Advancing In Vitro Tools for Oncologic Research in Cats and Dogs
Simple Summary
Abstract
1. Introduction
2. Conventional Two-Dimensional (2D) Culture
2.1. Overview of 2D Culture
2.2. Immortalization
2.3. Oncogenic Transformation
3. Three-Dimensional (3D) Culture
3.1. Overview of 3D Culture
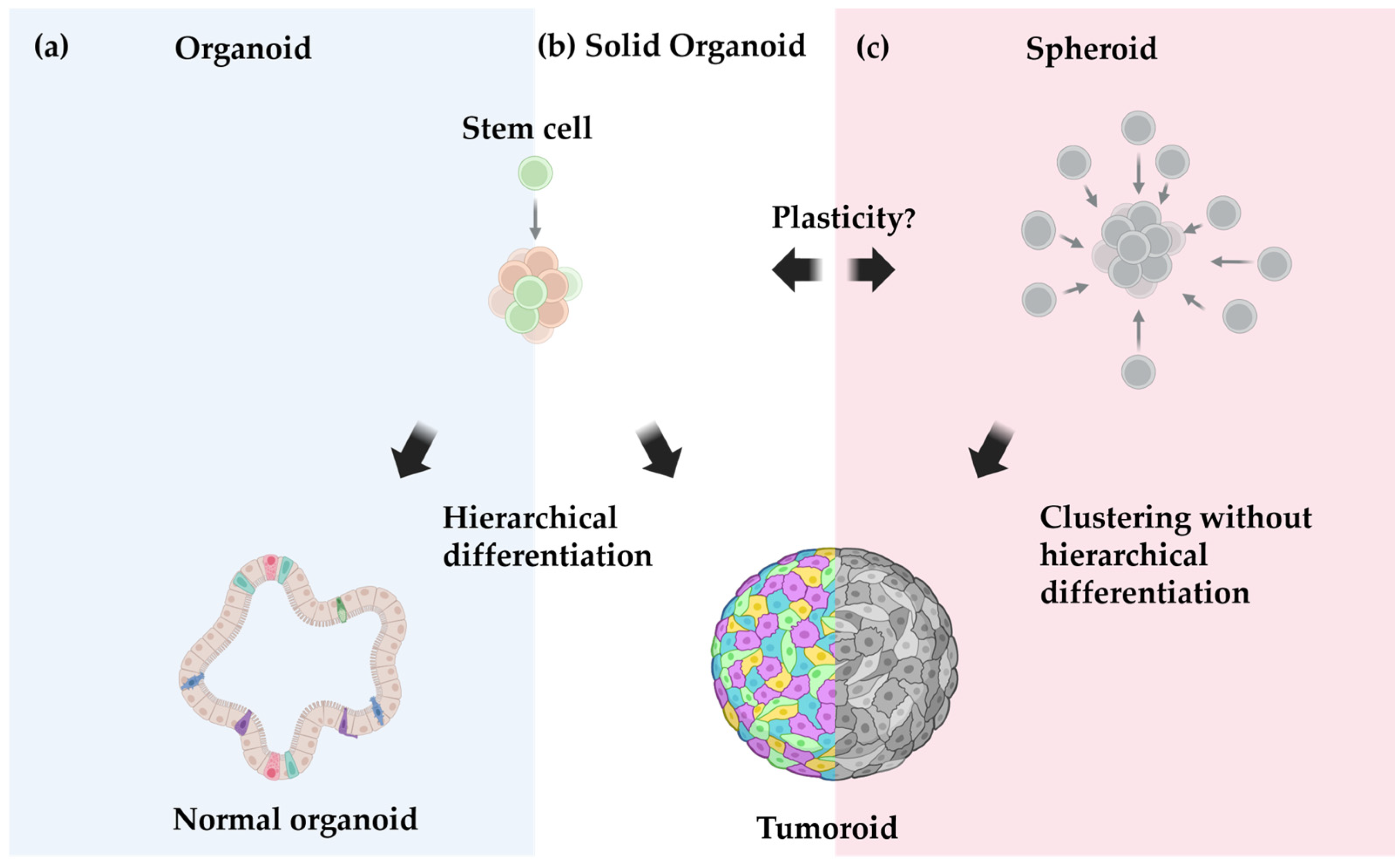
3.2. Organoids vs. Spheroids vs. Tumoroids: Definition Matters
4. Mammary Tumor Models
4.1. Advancements and Challenges in Establishing In Vitro Models for Mammary Tumors
| Species | Model Type | Tumor Source | Number of Animals | Reference | Highlights |
|---|---|---|---|---|---|
| Canine | 2D |
| 2 | Van der Burg, 1989 [102] |
|
| 1 | Priosoeryanto et al., 1995 [103] |
| ||
| 5 | Hellmén, 1992 [104] |
| ||
| 4 | Uyama et al., 2006 [105] |
| ||
| 1 | Caceres et al., 2015 [106] |
| ||
| 1 | Mei et al., 2021 [107] |
| ||
| 1 | Li et al., 2021 [108] |
| ||
| 10 out of 12 | Yeom et al., 2023 [109] |
| ||
| 8 | Park et al., 2024 [110] |
| ||
| Canine | 3D |
| 8 | Cocola et al., 2009 [94] |
|
| 16 | Inglebert et al., 2022 [88] |
| ||
| Feline | 2D |
| 4 out of 30 | Norval et al., 1985 [97] |
|
| 4 out of 135 | Minke et al., 1991 [96] |
| ||
| 5 out of 13 | Uyama et al., 2005 [51] |
| ||
| 1 | Borges et al., 2016 [111] |
| ||
| 1 | Granados-Soler et al., 2018 [112] |
|
4.2. Predictive Drug Response and Biomarkers in Mammary Tumor Models
4.3. Exploring Novel Biomarkers with CRISPR/Cas9 Screening
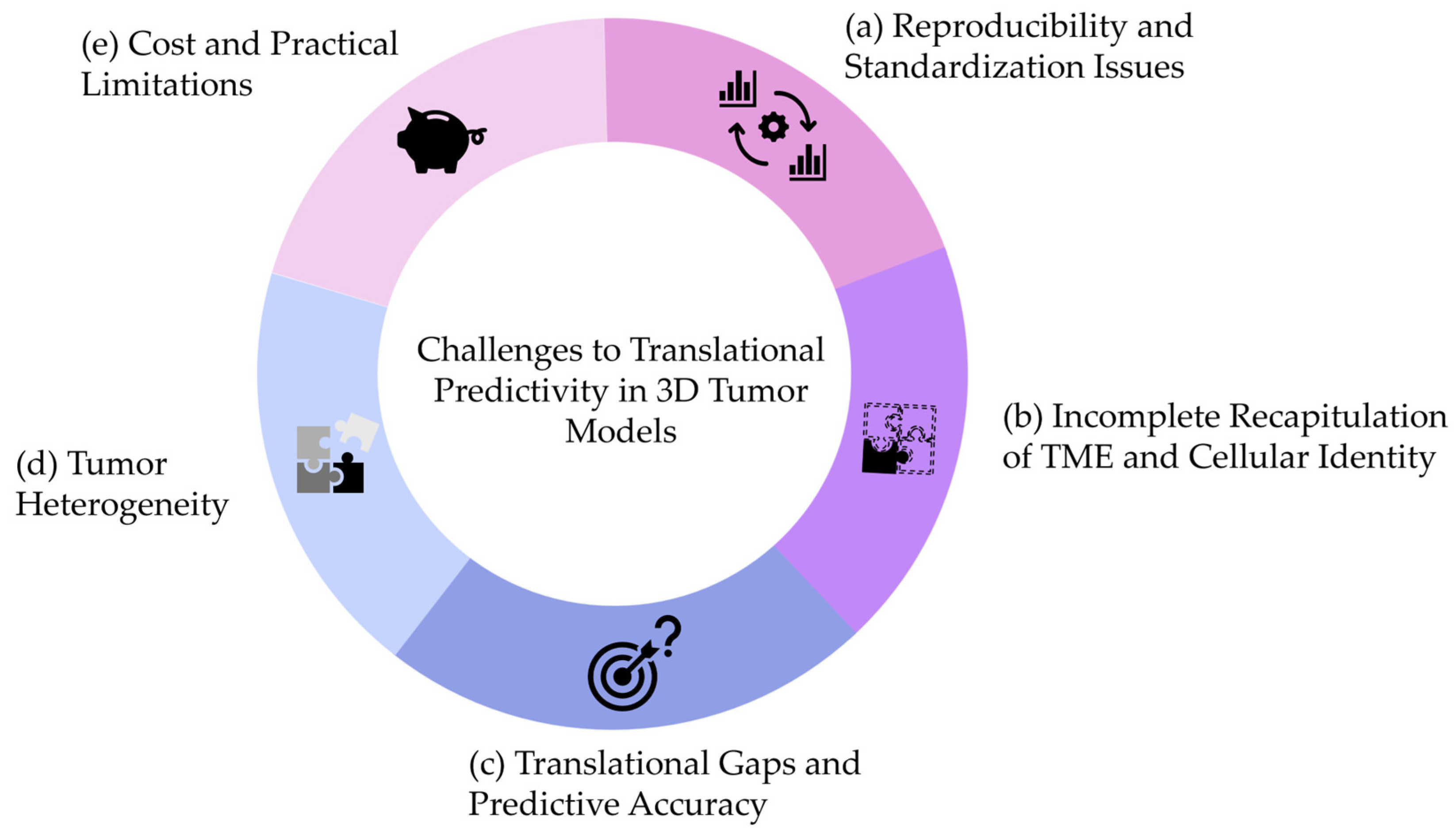
4.4. Lost in Translation
- (a)
- Reproducibility and Standardization Issues
- (b)
- Incomplete Recapitulation of the Native TME
- (c)
- Translational Gaps and Predictive Accuracy
- (d)
- Addressing Tumor Heterogeneity
- (e)
- Cost and Practical Limitations
5. Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Höxtermann, E. Cellular ‘Elementary Organisms’ In Vitro. The Early Vision of Gottlieb Haberlandt and Its Realization. Physiol. Plant. 1997, 100, 716–728. [Google Scholar] [CrossRef]
- Zhao, C. Cell Culture: In Vitro Model System and a Promising Path to In Vivo Applications. J. Histotechnol. 2023, 46, 1–4. [Google Scholar] [CrossRef]
- Lorvellec, M.; Pellegata, A.F.; Maestri, A.; Turchetta, C.; Alvarez Mediavilla, E.; Shibuya, S.; Jones, B.; Scottoni, F.; Perocheau, D.P.; Cozmescu, A.C.; et al. An In Vitro Whole-Organ Liver Engineering for Testing of Genetic Therapies. iScience 2020, 23, 101808. [Google Scholar] [CrossRef]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef]
- Brassard, J.A.; Nikolaev, M.; Hübscher, T.; Hofer, M.; Lutolf, M.P. Recapitulating Macro-Scale Tissue Self-Organization through Organoid Bioprinting. Nat. Mater. 2021, 20, 22–29. [Google Scholar] [CrossRef]
- Shin, W.; Kim, H.J. 3D In Vitro Morphogenesis of Human Intestinal Epithelium in a Gut-on-a-Chip or a Hybrid Chip with a Cell Culture Insert. Nat. Protoc. 2022, 17, 910–939. [Google Scholar] [CrossRef]
- Neufeld, L.; Yeini, E.; Reisman, N.; Shtilerman, Y.; Ben-Shushan, D.; Pozzi, S.; Madi, A.; Tiram, G.; Eldar-Boock, A.; Ferber, S.; et al. Microengineered Perfusable 3D-Bioprinted Glioblastoma Model for in Vivo Mimicry of Tumor Microenvironment. Sci. Adv. 2021, 7, eabi9119. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.N.; Ingber, D.E. Microfluidic Organs-on-Chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef]
