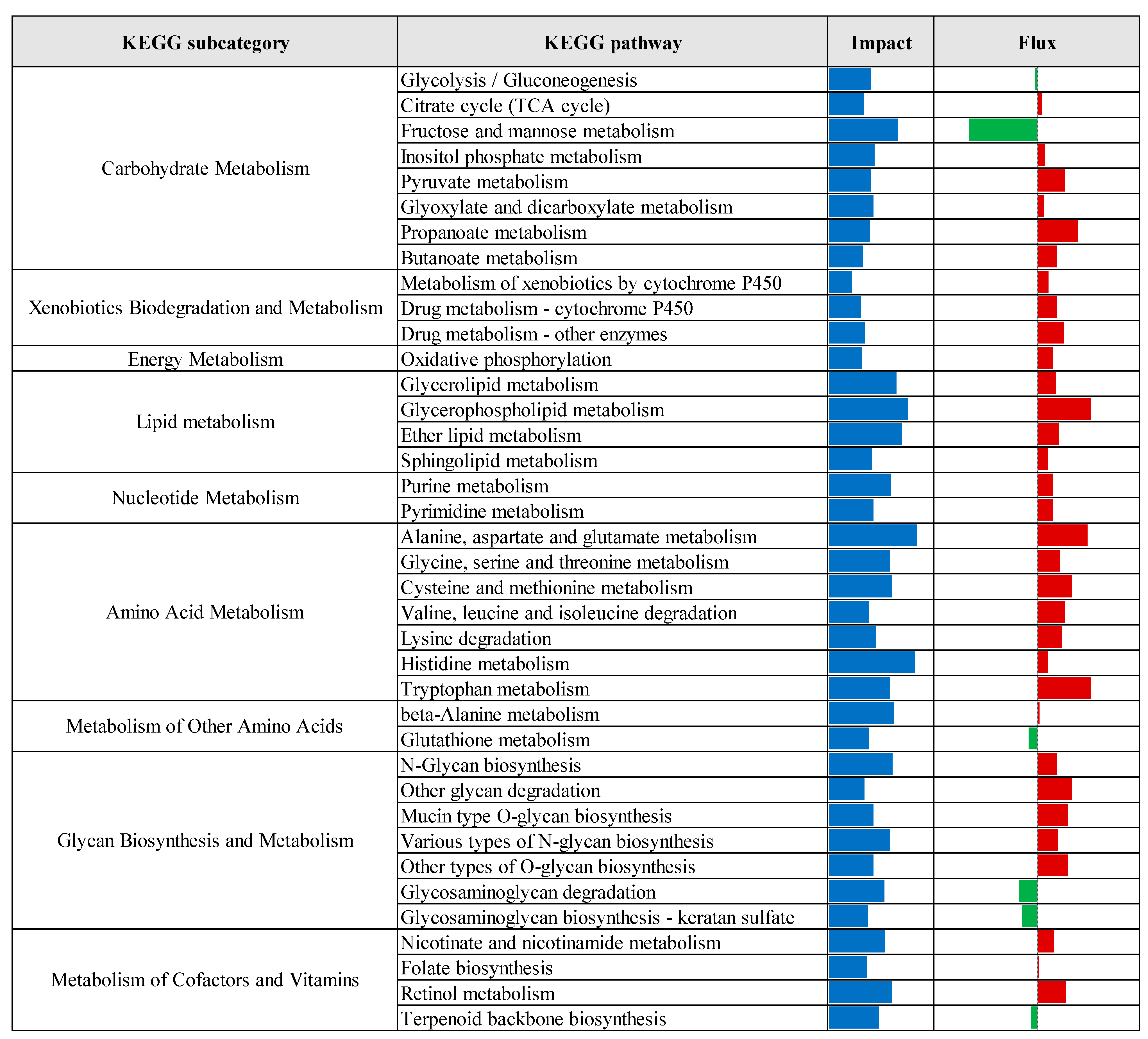
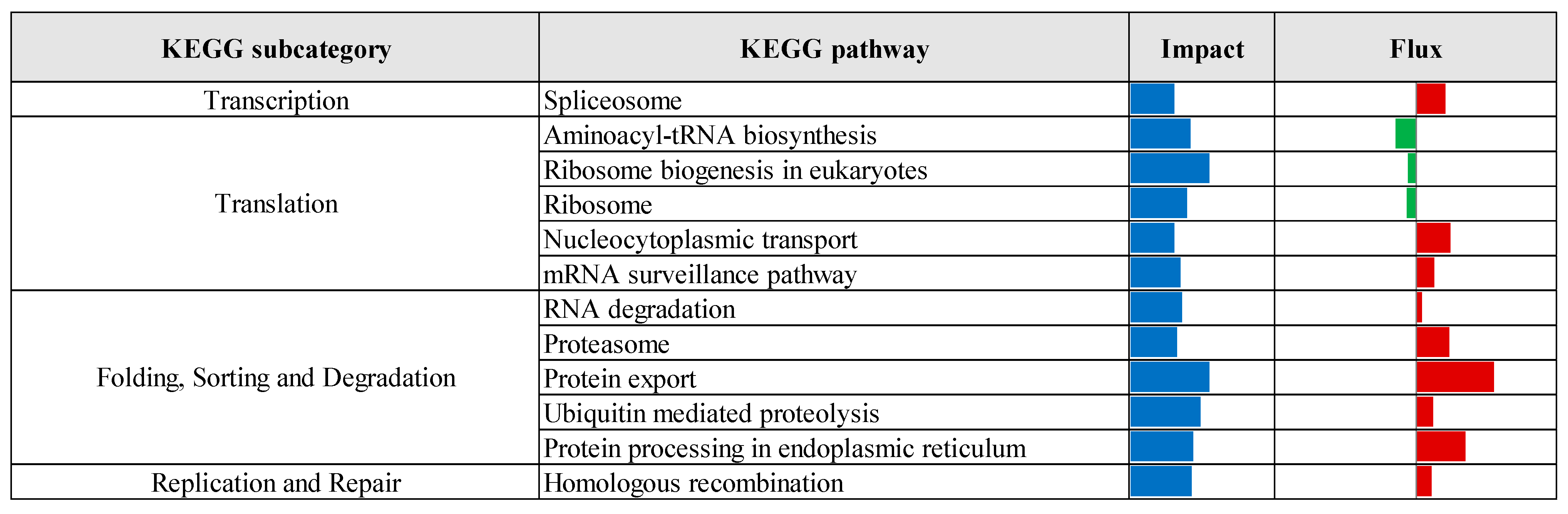
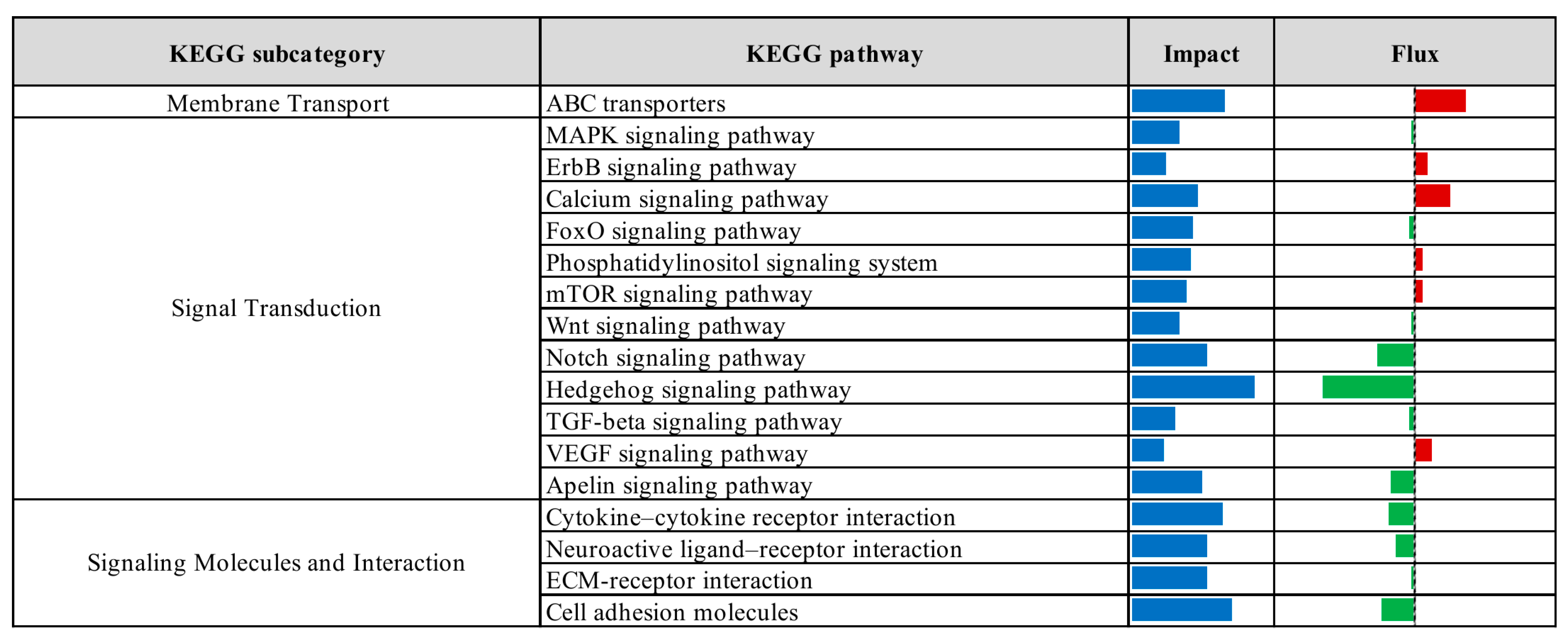
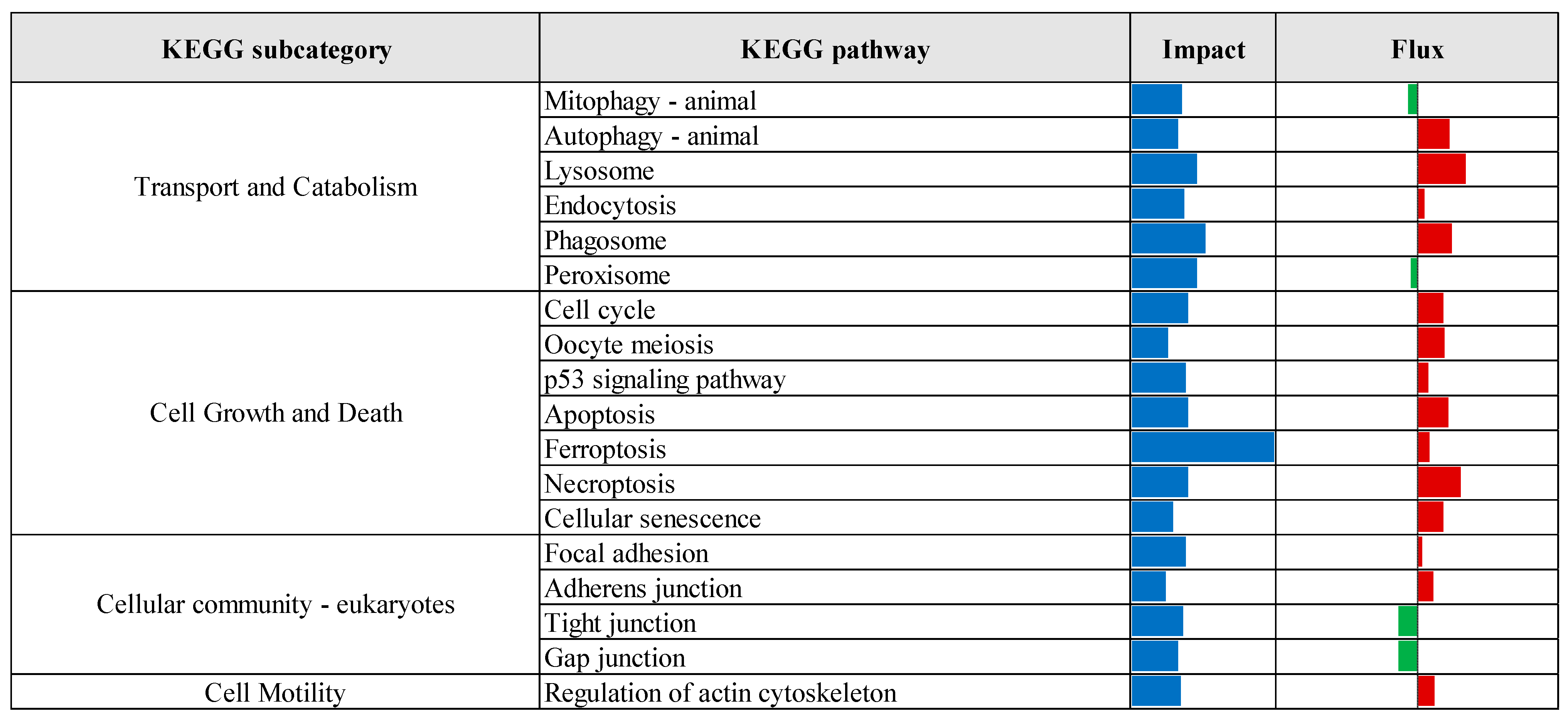
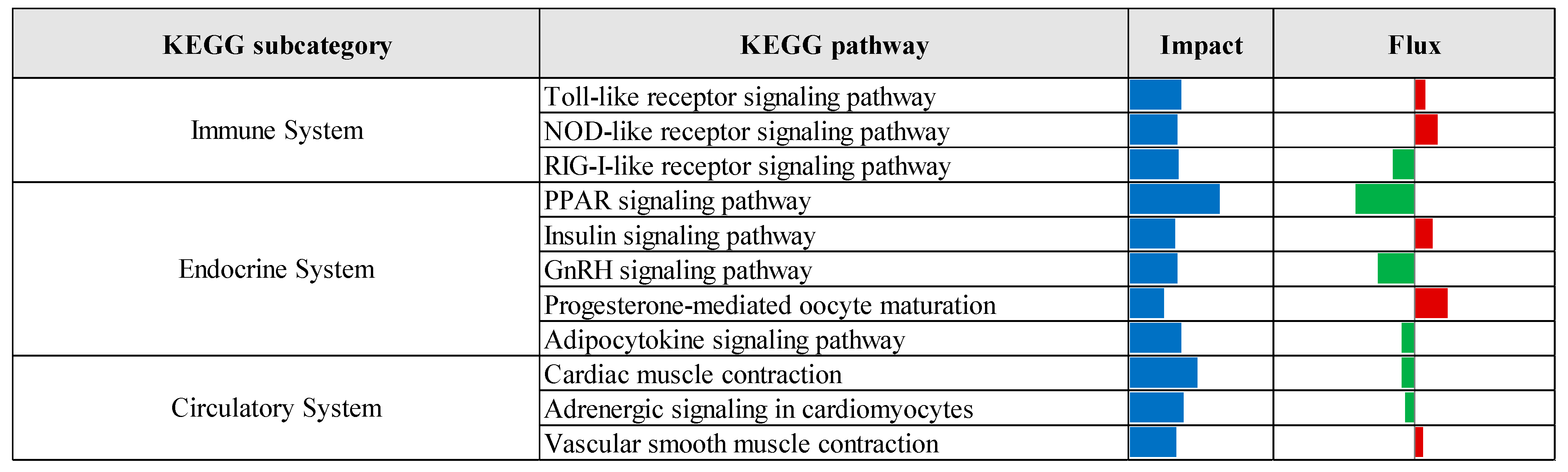
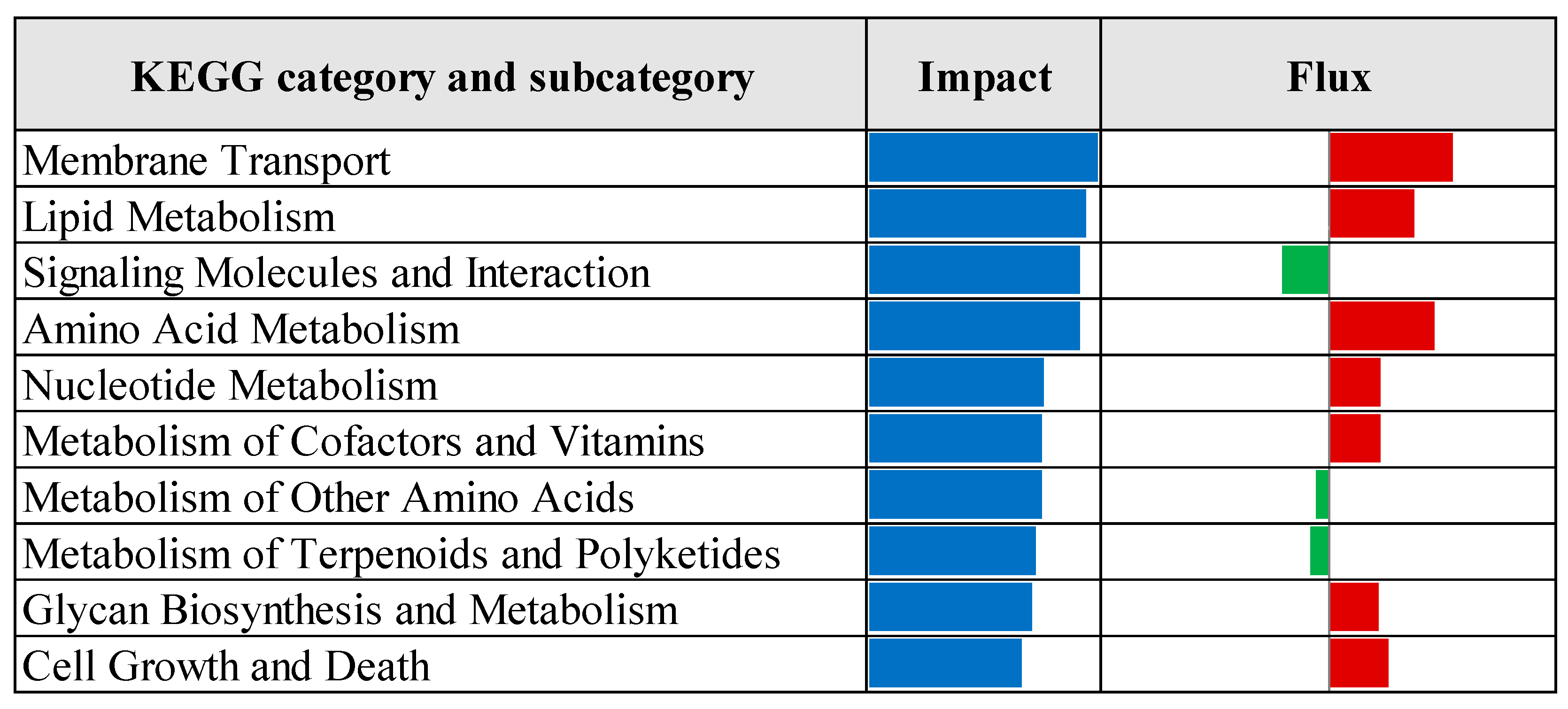
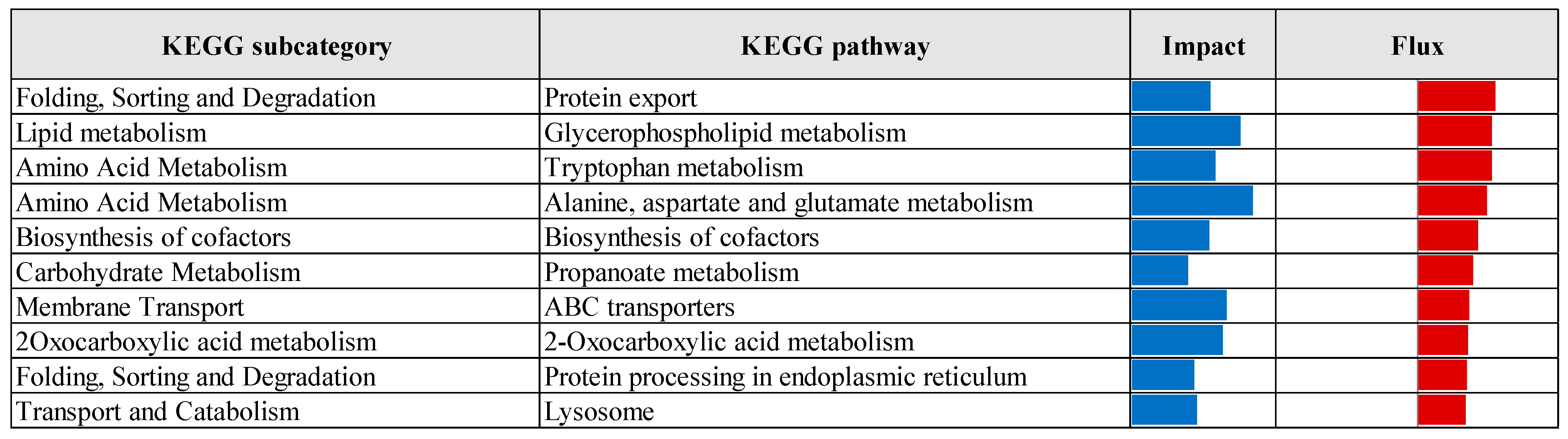
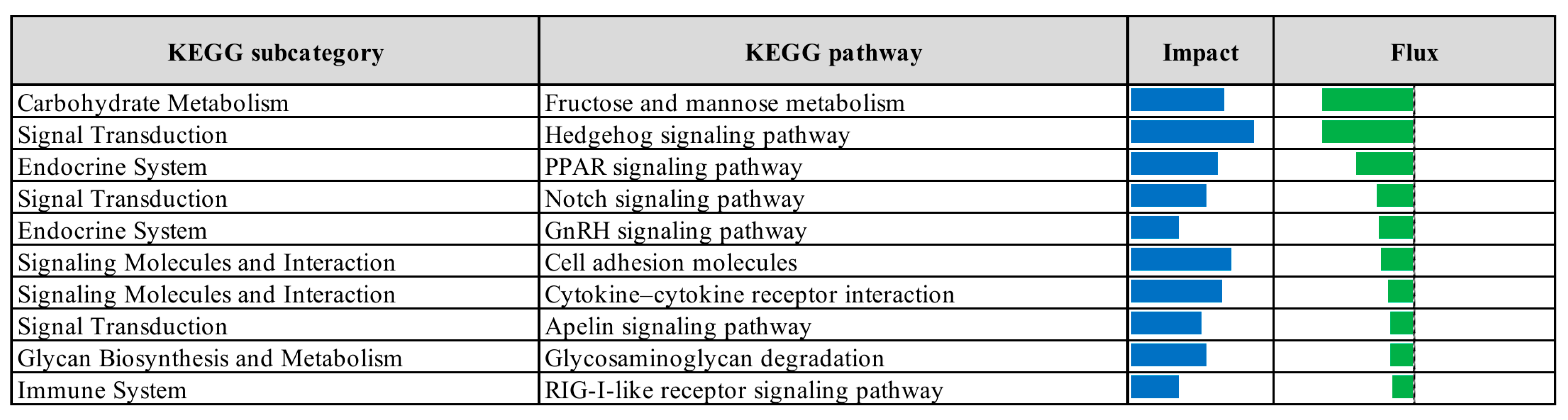
3.2. Pathway Enrichment Analysis of Differentially Expressed Genes
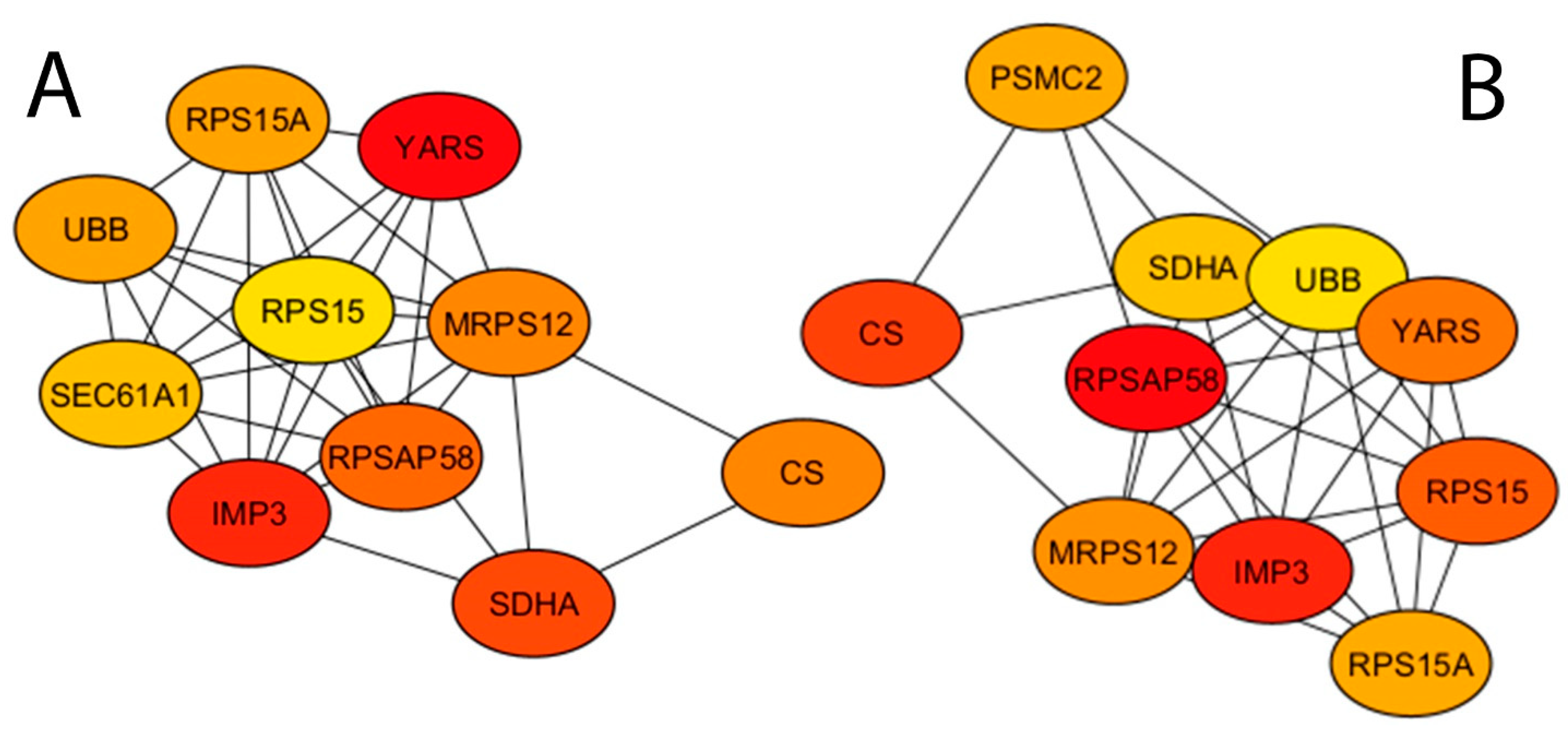
3.3. Construction of Protein–Protein Interaction Network and Screening of Hub Genes
3.4. Detection of Transcription Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gadde, U.; Kim, W.H.; Oh, S.T.; Lillehoj, H.S. Alternatives to Antibiotics for Maximizing Growth Performance and Feed Efficiency in Poultry: A Review. Anim. Health Res. Rev. 2017, 18, 26–45. [Google Scholar] [CrossRef]
- Low, C.X.; Tan, L.T.H.; Mutalib, N.S.A.; Pusparajah, P.; Goh, B.H.; Chan, K.G.; Letchumanan, V.; Lee, L.H. Unveiling the Impact of Antibiotics and Alternative Methods for Animal Husbandry: A Review. Antibiotics 2021, 10, 578. [Google Scholar] [CrossRef] [PubMed]
- Hakami, Z.M.; Alhotan, R.A.; Al Sulaiman, A.R.; Aljumaah, R.S.; Palombo, V.; D’Andrea, M.; Alharthi, A.S.; Abudabos, A.E. Enhancing Laying Hen Productivity and Health: Influence of Dietary Probiotic Bacillus Strains and Prebiotic Saccharomyces cerevisiae Yeast Cell Wall on Production Performance, Egg Quality, and Inflammatory Responses. Animals 2025, 15, 1398. [Google Scholar] [CrossRef] [PubMed]
- Ramlucken, U.; Lalloo, R.; Roets, Y.; Moonsamy, G.; van Rensburg, C.J.; Thantsha, M.S. Advantages of Bacillus-Based Probiotics in Poultry Production. Livest. Sci. 2020, 241, 104215. [Google Scholar] [CrossRef]
- Bahaddad, S.A.; Almalki, M.H.K.; Alghamdi, O.A.; Sohrab, S.S.; Yasir, M.; Azhar, E.I.; Chouayekh, H. Bacillus Species as Direct-Fed Microbial Antibiotic Alternatives for Monogastric Production. Probiotics Antimicrob. Proteins 2022, 15, 1–16. [Google Scholar] [CrossRef]
- Popov, I.V.; Algburi, A.; Prazdnova, E.V.; Mazanko, M.S.; Elisashvili, V.; Bren, A.B.; Chistyakov, V.A.; Tkacheva, E.V.; Trukhachev, V.I.; Donnik, I.M.; et al. A Review of the Effects and Production of Spore-Forming Probiotics for Poultry. Animals 2021, 11, 1941. [Google Scholar] [CrossRef]
- Grant, A.; Gay, C.G.; Lillehoj, H.S. Bacillus spp. as Direct-Fed Microbial Antibiotic Alternatives to Enhance Growth, Immunity, and Gut Health in Poultry. Avian Pathol. 2018, 47, 339–351. [Google Scholar] [CrossRef]
- Luise, D.; Bosi, P.; Raff, L.; Amatucci, L.; Virdis, S.; Trevisi, P. Bacillus spp. Probiotic Strains as a Potential Tool for Limiting the Use of Antibiotics, and Improving the Growth and Health of Pigs and Chickens. Front. Microbiol. 2022, 13, 801827. [Google Scholar] [CrossRef]
