Composition Heterogeneity and Low-Molecular-Weight Allergen Content of Dermatophagoides farinae House Dust Mite Allergen Extracts Used in Veterinary Medicine
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Allergen Extracts
2.3. Protein Content Determination
2.4. Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.5. Der f 1 and Der f 2 Quantification
2.6. ELISA
3. Results
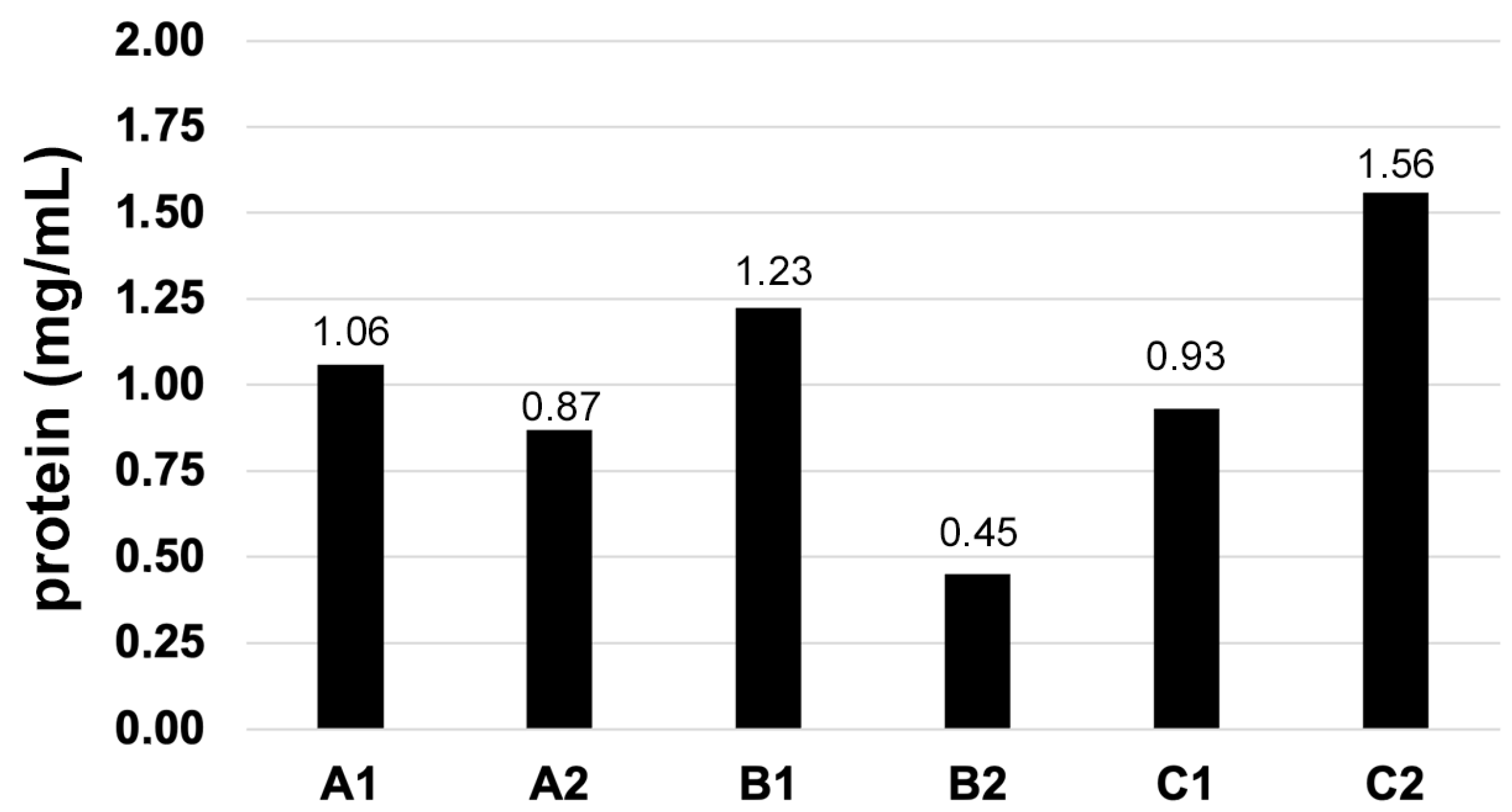
3.1. Protein Content
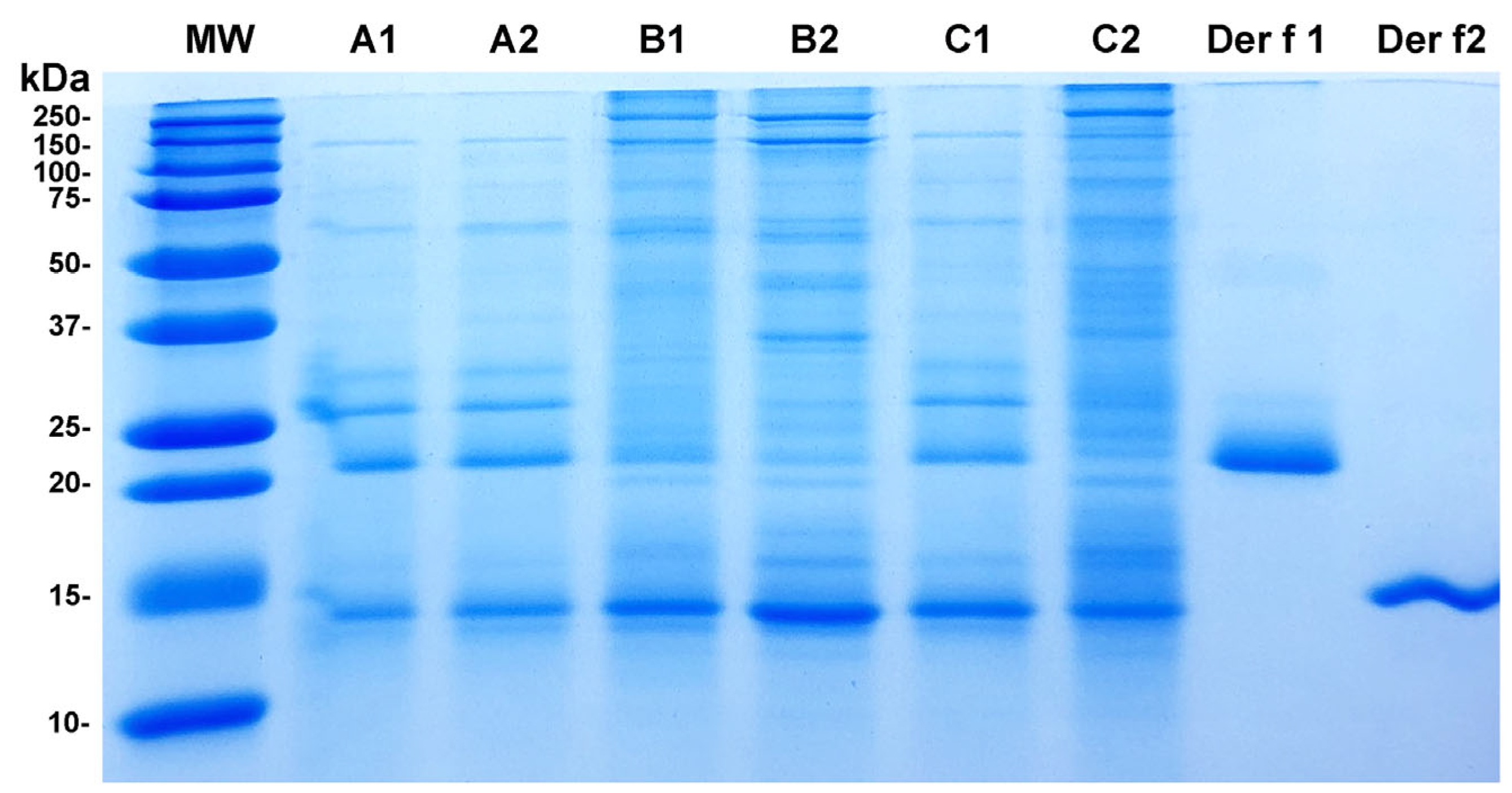
3.2. SDS-PAGE and Mass Spectrometry
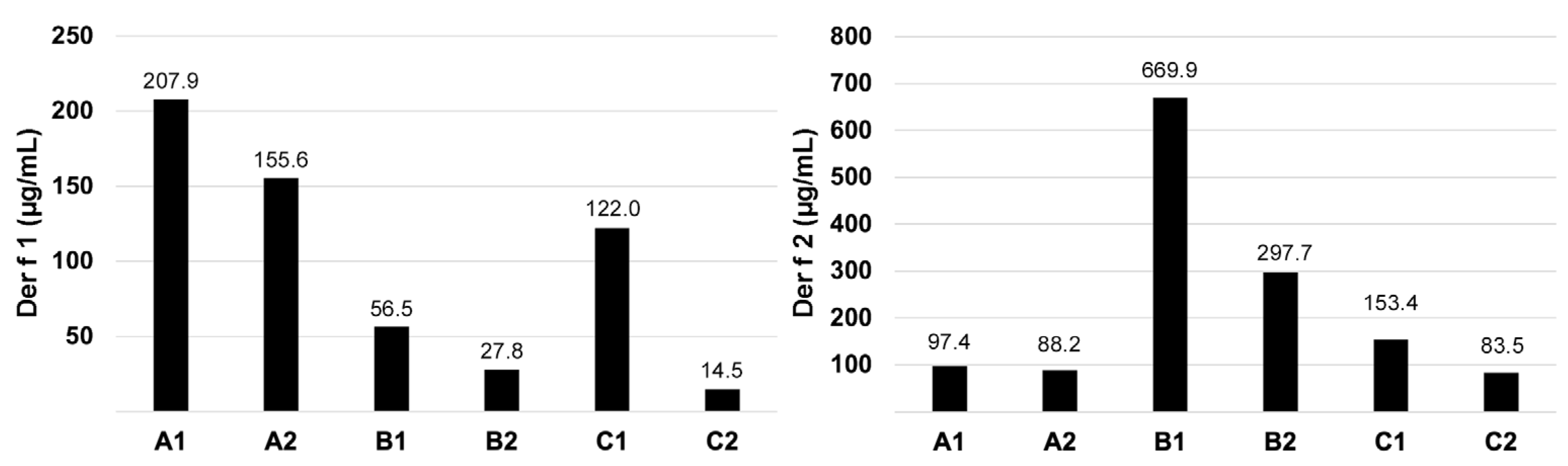
3.3. Der f 1 and Der f 2 Quantification
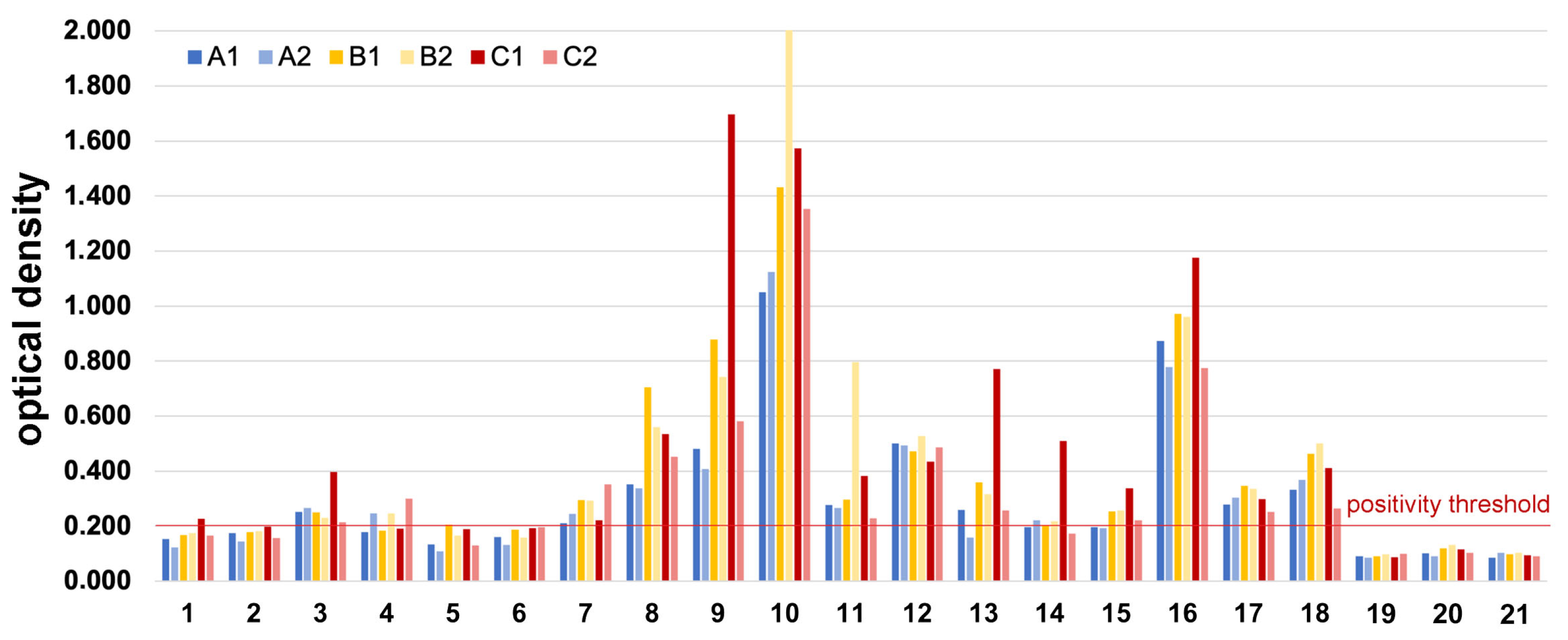
3.4. ELISA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIT | Allergen immunotherapy |
| ELISA | Enzyme-linked immunosorbent assay |
| HDM | House dust mites |
| PAGE | Polyacrylamide gel electrophoresis |
| PAX | Pet Allergy Xplorer |
| SDS | Sodium dodecyl sulfate |
| sIgE | Specific immunoglobulin E |
References
- Batard, T.; Canonica, W.G.; Pfaar, O.; Shamji, M.H.; O’Hehir, R.E.; van Zelm, M.C.; Mascarell, L. Current Advances in House Dust Mite Allergen Immunotherapy (AIT): Routes of Administration, Biomarkers and Molecular Allergen Profiling. Mol. Immunol. 2023, 155, 124–134. [Google Scholar] [CrossRef]
- van Ree, R. Indoor Allergens: Relevance of Major Allergen Measurements and Standardization. J. Allergy Clin. Immunol. 2007, 119, 270–279. [Google Scholar] [CrossRef]
- Brunetto, B.; Tinghino, R.; Braschi, M.C.; Antonicelli, L.; Pini, C.; Iacovacci, P. Characterization and Comparison of Commercially Available Mite Extracts for in Vivo Diagnosis. Allergy 2010, 65, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Perez, R.; Poza-Guedes, P.; Pino, Y.B.D.; Matheu, V.; Sanchez-Machin, I. Evaluation