- Ben-David, U.; Siranosian, B.; Ha, G.; Tang, H.; Oren, Y.; Hinohara, K.; Strathdee, C.A.; Dempster, J.; Lyons, N.J.; Burns, R.; et al. Genetic and Transcriptional Evolution Alters Cancer Cell Line Drug Response. Nature 2018, 560, 325–330. [Google Scholar] [CrossRef]
- Pinello, K.; Amorim, I.; Pires, I.; Canadas-Sousa, A.; Catarino, J.; Faísca, P.; Branco, S.; Peleteiro, M.C.; Silva, D.; Severo, M.; et al. Vet-OncoNet: Malignancy Analysis of Neoplasms in Dogs and Cats. Vet. Sci. 2022, 9, 535. [Google Scholar] [CrossRef]
- Graf, R.; Grüntzig, K.; Hässig, M.; Axhausen, K.W.; Fabrikant, S.; Welle, M.; Meier, D.; Guscetti, F.; Folkers, G.; Otto, V.; et al. Swiss Feline Cancer Registry: A Retrospective Study of the Occurrence of Tumours in Cats in Switzerland from 1965 to 2008. J. Comp. Pathol. 2015, 153, 266–277. [Google Scholar] [CrossRef]
- MacVean, D.W.; Monlux, A.W.; Anderson, P.S.; Silber Jr, S.L.; Roszel, J.F. Frequency of Canine and Feline Tumors in a Defined Population. Vet. Pathol. 1978, 15, 700–715. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, L.; Dobromylskyj, M.; Wood, G.A.; van der Weyden, L. Feline Oncogenomics: What Do We Know about the Genetics of Cancer in Domestic Cats? Vet. Sci. 2022, 9, 547. [Google Scholar] [CrossRef] [PubMed]
- Graf, R.; Pospischil, A.; Guscetti, F.; Meier, D.; Welle, M.; Dettwiler, M. Cutaneous Tumors in Swiss Dogs: Retrospective Data From the Swiss Canine Cancer Registry, 2008–2013. Vet. Pathol. 2018, 55, 809–820. [Google Scholar] [CrossRef]
- Merlo, D.F.; Rossi, L.; Pellegrino, C.; Ceppi, M.; Cardellino, U.; Capurro, C.; Ratto, A.; Sambucco, P.L.; Sestito, V.; Tanara, G.; et al. Cancer Incidence in Pet Dogs: Findings of the Animal Tumor Registry of Genoa, Italy. J. Vet. Intern. Med. 2008, 22, 976–984. [Google Scholar] [CrossRef]
- Dorn, C.R.; Taylor, D.O.N.; Schneider, R.; Hibbard, H.H.; Klauber, M.R. Survey of Animal Neoplasms In Alameda and Contra Costa Counties, California. II. Cancer Morbidity in DOls and Cats From Alameda Countyl,2. J. Natl. Cancer Inst. 1968, 40, 307–318. [Google Scholar]
- Grüntzig, K.; Graf, R.; Hässig, M.; Welle, M.; Meier, D.; Lott, G.; Erni, D.; Schenker, N.S.; Guscetti, F.; Boo, G.; et al. The Swiss Canine Cancer Registry: A Retrospective Study on the Occurrence of Tumours in Dogs in Switzerland from 1955 to 2008. J. Comp. Pathol. 2015, 152, 161–171. [Google Scholar] [CrossRef]
- Egenvall, A.; Bonnett, B.N.; Hedhammar, Å.; Olson, P. Mortality in over 350,000 Insured Swedish Dogs from 1995-2000: II. Breed-Specific Age and Survival Patterns and Relative Risk for Causes of Death. Acta Vet. Scand. 2005, 46, 121. [Google Scholar] [CrossRef]
- Dobson, J.M.; Samuel, S.; Milstein, H.; Rogers, K.; Wood, J.L.N. Canine Neoplasia in the UK: Estimates of Incidence Rates from a Population of Insured Dogs. J. Small Anim. Pract. 2002, 43, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.A.; Mooney, S. Feline Mammary Tumors. Vet. Clin. N. Am.—Small Anim. Pract. 1985, 15, 513–520. [Google Scholar] [CrossRef]
- Zappulli, V.; Rasotto, R.; Caliari, D.; Mainenti, M.; Peña, L.; Goldschmidt, M.H.; Kiupel, M. Prognostic Evaluation of Feline Mammary Carcinomas: A Review of the Literature. Vet. Pathol. 2015, 52, 46–60. [Google Scholar] [CrossRef]