- Zhou, Y.; Zeng, Z.; Xu, Y.; Ying, J.; Wang, B.; Majeed, M.; Majeed, S.; Pande, A.; Li, W. Application of Bacillus coagulans in Animal Husbandry and Its Underlying Mechanisms. Animals 2020, 10, 454. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, H.; Yu, Y.; Zhang, R.; Wu, Y.; Yue, M.; Yang, C. Effects of Bacillus coagulans on Growth Performance, Antioxidant Capacity, Immunity Function, and Gut Health in Broilers. Poult. Sci. 2021, 100, 101168. [Google Scholar] [CrossRef]
- Alkhulaifi, M.M.; Alqhtani, A.H.; Alharthi, A.S.; Al Sulaiman, A.R.; Abudabos, A.M. Influence of Prebiotic Yeast Cell Wall Extracts on Growth Performance, Carcase Attributes, Biochemical Metabolites, and Intestinal Morphology and Bacteriology of Broiler Chickens Challenged with Salmonella typhimurium and Clostridium perfringens. Ital. J. Anim. Sci. 2022, 21, 1190–1199. [Google Scholar] [CrossRef]
- Fathima, S.; Shanmugasundaram, R.; Sifri, M.; Selvaraj, R. Yeasts and Yeast-Based Products in Poultry Nutrition. J. Appl. Poult. Res. 2023, 32, 100345. [Google Scholar] [CrossRef]
- Bar-Dagan, H.; Gover, O.; Cohen, N.A.; Vetvicka, V.; Rozenboim, I.; Schwartz, B. Beta-Glucans Induce Cellular Immune Training and Changes in Intestinal Morphology in Poultry. Front. Vet. Sci. 2023, 9, 1092812. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Santamarina, A.; Mondragon, A.d.C.; Lamas, A.; Miranda, J.M.; Franco, C.M.; Cepeda, A. Animal-Origin Prebiotics Based on Chitin: An Alternative for the Future? A Critical Review. Foods 2020, 9, 782. [Google Scholar] [CrossRef]
- Alqhtani, A.H.; Al Sulaiman, A.R.; Alharthi, A.S.; Abudabos, A.E. Dietary Supplementation of Prebiotic Yeast Saccharomyces Cerevisiae Cell Wall Promotes Growth Performance and Intestinal Health in Broiler Chickens Challenged with Clostridium Perfringens. Br. Poult. Sci. 2024, 65, 129–136. [Google Scholar] [CrossRef]
- Amevor, F.K.; Uyanga, V.A.; Wu, L.; Xu, D.; Shu, G.; Wang, Y.; Zhao, X. Enhancing Poultry Health and Productivity through the Liver-Gut Axis with Integrated Nutritional and Immunological Approaches: A Mini-Review. Front. Physiol. 2025, 16, 1537099. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, J.; Ji, L.; Zhang, L.; Zhao, L.; Guo, Y.; Wei, H.; Lu, L. Bacillus subtilis Improves Antioxidant Capacity and Optimizes Inflammatory State in Broilers. Anim. Biosci. 2024, 37, 1041–1052. [Google Scholar] [CrossRef]
- Dong, W.; Liu, B.; Xiao, Y.; Liu, X.; Yang, M.; Yuan, X.; Zhang, Y.; Li, G.; Meng, K.; Liu, M.; et al. Isolation of Bacillus licheniformis and Its Protective Effect on Liver Oxidative Stress and Apoptosis Induced by Aflatoxin B1. Poult. Sci. 2024, 103, 104079. [Google Scholar] [CrossRef]
- El-Gendy, Z.A.; El-Marasy, S.A.; Ahmed, R.F.; El-Batran, S.A.; Abd El-Rahman, S.S.; Ramadan, A.; Youssef, S.A.H. Hepatoprotective Effect of Saccharomyces Cervisciae Cell Wall Extract against Thioacetamide-Induced Liver Fibrosis in Rats. Heliyon 2021, 7, e07159. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Poultry, 9th ed.; National Academies Press: Washington, DC, USA, 1994. [Google Scholar] [CrossRef]