of Major Mite Allergens from European Standardized Commercial Extracts for in Vivo Diagnosis: Addressing the Need for Precision Medicine. Clin. Transl. Allergy 2019, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Vailes, L.; Sridhara, S.; Cromwell, O.; Weber, B.; Breitenbach, M.; Chapman, M. Quantitation of the Major Fungal Allergens, Alt a 1 and Asp f 1, in Commercial Allergenic Products. J. Allergy Clin. Immunol. 2001, 107, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Mindaye, S.T.; Spiric, J.; David, N.A.; Rabin, R.L.; Slater, J.E. Accurate Quantification of 5 German Cockroach (GCr) Allergens in Complex Extracts Using Multiple Reaction Monitoring Mass Spectrometry (MRM MS). Clin. Exp. Allergy 2017, 47, 1661–1670. [Google Scholar] [CrossRef]
- Mindaye, S.T.; David, N.A.; Esfahani, S.A.Z.; Schal, C.; Matsui, E.C.; Rabin, R.L.; Slater, J.E. Measurement of German Cockroach Allergens and Their Isoforms in Allergen Extracts with Mass Spectrometry. Clin. Exp. Allergy 2020, 50, 741–751. [Google Scholar] [CrossRef]
- Duffort, O.; Palomares, O.; Lombardero, M.; Villalba, M.; Barber, D.; Rodriguez, R.; Polo, F. Variability of Ole e 9 Allergen in Olive Pollen Extracts: Relevance of Minor Allergens in Immunotherapy Treatments. Int. Arch. Allergy Immunol. 2006, 140, 131–138. [Google Scholar] [CrossRef]
- Focke, M.; Marth, K.; Flicker, S.; Valenta, R. Heterogeneity of Commercial Timothy Grass Pollen Extracts. Clin. Exp. Allergy 2008, 38, 1400–1408. [Google Scholar] [CrossRef]
- Focke, M.; Marth, K.; Valenta, R. Molecular Composition and Biological Activity of Commercial Birch Pollen Allergen Extracts. Eur. J. Clin. Investig. 2009, 39, 429–436. [Google Scholar] [CrossRef]