- Sorenmo, K.U.; Rasotto, R.; Zappulli, V.; Goldschmidt, M.H. Development, Anatomy, Histology, Lymphatic Drainage, Clinical Features, and Cell Differentiation Markers of Canine Mammary Gland Neoplasms. Vet. Pathol. 2011, 48, 85–97. [Google Scholar] [CrossRef]
- Soares, M.; Correia, J.; Peleteiro, M.C.; Ferreira, F. St Gallen Molecular Subtypes in Feline Mammary Carcinoma and Paired Metastases-Disease Progression and Clinical Implications from a 3-Year Follow-up Study. Tumor Biol. 2016, 37, 4053–4064. [Google Scholar] [CrossRef]
- Goldschmidt, M.H.; Peña, L.; Rasotto, R.; Zappulli, V. Classification and Grading of Canine Mammary Tumors. Vet. Pathol. 2011, 48, 117–131. [Google Scholar] [CrossRef]
- Goldschmidt, M.H.; Peña, L.; Zappulli, V. Tumors of the Mammary Gland. In Tumors in Domestic Animals; Wiley: Hoboken, NJ, USA, 2016; pp. 723–765. [Google Scholar]
- Salas, Y.; Márquez, A.; Diaz, D.; Romero, L. Epidemiological Study of Mammary Tumors in Female Dogs Diagnosed during the Period 2002–2012: A Growing Animal Health Problem. PLoS ONE 2015, 10, e0127381. [Google Scholar] [CrossRef]
- Hambly, J.N.; Ruby, C.E.; Mourich, D.V.; Bracha, S.; Dolan, B.P. Potential Promises and Perils of Human Biological Treatments for Immunotherapy in Veterinary Oncology. Vet. Sci. 2023, 10, 336. [Google Scholar] [CrossRef]
- Mestrinho, L.A.; Santos, R.R. Translational Oncotargets for Immunotherapy: From Pet Dogs to Humans. Adv. Drug Deliv. Rev. 2021, 172, 296–313. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, A.K.; Mazcko, C.N. Improving Human Cancer Therapy through the Evaluation of Pet Dogs. Nat. Rev. Cancer 2020, 20, 727–742. [Google Scholar] [CrossRef]
- Oh, J.H.; Cho, J.Y. Comparative Oncology: Overcoming Human Cancer through Companion Animal Studies. Exp. Mol. Med. 2023, 55, 725–734. [Google Scholar] [CrossRef]
- Garden, O.A.; Volk, S.W.; Mason, N.J.; Perry, J.A. Companion Animals in Comparative Oncology: One Medicine in Action. Vet. J. 2018, 240, 6–13. [Google Scholar] [CrossRef]
- Cannon, C.M. Cats, Cancer and Comparative Oncology. Vet. Sci. 2015, 2, 111–126. [Google Scholar] [CrossRef]
- London, C.A.; Hannah, A.L.; Zadovoskaya, R.; Chien, M.B.; Kollias-Baker, C.; Rosenberg, M.; Downing, S.; Post, G.; Boucher, J.; Shenoy, N.; et al. Phase I Dose-Escalating Study of SU11654, a Small Molecule Receptor Tyrosine Kinase Inhibitor, in Dogs with Spontaneous Malignancies12. Clin. Cancer Res. 2003, 9, 2755–2768. [Google Scholar] [PubMed]
- Liao, A.T.; Chien, M.B.; Shenoy, N.; Mendel, D.B.; McMahon, G.; Cherrington, J.M.; London, C.A. Inhibition of Constitutively Active Forms of Mutant Kit by Multitargeted Indolinone Tyrosine Kinase Inhibitors. Blood 2002, 100, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Karlseder, J.; Smogorzewska, A.; de Lange, T. Senescence Induced by Altered Telomere State, Not Telomere Loss. Science (1979) 2002, 295, 2446–2449. [Google Scholar] [CrossRef]
- Barthel, F.P.; Wei, W.; Tang, M.; Martinez-Ledesma, E.; Hu, X.; Amin, S.B.; Akdemir, K.C.; Seth, S.; Song, X.; Wang, Q.; et al. Systematic Analysis of Telomere Length and Somatic Alterations in 31 Cancer Types. Nat. Genet. 2017, 49, 349–357. [Google Scholar] [CrossRef]
- Yuan, X.; Xu, D. Telomerase Reverse Transcriptase (TERT) in Action: Cross-Talking with Epigenetics. Int. J. Mol. Sci. 2019, 20, 3338. [Google Scholar] [CrossRef]
- Hubbard, K.; Ozer, H.L. Mechanism of Immortalization. Age 1999, 22, 65–69. [Google Scholar] [CrossRef]