- Latimer, G.W., Jr. Official Methods of Analysis of AOAC International, 22nd ed.; AOAC International Publications: New York, NY, USA, 2023. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Bionaz, M.; Periasamy, K.; Rodriguez-Zas, S.L.; Hurley, W.L.; Loor, J.J. A Novel Dynamic Impact Approach (DIA) for Functional Analysis of Time-Course Omics Studies: Validation Using the Bovine Mammary Transcriptome. PLoS ONE 2012, 7, e32455. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Franceschini, A.; Kuhn, M.; Simonovic, M.; Roth, A.; Minguez, P.; Doerks, T.; Stark, M.; Muller, J.; Bork, P.; et al. The STRING Database in 2011: Functional Interaction Networks of Proteins, Globally Integrated and Scored. Nucleic Acids Res. 2011, 39, D561–D568. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.H.; Chen, S.H.; Wu, H.H.; Ho, C.W.; Ko, M.T.; Lin, C.Y. CytoHubba: Identifying Hub Objects and Sub-Networks from Complex Interactome. BMC Syst. Biol. 2014, 8 (Suppl. S4), S11. [Google Scholar] [CrossRef] [PubMed]
- Keenan, A.B.; Torre, D.; Lachmann, A.; Leong, A.K.; Wojciechowicz, M.L.; Utti, V.; Jagodnik, K.M.; Kropiwnicki, E.; Wang, Z.; Ma’ayan, A. ChEA3: Transcription Factor Enrichment Analysis by Orthogonal Omics Integration. Nucleic Acids Res. 2019, 47, W212–W224. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Hitz, B.C.; Gabdank, I.; Hilton, J.A.; Kagda, M.S.; Lam, B.; Myers, Z.; Sud, P.; Jou, J.; Lin, K.; et al. New Developments on the Encyclopedia of DNA Elements (ENCODE) Data Portal. Nucleic Acids Res. 2020, 48, D882–D889. [Google Scholar] [CrossRef]
- Alhotan, R.A.; Al Sulaiman, A.R.; Alharthi, A.S.; Abudabos, A.M. Protective Influence of Betaine on Intestinal Health by Regulating Inflammation and Improving Barrier Function in Broilers under Heat Stress. Poult. Sci. 2021, 100, 101337. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Staltner, R.; Burger, K.; Baumann, A.; Bergheim, I. Fructose: A Modulator of Intestinal Barrier Function and Hepatic Health? Eur. J. Nutr. 2023, 62, 3113–3124. [Google Scholar] [CrossRef]
- Krause, N.; Wegner, A. Fructose Metabolism in Cancer. Cells 2020, 9, 2635. [Google Scholar] [CrossRef]
- Guney, C.; Bal, N.B.; Akar, F. The Impact of Dietary Fructose on Gut Permeability, Microbiota, Abdominal Adiposity, Insulin Signaling and Reproductive Function. Heliyon 2023, 9, e18896. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Zhu, L.; Yang, X.; He, F.; Wang, T.; Bao, T.; Lu, H.; Wang, H.; Yang, S. Inulin Alleviates Inflammation of Alcoholic Liver Disease via SCFAs-Inducing Suppression of M1 and Facilitation of M2 Macrophages in Mice. Int. Immunopharmacol. 2020, 78, 106062. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, J.; Zhou, S.; Liao, J.; Ye, Z.; Mao, L. Efficacy of Probiotics on Nonalcoholic Fatty Liver Disease: A Meta-Analysis. Medicine 2023, 102, E32734. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.; Qi, G.; Wang, J.; Zhang, H.; Qiu, K.; Wu, S. Intestinal Microbiota of Layer Hens and Its Association with Egg Quality and Safety. Poult. Sci. 2022, 101, 102008. [Google Scholar] [CrossRef] [PubMed]