- Abrams, S.B.; Brock, G.N.; Palettas, M.; Bolner, M.L.; Moore-Sowers, T.; Plunkett, G.A.; Cole, L.K.; Diaz, S.F.; Lorch, G. An Evaluation of Veterinary Allergen Extract Content and Resultant Canine Intradermal Threshold Concentrations. Vet. Dermatol. 2018, 29, 496-e167. [Google Scholar] [CrossRef] [PubMed]
- Valenta, R.; Karaulov, A.; Niederberger, V.; Zhernov, Y.; Elisyutina, O.; Campana, R.; Focke-Tejkl, M.; Curin, M.; Namazova-Baranova, L.; Wang, J.-Y.; et al. Allergen Extracts for in Vivo Diagnosis and Treatment of Allergy: Is There a Future? J. Allergy Clin. Immunol. Pract. 2018, 6, 1845–1855.e2. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cai, Z.; Fan, D.; Hu, J.; Hou, Y.; He, Y.; Zhang, Z.; Zhao, Z.; Gao, P.; Hu, W.; et al. Chromosome-Level Assembly of Dermatophagoides farinae Genome and Transcriptome Reveals Two Novel Allergens Der f 37 and Der f 39. World Allergy Organ. J. 2021, 14, 100590. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Huang, H.; Zhou, Y.; Liu, Y.; Ren, Y.; Liao, Y.; Yuan, C.; Gu, X.; Cui, Y. A Chromosomal-Level Genome of Dermatophagoides farinae, a Common Allergenic Mite Species. Int. J. Genom. 2024, 2024, 3779688. [Google Scholar] [CrossRef]
- WHO/IUIS Allergen Nomenclature Sub-Committee Allergen Nomenclature. Available online: https://www.allergen.org/ (accessed on 12 August 2025).
- Mueller, R.S.; Janda, J.; Jensen-Jarolim, E.; Rhyner, C.; Marti, E. Allergens in Veterinary Medicine. Allergy 2016, 71, 27–35. [Google Scholar] [CrossRef]
- Favrot, C.; Olivry, T.; Iwasaki, T. An International Seroprevalence Survey of the IgE Sensitisation to the Dermatophagoides farinae House Dust Mite and Two of Its Major Allergens (Der f 2, Zen 1) in Atopic Dogs. Vet. Dermatol. 2022, 33, 117-e34. [Google Scholar] [CrossRef]