- Wong, K.; Ludwig, L.; Krijgsman, O.; Adams, D.J.; Wood, G.A.; Van Der Weyden, L. Comparison of the Oncogenomic Landscape of Canine and Feline Hemangiosarcoma Shows Novel Parallels with Human Angiosarcoma. DMM Dis. Models Mech. 2021, 14, dmm049044. [Google Scholar] [CrossRef]
- Wong, K.; Abascal, F.; Ludwig, L.; Aupperle-Lellbach, H.; Grassinger, J.; Wright, C.W.; Allison, S.J.; Pinder, E.; Phillips, R.M.; Romero, L.P.; et al. Cross-Species Oncogenomics Offers Insight into Human Muscle-Invasive Bladder Cancer. Genome Biol. 2023, 24, 191. [Google Scholar] [CrossRef]
- Van Leeuwen, I.S.; Hellmèn, E.; Cornelisse, C.J.; Van den Burgh, B.; Rutteman, G.R. P53 Mutations in Mammary Tumor Cell Lines and Corresponding Tumor Tissues in the Dog. Anticancer Res. 1996, 16, 3737–3744. [Google Scholar]
- You, S.; Moon, J.-H.; Kim, T.-K.; Kim, S.-C.; Kim, J.-W.; Yoon, D.-H.; Kwak, S.; Hong, K.-C.; Choi, Y.-J.; Kim, H. Cellular Characteristics of Primary and Immortal Canine Embryonic Fibroblast Cells. Exp. Mol. Med. 2004, 36, 325–335. [Google Scholar] [CrossRef]
- Lee, Y.; Berríos-Vázquez, G.; Maes, R.K.; Kiupel, M.; Desmarets, L.M.B.; Nauwynck, H.J.; Soboll Hussey, G. Development of Immortalized Feline Respiratory Epithelial Cells in an Air-Liquid-Interface Culture System for Feline Herpesvirus-1 Study. Virus Res. 2023, 326, 199063. [Google Scholar] [CrossRef]
- Pelst, M.; Höbart, C.; de Rooster, H.; Devriendt, B.; Cox, E. Immortalised Canine Buccal Epithelial Cells’ CXCL8 Secretion Is Affected by Allergen Extracts, Toll-like Receptor Ligands, IL-17A and Calcitriol. Vet. Res. 2022, 53, 72. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wang, Z.; Li, J.; Li, J.; Cui, L.; Dong, J.; Meng, X.; Qian, C.; Wang, H. Immortalization Effect of SV40T Lentiviral Vectors on Canine Corneal Epithelial Cells. BMC Vet. Res. 2022, 18, 181. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, T.; Takesue, M.; Westerman, K.A.; Okitsu, T.; Sakaguchi, M.; Fukazawa, T.; Totsugawa, T.; Noguchi, H.; Yamamoto, S.; Stolz, D.B.; et al. Establishment of an Immortalized Human-Liver Endothelial Cell Line with SV40T and HTERT. Transplantation 2004, 77, 1357–1365. [Google Scholar] [CrossRef]
- Yasumura, Y.; Teshima, T.; Nagashima, T.; Takano, T.; Michishita, M.; Taira, Y.; Suzuki, R.; Matsumoto, H. Immortalized Canine Adipose-Derived Mesenchymal Stem Cells as a Novel Candidate Cell Source for Mesenchymal Stem Cell Therapy. Int. J. Mol. Sci. 2023, 24, 2250. [Google Scholar] [CrossRef] [PubMed]
- López, S.M.; Balog-Alvarez, C.; Canessa, E.H.; Hathout, Y.; Brown, K.J.; Vitha, S.; Bettis, A.K.; Boehler, J.; Kornegay, J.N.; Nghiem, P.P. Creation and Characterization of an Immortalized Canine Myoblast Cell Line: Myok9. Mamm. Genome 2020, 31, 95–109. [Google Scholar] [CrossRef]
- Desmarets, L.M.; Theuns, S.; Olyslaegers, D.A.; Dedeurwaerder, A.; Vermeulen, B.L.; Roukaerts, I.D.; Nauwynck, H.J. Establishment of Feline Intestinal Epithelial Cell Cultures for the Propagation and Study of Feline Enteric Coronaviruses. Vet. Res. 2013, 44, 71. [Google Scholar] [CrossRef]
- Olyslaegers, D.A.J.; Desmarets, L.M.B.; Dedeurwaerder, A.; Dewerchin, H.L.; Nauwynck, H.J. Generation and Characterization of Feline Arterial and Venous Endothelial Cell Lines for the Study of the Vascular Endothelium. BMC Vet. Res. 2013, 9, 170. [Google Scholar] [CrossRef]