- Pineda-Quiroga, C.; Borda-Molina, D.; Chaves-Moreno, D.; Ruiz, R.; Atxaerandio, R.; Camarinha-Silva, A.; García-Rodríguez, A. Microbial and Functional Profile of the Ceca from Laying Hens Affected by Feeding Prebiotics, Probiotics, and Synbiotics. Microorganisms 2019, 7, 123. [Google Scholar] [CrossRef]
- Khan, S.; Moore, R.J.; Stanley, D.; Chousalkar, K.K. The Gut Microbiota of Laying Hens and Its Manipulation with Prebiotics and Probiotics to Enhance Gut Health and Food Safety. Appl. Environ. Microbiol. 2020, 86, 20. [Google Scholar] [CrossRef]
- Morris, G.; Berk, M.; Carvalho, A.; Caso, J.R.; Sanz, Y.; Walder, K.; Maes, M. The Role of the Microbial Metabolites Including Tryptophan Catabolites and Short Chain Fatty Acids in the Pathophysiology of Immune-Inflammatory and Neuroimmune Disease. Mol. Neurobiol. 2016, 54, 4432–4451. [Google Scholar] [CrossRef]
- Heidari, M.; Soleyman-Nejad, M.; Isazadeh, A.; Shapouri, J.; Taskhiri, M.H.; Ahangari, R.; Mohamadi, A.R.; Ebrahimi, M.; Karimi, H.; Bolhassani, M.; et al. Association of a Novel Homozygous Mutation in the HMGCS2 Gene with an HMGCSD in an Iranian Patient. Mol. Genet. Genom. Med. 2020, 8, e1507. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-Generated Metabolites Promote Metabolic Benefits via Gut-Brain Neural Circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef]
- Feng, J.; Lu, M.; Wang, J.; Zhang, H.; Qiu, K.; Qi, G.; Wu, S. Dietary Oregano Essential Oil Supplementation Improves Intestinal Functions and Alters Gut Microbiota in Late-Phase Laying Hens. J. Anim. Sci. Biotechnol. 2021, 12, 72. [Google Scholar] [CrossRef]
- Luo, G.; Niu, M.; Li, Y.; Cui, N.; Huang, S.; Yang, X. Cholesterol Metabolism and Its Role in Type 2 Diabetes Mellitus Development. Food Nutr. Health 2025, 2, 15. [Google Scholar] [CrossRef]
- Gonen, A.; Miller, Y.I. From Inert Storage to Biological Activity—In Search of Identity for Oxidized Cholesteryl Esters. Front. Endocrinol. 2020, 11, 602252. [Google Scholar] [CrossRef] [PubMed]
- Kriaa, A.; Bourgin, M.; Potiron, A.; Mkaouar, H.; Jablaoui, A.; Gérard, P.; Maguin, E.; Rhimi, M. Microbial Impact on Cholesterol and Bile Acid Metabolism: Current Status and Future Prospects. J. Lipid Res. 2019, 60, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Seltman, H.; Diven, W.; Rizk, M.; Noland, B.J.; Chanderbhan, R.; Scallen, T.J.; Vahouny, G.; Sanghvi, A. Regulation of Bile-Acid Synthesis. Role of Sterol Carrier Protein2 in the Biosynthesis of 7α-Hydroxycholesterol. Biochem. J. 1985, 230, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Kannan, M.; Karunakaran, R.; Balakrishnan, V.; Prabhakar, T.G. Influence of Prebiotics Supplementation on Lipid Profile of Broilers. Int. J. Poult. Sci. 2005, 4, 994–997. [Google Scholar] [CrossRef]