- Huang, H.-J.; Sarzsinszky, E.; Vrtala, S. House Dust Mite Allergy: The Importance of House Dust Mite Allergens for Diagnosis and Immunotherapy. Mol. Immunol. 2023, 158, 54–67. [Google Scholar] [CrossRef]
- Thomas, W.R. Hierarchy and Molecular Properties of House Dust Mite Allergens. Allergol. Int. 2015, 64, 304–311. [Google Scholar] [CrossRef]
- Masuda, K.; Tsujimoto, H.; Fujiwara, S.; Kurata, K.; Hasegawa, A.; Yasueda, H.; Yamashita, K.; DeBoer, D.J.; deWeck, A.L.; Sakaguchi, M. IgE Sensitivity and Cross-Reactivity to Crude and Purified Mite Allergens (Der f 1, Der f 2, Der p 1, Der p 2) in Atopic Dogs Sensitive to Dermatophagoides Mite Allergens. Vet. Immunol. Immunopathol. 1999, 72, 303–313. [Google Scholar] [CrossRef]
- Yamashita, K.; Fujiwara, C.; Azuma, R.; Sawazaki, T.; Nakao, Y.; Hasegawa, A. Determination of Antigenic Proteins of Housedust Mites in 90 Dogs Suffering from Atopic Dermatitis. J. Vet. Med. Sci. 2002, 64, 673–676. [Google Scholar] [CrossRef]
- Nuttall, T.J.; Lamb, J.R.; Hill, P.B. Characterisation of Major and Minor Dermatophagoides Allergens in Canine Atopic Dermatitis. Res. Vet. Sci. 2001, 71, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Olivry, T.; Dunston, S.M.; Favrot, C.; Prelaud, P.; Tsukui, T. The Novel High Molecular Weight Dermatophagoides farinae Protein Zen-1 Is a Major Allergen in North American and European Mite Allergic Dogs with Atopic Dermatitis. Vet. Dermatol. 2017, 28, 177-e38. [Google Scholar] [CrossRef] [PubMed]