- Uyama, R.; Hong, S.-H.; Nakagawa, T.; Yazawa, M.; Kadosawa, T.; Mochizuki, M.; Tsujimoto, H.; Nishimura, R.; Sasaki, N. Establishment and Characterization of Eight Feline Mammary Adenocarcinoma Cell Lines. J. Veter-Med. Sci. 2005, 67, 1273–1276. [Google Scholar] [CrossRef]
- Gorbunova, V.; Seluanov, A. Coevolution of Telomerase Activity and Body Mass in Mammals: From Mice to Beavers. Mech. Ageing Dev. 2009, 130, 3–9. [Google Scholar] [CrossRef]
- Prowse, K.R.; Greider, C.W. Developmental and Tissue-Specific Regulation of Mouse Telomerase and Telomere Length. Proc. Natl. Acad. Sci. USA 1995, 92, 4818–4822. [Google Scholar] [CrossRef]
- Pfeiffer, V.; Lingner, J. Replication of Telomeres and the Regulation of Telomerase. Cold Spring Harb Perspect. Biol. 2013, 5, a010405. [Google Scholar] [CrossRef]
- McKevitt, T.; Nasir, L.; Wallis, C.; Argyle, D. A Cohort Study of Telomere and Telomerase Biology in Cats. Am. J. Vet. Res. 2003, 12, 1496–1499. [Google Scholar] [CrossRef]
- Brümmendorf, T.H.; Mak, J.; Sabo, K.M.; Baerlocher, G.M.; Dietz, K.; Abkowitz, J.L.; Lansdorp, P.M. Longitudinal Studies of Telomere Length in Feline Blood Cells: Implications for Hematopoietic Stem Cell Turnover in Vivo. Exp. Hematol. 2002, 30, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Fick, L.J.; Fick, G.H.; Li, Z.; Cao, E.; Bao, B.; Heffelfinger, D.; Parker, H.G.; Ostrander, E.A.; Riabowol, K. Telomere Length Correlates with Life Span of Dog Breeds. Cell Rep. 2012, 2, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, R.A.; Allsopp, R.C.; Chin, L.; Morin, G.B.; DePinho, R.A. Expression of Mouse Telomerase Reverse Transcriptase during Development, Differentiation and Proliferation. Oncogene 1998, 16, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.-J.; Grandori, C.; Amacker, M.; Simon-Vermot, N.; Polack, A.; Lingner, J.; Dalla-Favera, R. Direct Activation of TERT Transcription by C-MYC. Nat. Genet. 1999, 21, 220–224. [Google Scholar] [CrossRef]
- Pavel, M.; Renna, M.; Park, S.J.; Menzies, F.M.; Ricketts, T.; Füllgrabe, J.; Ashkenazi, A.; Frake, R.A.; Lombarte, A.C.; Bento, C.F.; et al. Contact Inhibition Controls Cell Survival and Proliferation via YAP/TAZ-Autophagy Axis. Nat. Commun. 2018, 9, 2961. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Heimann, R.; Rice, R.H. Rat Esophageal and Epidermal Keratinocytes: Intrinsic Differences in Culture and Derivation of Continuous Lines. J. Cell Physiol. 1983, 117, 362–367. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, Q.; Luo, Z.; Yu, Y.; Xiao, N.A.; Sun, X.; Cheng, L. Spontaneous Immortalization of Mouse Liver Sinusoidal Endothelial Cells. Int. J. Mol. Med. 2015, 35, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Hassan, B.B.; Elshafae, S.M.; Supsavhad, W.; Simmons, J.K.; Dirksen, W.P.; Sokkar, S.M.; Rosol, T.J. Feline Mammary Cancer: Novel Nude Mouse Model and Molecular Characterization of Invasion and Metastasis Genes. Vet. Pathol. 2017, 54, 32–43. [Google Scholar] [CrossRef]
- Chen, C.; Lin, W.; Huang, Y.; Chen, X.; Wang, H.; Teng, L. The Essential Factors of Establishing Patient-Derived Tumor Model. J. Cancer 2021, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Rangarajan, A.; Hong, S.J.; Gifford, A.; Weinberg, R.A. Species-and Cell Type-Specific Requirements for Cellular Transformation. Cancer Cell 2013, 3, 171–183. [Google Scholar] [CrossRef]