- Wilson, T.A.; Nicolosi, R.J.; Rogers, E.J.; Sacchiero, R.; Goldberg, D.J. Studies of Cholesterol and Bile Acid Metabolism, and Early Atherogenesis in Hamsters Fed GT16-239, a Novel Bile Acid Sequestrant (BAS). Atherosclerosis 1998, 140, 315–324. [Google Scholar] [CrossRef]
- Letexier, D.; Diraison, F.; Beylot, M. Addition of Inulin to a Moderately High-Carbohydrate Diet Reduces Hepatic Lipogenesis and Plasma Triacylglycerol Concentrations in Humans. Am. J. Clin. Nutr. 2003, 77, 559–564. [Google Scholar] [CrossRef]
- Xie, C.; Zhu, X.; Xu, B.; Niu, Y.; Zhang, X.; Ma, L.; Yan, X. Integrated Analysis of Multi-Tissues Lipidome and Gut Microbiome Reveals Microbiota-Induced Shifts on Lipid Metabolism in Pigs. Anim. Nutr. 2022, 10, 280–293. [Google Scholar] [CrossRef]
- White, C.J.; Ellis, J.M.; Wolfgang, M.J. The Role of Ethanolamine Phosphate Phospholyase in Regulation of Astrocyte Lipid Homeostasis. J. Biol. Chem. 2021, 297, 100830. [Google Scholar] [CrossRef]
- Korbecki, J.; Bosiacki, M.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. Biosynthesis and Significance of Fatty Acids, Glycerophospholipids, and Triacylglycerol in the Processes of Glioblastoma Tumorigenesis. Cancers 2023, 15, 2183. [Google Scholar] [CrossRef]
- Huang, L.; Wu, H.; Li, H.; Hou, Y.; Hu, J.; Huang, L.; Lu, Y.; Liu, X. Hepatic Glycerolipid Metabolism Is Critical to the Egg Laying Rate of Guangxi Ma Chickens. Gene 2022, 830, 146500. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhu, W.; Du, Y.; Liu, X.; Geng, Z. Genetic Parameters for Yolk Cholesterol and Transcriptional Evidence Indicate a Role of Lipoprotein Lipase in the Cholesterol Metabolism of the Chinese Wenchang Chicken. Front. Genet. 2019, 10, 902. [Google Scholar] [CrossRef] [PubMed]
- Wlcek, K.; Stieger, B. ATP-Binding Cassette Transporters in Liver. BioFactors 2014, 40, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Varga, T.; Czimmerer, Z.; Nagy, L. PPARs Are a Unique Set of Fatty Acid Regulated Transcription Factors Controlling Both Lipid Metabolism and Inflammation. BBA-Mol. Basis Dis. 2011, 1812, 1007–1022. [Google Scholar] [CrossRef]
- Mardinoglu, A.; Shoaie, S.; Bergentall, M.; Ghaffari, P.; Zhang, C.; Larsson, E.; Bäckhed, F.; Nielsen, J. The Gut Microbiota Modulates Host Amino Acid and Glutathione Metabolism in Mice. Mol. Syst. Biol. 2015, 11, 834. [Google Scholar] [CrossRef]
- Davila, A.M.; Blachier, F.; Gotteland, M.; Andriamihaja, M.; Benetti, P.H.; Sanz, Y.; Tomé, D. Re-Print of “Intestinal Luminal Nitrogen Metabolism: Role of the Gut Microbiota and Consequences for the Host”. Pharmacol. Res. 2013, 69, 114–126. [Google Scholar] [CrossRef]