- Noli, C.; Bernadina, W.E.; Willemse, T. The Significance of Reactions to Purified Fractions of Dermatophagoides pteronyssinus and Dermatophagoides farinae in Canine Atopic Dermatitis. Vet. Immunol. Immunopathol. 1996, 52, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Moya, R.; Carnes, J.; Sinovas, N.; Ramio, L.; Brazis, P.; Puigdemont, A. Immunoproteomic Characterization of a Dermatophagoides farinae Extract Used in the Treatment of Canine Atopic Dermatitis. Vet. Immunol. Immunopathol. 2016, 180, 1–8. [Google Scholar] [CrossRef]
- McCall, C.; Hunter, S.; Stedman, K.; Weber, E.; Hillier, A.; Bozic, C.; Rivoire, B.; Olivry, T. Characterization and Cloning of a Major High Molecular Weight House Dust Mite Allergen (Der f 15) for Dogs. Vet. Immunol. Immunopathol. 2001, 78, 231–247. [Google Scholar] [CrossRef]
- Weber, E.; Hunter, S.; Stedman, K.; Dreitz, S.; Olivry, T.; Hillier, A.; McCall, C. Identification, Characterization, and Cloning of a Complementary DNA Encoding a 60-Kd House Dust Mite Allergen (Der f 18) for Human Beings and Dogs. J. Allergy Clin. Immunol. 2003, 112, 79–86. [Google Scholar] [CrossRef]
- Tsukui, T.; Sakaguchi, M.; Kurata, K.; Maeda, S.; Ohmori, K.; Koyanagi, M.; Masuda, K.; Ohno, K.; Tsujimoto, H.; Iwabuchi, S. Analysis of House Dust Mite (D. farinae) Allergens in Canine Atopic Dermatitis. Vet. Dermatol. 2007, 18, 194–195. [Google Scholar] [CrossRef]
- Olivry, T.; Fontao, A.M.; Jacquenet, S.; Aumayr, M.; Tsukui, T.; Gomord, V.; Faye, L.; Favrot, C. Identification of Cross-Reactive Allergens between the Dermatophagoides farinae House Dust Mite and the Toxocara canis Nematode in Dogs with Suspected Allergies. Vet. Dermatol. 2024, 35, 662–671. [Google Scholar] [CrossRef]
- Olivry, T.; Fontao, A.M.; Aumayr, M.; Ivanovova, N.P.; Mitterer, G.; Harwanegg, C. Validation of a Multiplex Molecular Macroarray for the Determination of Allergen-Specific IgE Sensitizations in Dogs. Vet. Sci. 2024, 11, 482. [Google Scholar] [CrossRef]