- Hahn, W.C.; Dessain, S.K.; Brooks, M.W.; King, J.E.; Elenbaas, B.; Sabatini, D.M.; DeCaprio, J.A.; Weinberg, R.A. Enumeration of the Simian Virus 40 Early Region Elements Necessary for Human Cell Transformation. Mol. Cell Biol. 2002, 22, 2111–2123. [Google Scholar] [CrossRef]
- Firsanov, D.; Zacher, M.; Tian, X.; Zhao, Y.; George, J.C.; Sformo, T.L.; Ali Biashad, S.; Gilman, A.; Hamilton, N.; Patel, A.; et al. DNA Repair and Anti-Cancer Mechanisms in the Longest-Living Mammal: The 1 Bowhead Whale 2 3. BioRxiv 2023. [Google Scholar] [CrossRef]
- Vincze, O.; Colchero, F.; Lemaître, J.F.; Conde, D.A.; Pavard, S.; Bieuville, M.; Urrutia, A.O.; Ujvari, B.; Boddy, A.M.; Maley, C.C.; et al. Cancer Risk across Mammals. Nature 2022, 601, 263–267. [Google Scholar] [CrossRef]
- Eun, K.; Park, M.G.; Jeong, Y.W.; Jeong, Y.I.; Hyun, S.H.; Hwang, W.S.; Kim, S.H.; Kim, H. Establishment of TP53-Knockout Canine Cells Using Optimized CRIPSR/Cas9 Vector System for Canine Cancer Research. BMC Biotechnol. 2019, 19, 1. [Google Scholar] [CrossRef]
- Yelle, J.; Lussier, G.; Pramatarova, A.; Hamelin, C. Low Tumorigenicity of Canine Cells Transformed by the Human Cytomegalovirus. Biol. Cell 1990, 70, 9–18. [Google Scholar] [CrossRef]
- Geder, L.; Lausch, R.; O’Neill, F.; Rapp, F. Oncogenic Transformation of Human Embryo Lung Cells by Human Cytomegalovirus. Science (1979) 1976, 192, 1134–1137. [Google Scholar] [CrossRef] [PubMed]
- Morel, A.P.; Hinkal, G.W.; Thomas, C.; Fauvet, F.; Courtois-Cox, S.; Wierinckx, A.; Devouassoux-Shisheboran, M.; Treilleux, I.; Tissier, A.; Gras, B.; et al. EMT Inducers Catalyze Malignant Transformation of Mammary Epithelial Cells and Drive Tumorigenesis towards Claudin-Low Tumors in Transgenic Mice. PLoS Genet. 2012, 8, e1002723. [Google Scholar] [CrossRef] [PubMed]
- Meyers, J.; Craig, J.; Odde, D.J.J. Potential for Control of Signaling Pathways via Cell Size and Shape. Curr. Biol. 2006, 16, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- De Belly, H.; Stubb, A.; Yanagida, A.; Labouesse, C.; Jones, P.H.; Paluch, E.K.; Chalut, K.J. Membrane Tension Gates ERK-Mediated Regulation of Pluripotent Cell Fate. Cell Stem Cell 2021, 28, 273–284.e6. [Google Scholar] [CrossRef]
- Wrzesinski, K.; Rogowska-Wrzesinska, A.; Kanlaya, R.; Borkowski, K.; Schwämmle, V.; Dai, J.; Joensen, K.E.; Wojdyla, K.; Carvalho, V.B.; Fey, S.J. The Cultural Divide: Exponential Growth in Classical 2D and Metabolic Equilibrium in 3D Environments. PLoS ONE 2014, 9, e106973. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, X.; Dowbaj, A.M.; Sljukic, A.; Bratlie, K.; Lin, L.; Fong, E.L.S.; Balachander, G.M.; Chen, Z.; Soragni, A.; et al. Organoids. Nat. Rev. Methods Primers 2022, 2, 94. [Google Scholar] [CrossRef]
- McKee, C.; Chaudhry, G.R. Advances and Challenges in Stem Cell Culture. Colloids Surf. B Biointerfaces 2017, 159, 62–77. [Google Scholar] [CrossRef]
- Cocola, C.; Molgora, S.; Piscitelli, E.; Veronesi, M.C.; Greco, M.; Bragato, C.; Moro, M.; Crosti, M.; Gray, B.; Milanesi, L.; et al. FGF2 and EGF Are Required for Self-Renewal and Organoid Formation of Canine Normal and Tumor Breast Stem Cells. J. Cell Biochem. 2017, 118, 570–584. [Google Scholar] [CrossRef]