- Beckwith, J. The Sec-Dependent Pathway. Res. Microbiol. 2013, 164, 497–504. [Google Scholar] [CrossRef]
- Duwaerts, C.C.; Maiers, J.L. ER Disposal Pathways in Chronic Liver Disease: Protective, Pathogenic, and Potential Therapeutic Targets. Front. Mol. Biosci. 2022, 8, 804097. [Google Scholar] [CrossRef]
- Aebi, M.; Bernasconi, R.; Clerc, S.; Molinari, M. N-Glycan Structures: Recognition and Processing in the ER. Trends Biochem. Sci. 2010, 35, 74–82. [Google Scholar] [CrossRef]
- Macelline, S.P.; Toghyani, M.; Chrystal, P.V.; Selle, P.H.; Liu, S.Y. Amino Acid Requirements for Laying Hens: A Comprehensive Review. Poult. Sci. 2021, 100, 101036. [Google Scholar] [CrossRef]
- Gostner, J.M.; Geisler, S.; Stonig, M.; Mair, L.; Sperner-Unterweger, B.; Fuchs, D. Tryptophan Metabolism and Related Pathways in Psychoneuroimmunology: The Impact of Nutrition and Lifestyle. Neuropsychobiology 2020, 79, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Le Floc’h, N.; Seve, B. Biological Roles of Tryptophan and Its Metabolism: Potential Implications for Pig Feeding. Livest. Sci. 2007, 112, 23–32. [Google Scholar] [CrossRef]
- Yang, Y.; Borel, T.; De Azambuja, F.; Johnson, D.; Sorrentino, J.P.; Udokwu, C.; Davis, I.; Liu, A.; Altman, R.A. Diflunisal Derivatives as Modulators of ACMS Decarboxylase Targeting the Tryptophan-Kynurenine Pathway. J. Med. Chem. 2021, 64, 797–811. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Mu, C.L.; Farzi, A.; Zhu, W.Y. Tryptophan Metabolism: A Link Between the Gut Microbiota and Brain. Adv. Nutr. 2020, 11, 709–723. [Google Scholar] [CrossRef]
- Sorgdrager, F.J.H.; Naudé, P.J.W.; Kema, I.P.; Nollen, E.A.; De Deyn, P.P. Tryptophan Metabolism in Inflammaging: From Biomarker to Therapeutic Target. Front. Immunol. 2019, 10, 2565. [Google Scholar] [CrossRef]
- He, W.; Li, P.; Wu, G. Amino Acid Nutrition and Metabolism in Chickens. Adv. Exp. Med. Biol. 2021, 1285, 109–131. [Google Scholar] [CrossRef]
- Omenetti, A.; Choi, S.; Michelotti, G.; Diehl, A.M. Hedgehog Signaling in the Liver. J. Hepatol. 2011, 54, 366–373. [Google Scholar] [CrossRef]
- Katoh, Y.; Katoh, M. Hedgehog Signaling, Epithelial-to-Mesenchymal Transition and MiRNA (Review). Int. J. Mol. Med. 2008, 22, 271–275. [Google Scholar] [CrossRef]
- Katoh, Y.; Katoh, M. Hedgehog Target Genes: Mechanisms of Carcinogenesis Induced by Aberrant Hedgehog Signaling Activation. Curr. Mol. Med. 2009, 9, 873–886. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Wei, P.; Ye, F.; Chen, Y.; Shi, L.; Zhang, X.; Li, J.; Lin, S.; Yang, X. SPP1 and CXCL9 Promote Non-Alcoholic Steatohepatitis Progression Based on Bioinformatics Analysis and Experimental Studies. Front. Med. 2022, 9, 862278. [Google Scholar] [CrossRef]