- Glesner, J.; Filep, S.; Vailes, L.D.; Wünschmann, S.; Chapman, M.D.; Birrueta, G.; Frazier, A.; Jeong, K.Y.; Schal, C.; Bacharier, L.; et al. Allergen Content in German Cockroach Extracts and Sensitization Profiles to a New Expanded Set of Cockroach Allergens Determine in Vitro Extract Potency for IgE Reactivity. J. Allergy Clin. Immunol. 2019, 143, 1474–1481.e8. [Google Scholar] [CrossRef]
- Hatzler, L.; Panetta, V.; Lau, S.; Wagner, P.; Bergmann, R.L.; Illi, S.; Bergmann, K.E.; Keil, T.; Hofmaier, S.; Rohrbach, A.; et al. Molecular Spreading and Predictive Value of Preclinical IgE Response to Phleum Pratense in Children with Hay Fever. J. Allergy Clin. Immunol. 2012, 130, 894–901.e5. [Google Scholar] [CrossRef]
- Custovic, A.; Sonntag, H.-J.; Buchan, I.E.; Belgrave, D.; Simpson, A.; Prosperi, M.C.F. Evolution Pathways of IgE Responses to Grass and Mite Allergens throughout Childhood. J. Allergy Clin. Immunol. 2015, 136, 1645–1652.e8. [Google Scholar] [CrossRef]
- Posa, D.; Perna, S.; Resch, Y.; Lupinek, C.; Panetta, V.; Hofmaier, S.; Rohrbach, A.; Hatzler, L.; Grabenhenrich, L.; Tsilochristou, O.; et al. Evolution and Predictive Value of IgE Responses toward a Comprehensive Panel of House Dust Mite Allergens during the First 2 Decades of Life. J. Allergy Clin. Immunol. 2017, 139, 541–549.e8. [Google Scholar] [CrossRef] [PubMed]
- Plant, J.D.; Neradelik, M.B.; Polissar, N.L.; Fadok, V.A.; Scott, B.A. Agreement between Allergen-Specific IgE Assays and Ensuing Immunotherapy Recommendations from Four Commercial Laboratories in the USA. Vet. Dermatol. 2014, 25, 15-e6. [Google Scholar] [CrossRef]