- Sato, T.; Vries, R.G.; Snippert, H.J.; Van De Wetering, M.; Barker, N.; Stange, D.E.; Van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 Stem Cells Build Crypt-Villus Structures in Vitro without a Mesenchymal Niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef]
- Sato, T.; Clevers, H. Growing Self-Organizing Mini-Guts from a Single Intestinal Stem Cell: Mechanism and Applications. Science 2013, 340, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Kessler, M.; Hoffmann, K.; Brinkmann, V.; Thieck, O.; Jackisch, S.; Toelle, B.; Berger, H.; Mollenkopf, H.J.; Mangler, M.; Sehouli, J.; et al. The Notch and Wnt Pathways Regulate Stemness and Differentiation in Human Fallopian Tube Organoids. Nat. Commun. 2015, 6, 8989. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Renner, M.; Martin, C.A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral Organoids Model Human Brain Development and Microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef]
- Sutherland, R.M.; McCredie, J.A.; Inch, W.R. Growth of Multicell Spheroids in Tissue Culture as a Model of Nodular Carcinomas2. J. Natl. Cancer Inst. 1971, 46, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Hainline, K.M.; Gu, F.; Handley, J.F.; Tian, Y.F.; Wu, Y.; de Wet, L.; Vander Griend, D.J.; Collier, J.H. Self-Assembling Peptide Gels for 3D Prostate Cancer Spheroid Culture. Macromol. Biosci. 2019, 19, e1800249. [Google Scholar] [CrossRef]
- Dorrigiv, D.; Goyette, P.A.; St-Georges-Robillard, A.; Mes-Masson, A.M.; Gervais, T. Pixelated Microfluidics for Drug Screening on Tumour Spheroids and Ex Vivo Microdissected Tumour Explants. Cancers 2023, 15, 1060. [Google Scholar] [CrossRef]
- Petersen, O.W.; R0nnov-Jessen, L.; Howlettt, A.R.; Bissellt, M.J. Interaction with Basement Membrane Serves to Rapidly Distinguish Growth and Differentiation Pattern of Normal and Malignant Human Breast Epithelial Cells (Extracelular Matrix/Rapid Transformation Assay/Breast Cancer/Tissue Structure and Function). Proc. Natl. Acad. Sci. USA 1992, 89, 9064–9068. [Google Scholar] [CrossRef]
- Inglebert, M.; Dettwiler, M.; Hahn, K.; Letko, A.; Drogemuller, C.; Doench, J.; Brown, A.; Memari, Y.; Davies, H.R.; Degasperi, A.; et al. A Living Biobank of Canine Mammary Tumor Organoids as a Comparative Model for Human Breast Cancer. Sci. Rep. 2022, 12, 18051. [Google Scholar] [CrossRef]
- Elbadawy, M.; Fujisaka, K.; Yamamoto, H.; Tsunedomi, R.; Nagano, H.; Ayame, H.; Ishihara, Y.; Mori, T.; Azakami, D.; Uchide, T.; et al. Establishment of an Experimental Model of Normal Dog Bladder Organoid Using a Three-Dimensional Culture Method. Biomed. Pharmacother. 2022, 151, 113105. [Google Scholar] [CrossRef]
- Goldhammer, N.; Kim, J.; Timmermans-Wielenga, V.; Petersen, O.W. Characterization of Organoid Cultured Human Breast Cancer. Breast Cancer Res. 2019, 21, 141. [Google Scholar] [CrossRef] [PubMed]
- Schwerd-Kleine, P.; Würth, R.; Cheytan, T.; Michel, L.; Thewes, V.; Gutjahr, E.; Seker-Cin, H.; Kazdal, D.; Neuberth, S.-J.; Thiel, V.; et al. Biopsy-Derived Organoids in Personalised Early Breast Cancer Care: Challenges of Tumour Purity and Normal Cell Overgrowth Cap Their Practical Utility. Int. J. Cancer 2025, 156, 2200–2209. [Google Scholar] [CrossRef]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective Identification of Tumorigenic Breast Cancer Cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef]