- Syn, W.K.; Choi, S.S.; Liaskou, E.; Karaca, G.F.; Agboola, K.M.; Oo, Y.H.; Mi, Z.; Pereira, T.A.; Zdanowicz, M.; Malladi, P.; et al. Osteopontin Is Induced by Hedgehog Pathway Activation and Promotes Fibrosis Progression in Nonalcoholic Steatohepatitis. Hepatology 2011, 53, 106–115. [Google Scholar] [CrossRef]
- Eferl, R.; Sibilia, M.; Hilberg, F.; Fuchsbichler, A.; Kufferath, I.; Guertl, B.; Zenz, R.; Wagner, E.F.; Zatloukal, K. Functions of C-Jun in Liver and Heart Development. J. Cell Biol. 1999, 145, 1049–1061. [Google Scholar] [CrossRef]
- Alzaid, F.; Lagadec, F.; Albuquerque, M.; Ballaire, R.; Orliaguet, L.; Hainault, I.; Blugeon, C.; Lemoine, S.; Lehuen, A.; Saliba, D.G.; et al. IRF5 Governs Liver Macrophage Activation That Promotes Hepatic Fibrosis in Mice and Humans. JCI Insight 2016, 1, 88689. [Google Scholar] [CrossRef]
- Quan, T.; Li, R.; Chen, Y.; Gao, T. Regulatory Mechanism of Intestinal Stem Cells Based on Hippo Pathway and Signaling Crosstalk in Chicken. Int. J. Mol. Sci. 2025, 26, 5067. [Google Scholar] [CrossRef]
- Miretti, S.; Lecchi, C.; Ceciliani, F.; Baratta, M. MicroRNAs as Biomarkers for Animal Health and Welfare in Livestock. Front. Vet. Sci. 2020, 7, 578193. [Google Scholar] [CrossRef]
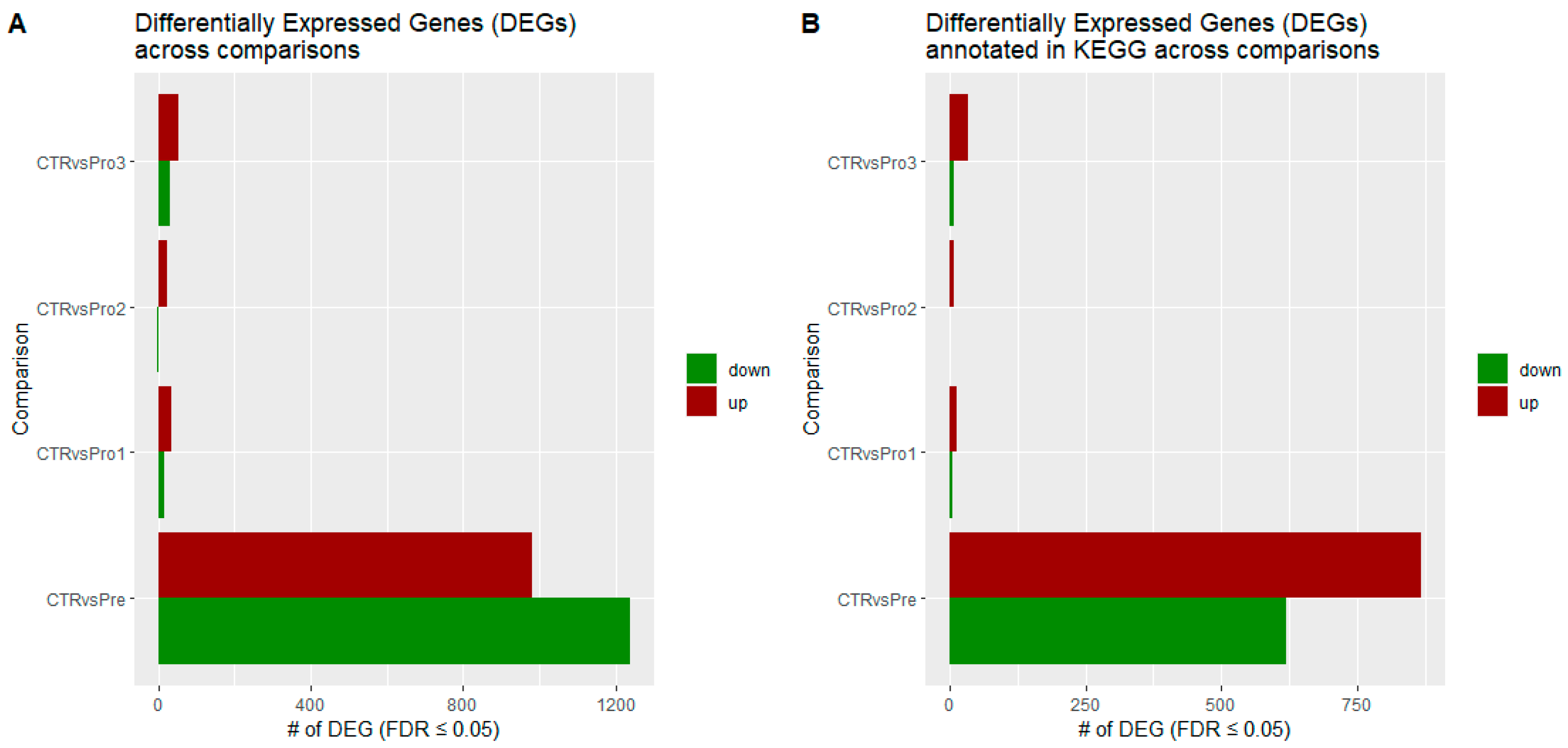
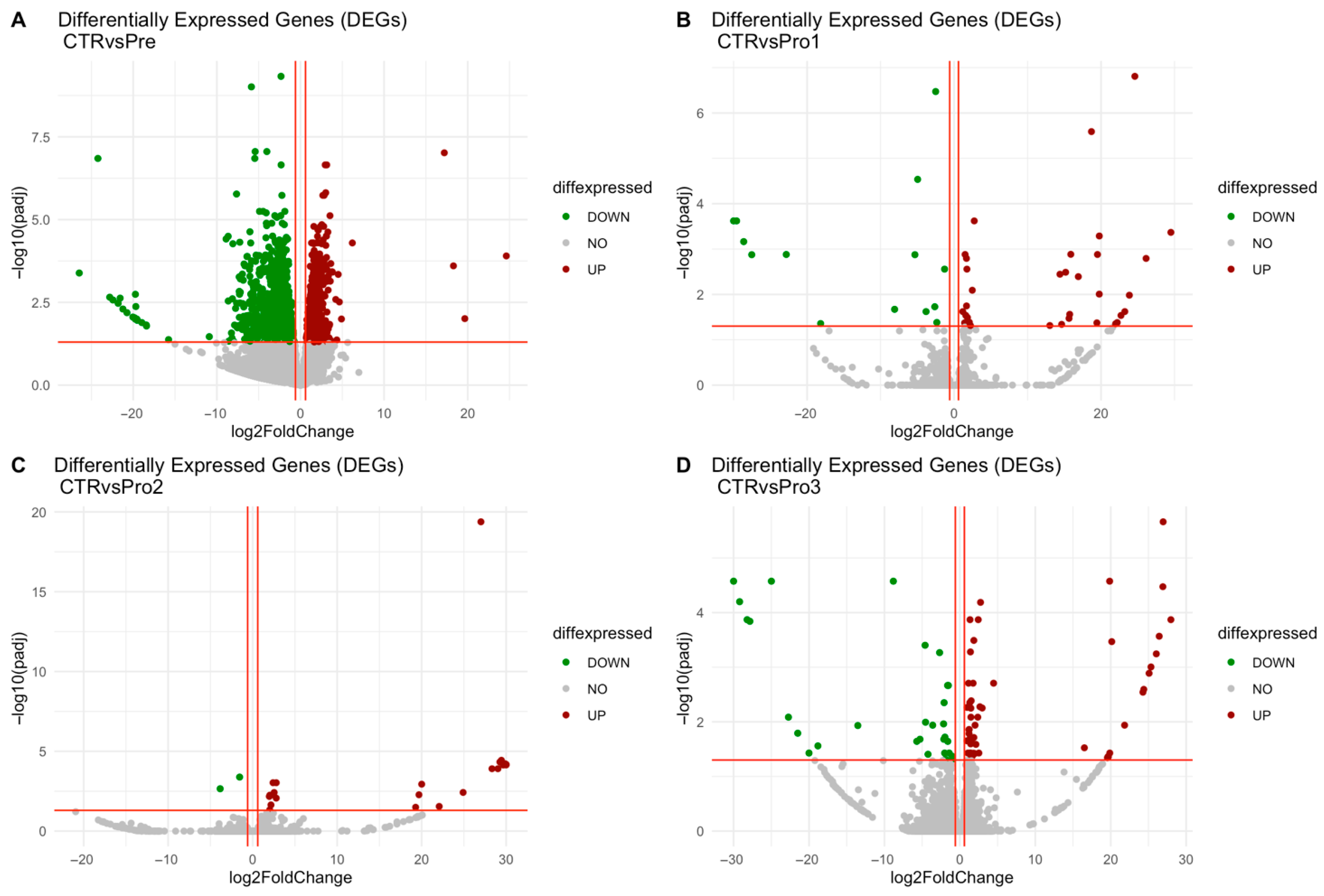
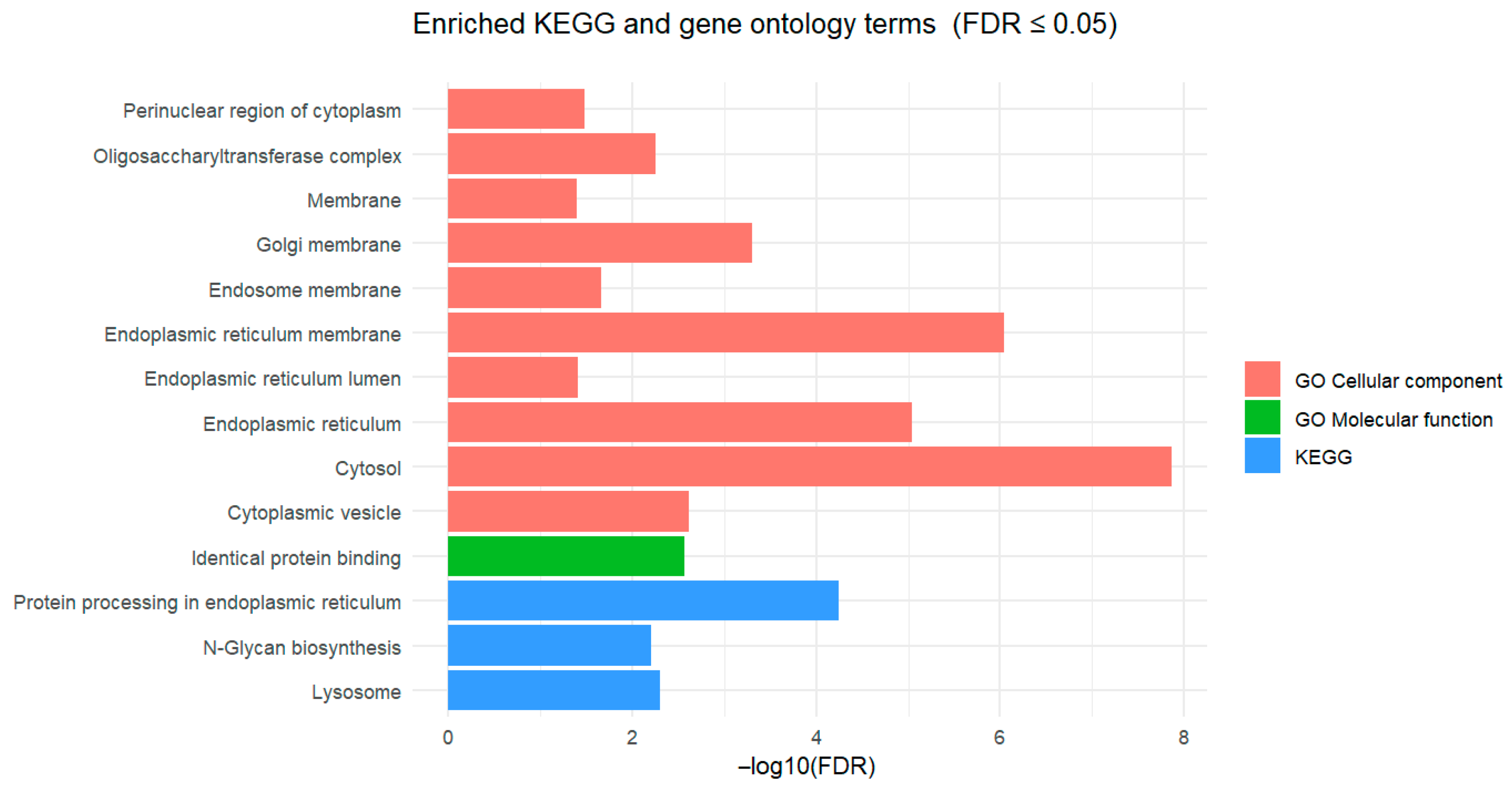
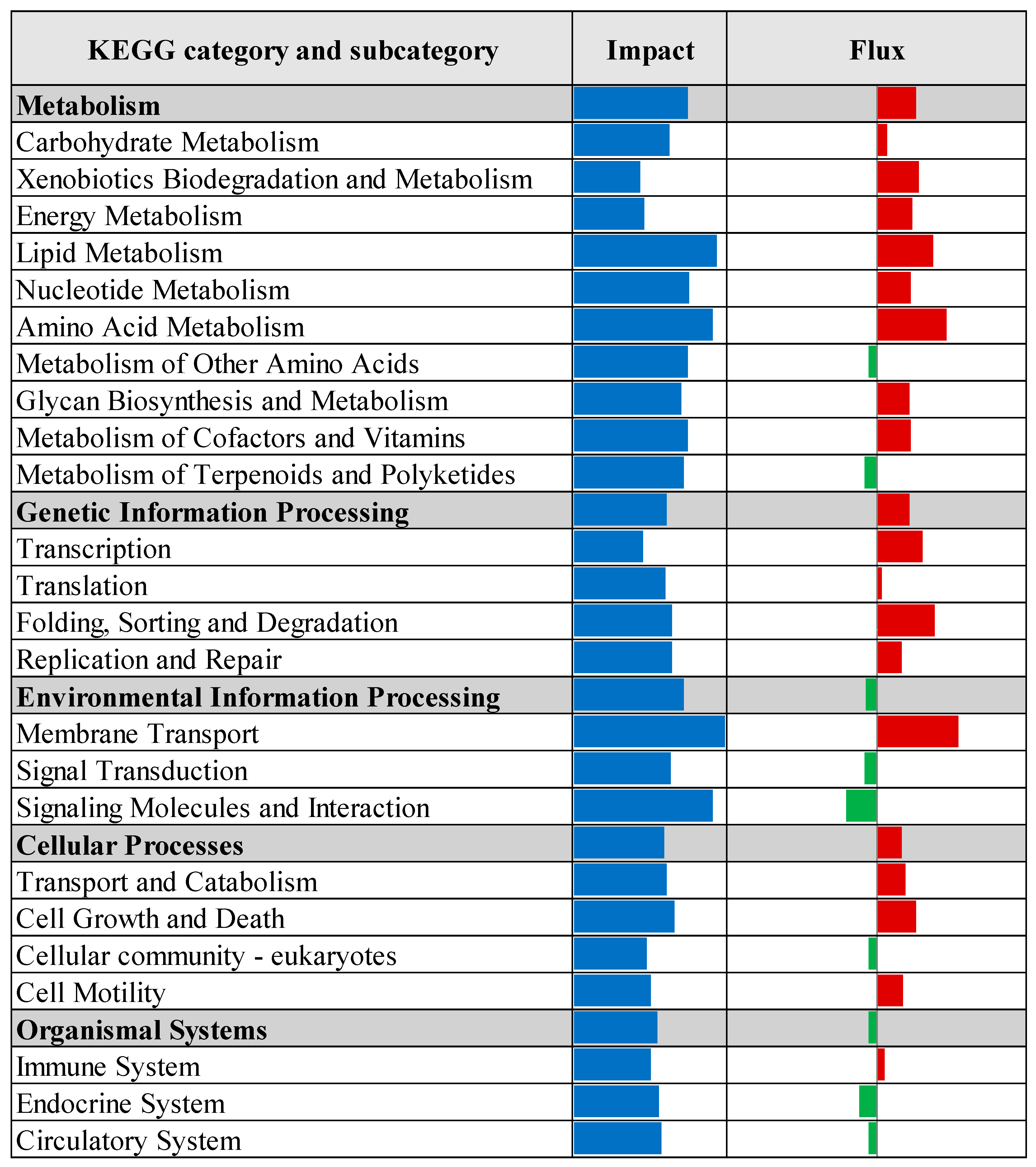
| Rank | Expression | CTR vs. Pre 1 | CTR vs. Pro1 | CTR vs. Pro2 | CTR vs. Pro3 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Gene | log2FC 2 | Gene | log2FC | Gene | log2FC | Gene | log2FC | ||
| 1 | upregulated | ETNPPL | 6.2 | LECT2 | 6.18 | ABCG8 | 2.79 | NHLH1 | 19.87 |
| 2 | CCNH | 4.65 | CKMT2 | 4.38 | PPAT | 2.54 | KLHL30 | 19.68 | |
| 3 | MRPS18C | 4.22 | FKBP5 | 7.14 | GPT2 | 2.41 | CLCA1 | 19.56 | |
| 4 | MOGAT1 | 3.59 | LPIN1 | 4.14 | FKBP5 | 2.41 | LPIN1 | 2.74 | |
| 5 | ACMSD | 3.56 | HSPH1 | 3.99 | PAICS | 2.16 | ABHD3 | 2.65 | |
| 1 | downregulated | LRRC4C | −8.63 | ELF3 | −5.33 | PAFAH2 | −1.55 | HEYL | −4.61 |
| 2 | DHH | −7.3 | KANK4 | −2.63 | - | - | ELF3 | −4.55 | |
| 3 | SOX17 | −6.3 | PDE4D | −2.51 | - | - | KIF21B | −1.95 | |
| 4 | CARNS1 | −6.16 | TRPM1 | −2.34 | - | - | CYP4A22 | −1.83 | |
| 5 | DTX1 | −5.97 | ENPP2 | −1.29 | - | - | ARHGAP45 | −1.63 | |
| Comparison 1 | Database 2 | Pathway/Term | p-Value | Fold Enrichment | FDR 3 |
|---|---|---|---|---|---|
| CTR vs. Pre | KEGG | Protein processing in endoplasmic reticulum | 3.70 × 10−7 | 2.24 | 5.77 × 10−5 |
| Lysosome | 6.48 × 10−5 | 2.09 | 5.05 × 10−3 | ||
| N-Glycan biosynthesis | 1.19 × 10−4 | 2.86 | 6.17 × 10−3 | ||
| GO-MF | Identical protein binding | 2.37 × 10−6 | 1.55 | 2.73 × 10−3 | |
| GO-CC | Cytosol | 1.98 × 10−11 | 1.41 | 1.38 × 10−8 | |
| Endoplasmic reticulum membrane | 2.57 × 10−9 | 2.06 | 8.95 × 10−7 | ||
| Endoplasmic reticulum | 3.96 × 10−8 | 1.78 | 9.20 × 10−6 | ||