- Baumann, K.N.; Gedon, N.K.Y.; Boehm, T.M.S.A.; Udraite-Vovk, L.; Mueller, R.S. Reproducibility of Serum Testing for Environmental Allergen-Specific IgE in Dogs in Europe. Vet. Dermatol. 2021, 32, 251-e67. [Google Scholar] [CrossRef]
- Chong, E.; Austel, M.; Banovic, F. Evaluation of the Correlation of Serological and Intradermal Allergen Testing with Clinical History in 29 Dogs with Atopic Dermatitis. Vet. Dermatol. 2024, 35, 516–523. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Welters, M.; Mas-Fontao, A.; Auxilia, S.T.; Olivry, T. Composition Heterogeneity and Low-Molecular-Weight Allergen Content of Dermatophagoides farinae House Dust Mite Allergen Extracts Used in Veterinary Medicine. Vet. Sci. 2025, 12, 824. https://doi.org/10.3390/vetsci12090824
Welters M, Mas-Fontao A, Auxilia ST, Olivry T. Composition Heterogeneity and Low-Molecular-Weight Allergen Content of Dermatophagoides farinae House Dust Mite Allergen Extracts Used in Veterinary Medicine. Veterinary Sciences. 2025; 12(9):824. https://doi.org/10.3390/vetsci12090824
Chicago/Turabian StyleWelters, Marie, Ana Mas-Fontao, Silvia T. Auxilia, and Thierry Olivry. 2025. "Composition Heterogeneity and Low-Molecular-Weight Allergen Content of Dermatophagoides farinae House Dust Mite Allergen Extracts Used in Veterinary Medicine" Veterinary Sciences 12, no. 9: 824. https://doi.org/10.3390/vetsci12090824
APA StyleWelters, M., Mas-Fontao, A., Auxilia, S. T., & Olivry, T. (2025). Composition Heterogeneity and Low-Molecular-Weight Allergen Content of Dermatophagoides farinae House Dust Mite Allergen Extracts Used in Veterinary Medicine. Veterinary Sciences, 12(9), 824. https://doi.org/10.3390/vetsci12090824