| Golgi membrane | 2.86 × 10−6 | 2.10 | 4.97 × 10−4 | ||
| Cytoplasmic vesicle | 1.75 × 10−5 | 2.28 | 2.44 × 10−3 | ||
| Oligosaccharyltransferase complex | 4.81 × 10−5 | 8.16 | 5.58 × 10−3 | ||
| Endosome membrane | 2.17 × 10−4 | 2.62 | 2.16 × 10−2 | ||
| Perinuclear region of cytoplasm | 3.82 × 10−4 | 1.65 | 3.32 × 10−2 | ||
| Endoplasmic reticulum lumen | 5.00 × 10−4 | 3.00 | 3.87 × 10−2 | ||
| Lysosomal membrane | 5.79 × 10−4 | 1.41 | 4.03 × 10−2 | ||
| CTR vs. Pro3 | GO-MF | Phosphatidate phosphatase activity | 4.72 × 10−4 | 2.65 | 2.83 × 10−2 |
| GO-CC | Cytosol | 4.93 × 10−4 | 8.70 × 101 | 5.03 × 10−2 |
| Rank | Hub by Degree Method 1 | Hub by Radiality Method |
|---|---|---|
| 1 | YARS | RPSAP58 |
| 2 | IMP3 | IMP3 |
| 3 | SDHA | CS |
| 4 | RPSAP58 | RPS15 |
| 5 | MRPS12 | YARS |
| 6 | CS | MRPS12 |
| 7 | UBB | RPS15A |
| 8 | RPS15A | PSMC2 |
| 9 | SEC61A1 | SDHA |
| 10 | RPS15 | UBB |
| KEGG Pathway | Number of Genes | p-Value | FDR | Genes 1 |
|---|---|---|---|---|
| Ribosome | 109 | 3.96 × 10−5 | 0.00658 | MRPS12, RPS15, RPSAP58, RPS15A |
| Citrate cycle (TCA cycle) | 27 | 0.0107 | 0.024 | SDHA, CS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abudabos, A.E.; Hakami, Z.M.; Al Sulaiman, A.R.; Aljumaah, R.S.; Palombo, V.; Aljumaah, M.R.; D’Andrea, M.; Alharthi, A.S.; Alhotan, R.A. RNA Sequencing-Based Transcriptome Analysis of Liver in Laying Hens Supplemented with Dietary Probiotic Bacillus Species and Prebiotic Yeast (Saccharomyces cerevisiae) Cell Walls. Vet. Sci. 2025, 12, 822. https://doi.org/10.3390/vetsci12090822
Abudabos AE, Hakami ZM, Al Sulaiman AR, Aljumaah RS, Palombo V, Aljumaah MR, D’Andrea M, Alharthi AS, Alhotan RA. RNA Sequencing-Based Transcriptome Analysis of Liver in Laying Hens Supplemented with Dietary Probiotic Bacillus Species and Prebiotic Yeast (Saccharomyces cerevisiae) Cell Walls. Veterinary Sciences. 2025; 12(9):822. https://doi.org/10.3390/vetsci12090822
Chicago/Turabian StyleAbudabos, Ala E., Zafar M. Hakami, Ali R. Al Sulaiman, Riyadh S. Aljumaah, Valentino Palombo, Mashael R. Aljumaah, Mariasilvia D’Andrea, Abdulrahman S. Alharthi, and Rashed A. Alhotan. 2025. "RNA Sequencing-Based Transcriptome Analysis of Liver in Laying Hens Supplemented with Dietary Probiotic Bacillus Species and Prebiotic Yeast (Saccharomyces cerevisiae) Cell Walls" Veterinary Sciences 12, no. 9: 822. https://doi.org/10.3390/vetsci12090822
APA StyleAbudabos, A. E., Hakami, Z. M., Al Sulaiman, A. R., Aljumaah, R. S., Palombo, V., Aljumaah, M. R., D’Andrea, M., Alharthi, A. S., & Alhotan, R. A. (2025). RNA Sequencing-Based Transcriptome Analysis of Liver in Laying Hens Supplemented with Dietary Probiotic Bacillus Species and Prebiotic Yeast (Saccharomyces cerevisiae) Cell Walls. Veterinary Sciences, 12(9), 822. https://doi.org/10.3390/vetsci12090822