- Singh, S.K.; Hawkins, C.; Clarke, I.D.; Squire, J.A.; Bayani, J.; Hide, T.; Henkelman, R.M.; Cusimano, M.D.; Dirks, P.B. Identification of Human Brain Tumour Initiating Cells. Nature 2004, 432, 396–401. [Google Scholar] [CrossRef]
- Cocola, C.; Anastasi, P.; Astigiano, S.; Piscitelli, E.; Pelucchi, P.; Vilardo, L.; Bertoli, G.; Beccaglia, M.; Veronesi, M.C.; Sanzone, S.; et al. Isolation of Canine Mammary Cells with Stem Cell Properties and Tumour-Initiating Potential. Reprod. Domest. Anim. 2009, 44, 214–217. [Google Scholar] [CrossRef]
- Ward, R.J.; Dirks, P.B. Cancer Stem Cells: At the Headwaters of Tumor Development. Annu. Rev. Pathol. 2007, 2, 175–189. [Google Scholar] [CrossRef]
- Goldschneider, D.; Mehlen, P. Dependence Receptors: A New Paradigm in Cell Signaling and Cancer Therapy. Oncogene 2010, 29, 1865–1882. [Google Scholar] [CrossRef]
- Norval, M.; Maingay, J.; Else, R.W. Characteristics of a Feline Mammary Carcinoma Cell Line. Res. Vet. Sci. 1985, 2, 157–164. [Google Scholar] [CrossRef]
- Minke, J.M.; Schuuring, E.; van den Berghe, R.; Stolwijk, J.A.; Boonstra, J.; Cornelisse, C.; Hilkens, J.; Misdorp, W. Isolation of Two Distinct Epithelial Cell Lines from a Single Feline Mammary Carcinoma with Different Tumorigenic Potential in Nude Mice and Expressing Different Levels of Epidermal Growth Factor Receptors. Cancer Res. 1991, 51, 4028–4037. [Google Scholar]
- Kruitwagen, H.S.; Oosterhoff, L.A.; Vernooij, I.G.W.H.; Schrall, I.M.; van Wolferen, M.E.; Bannink, F.; Roesch, C.; van Uden, L.; Molenaar, M.R.; Helms, J.B.; et al. Long-Term Adult Feline Liver Organoid Cultures for Disease Modeling of Hepatic Steatosis. Stem Cell Rep. 2017, 8, 822–830. [Google Scholar] [CrossRef]
- Haaker, M.W.; Kruitwagen, H.S.; Vaandrager, A.B.; Houweling, M.; Penning, L.C.; Molenaar, M.R.; van Wolferen, M.E.; Oosterhoff, L.A.; Spee, B.; Helms, J.B. Identification of Potential Drugs for Treatment of Hepatic Lipidosis in Cats Using an in Vitro Feline Liver Organoid System. J. Vet. Intern. Med. 2020, 34, 132–138. [Google Scholar] [CrossRef]
- Tekes, G.; Ehmann, R.; Boulant, S.; Stanifer, M.L. Development of Feline Ileum-and Colon-Derived Organoids and Their Potential Use to Support Feline Coronavirus Infection. Cells 2020, 9, 2085. [Google Scholar] [CrossRef]
- van der Burg, B.; van Selm-Miltenburg, A.J.; van Maurik, P.; Rutteman, G.R.; Misdorp, W.; de Laat, S.W.; van Zoelen, E.J. Isolation of Autonomously Growing Dog Mammary Tumor Cell Lines Cultured in Medium Supplemented with Serum Treated to Inactivate Growth Factors. J. Natl. Cancer Inst. 1989, 81, 1545–1551. [Google Scholar] [CrossRef]
- Priosoeryanto, B.P.; Tateyama, S.; Yamaguchi, R.; Uchida, K. Establishment of a Cell Line (MCM-B2) from a Benign Mixed Tumour of Canine Mammary Gland. Res. Vet. Sci. 1995, 58, 272–276. [Google Scholar] [CrossRef]
- Hellmén, E. Characterization of Four in Vitro Established Canine Mammary Carcinoma and One Atypical Benign Mixed Tumor Cell Lines. Vitr. Cell Dev. Biol. 1992, 28, 309–319. [Google Scholar] [CrossRef]
- Uyama, R.; Nakagawa, T.; Hong, S.-H.; Mochizuki, M.; Nishimura, R.; Sasaki, N. Establishment of Four Pairs of Canine Mammary Tumour Cell Lines Derived from Primary and Metastatic Origin and Their E-Cadherin Expression. Vet. Comp. Oncol. 2006, 4, 104–113. [Google Scholar] [CrossRef]
- Caceres, S.; Peña, L.; DeAndres, P.J.; Illera, M.J.; Lopez, M.S.; Woodward, W.A.; Reuben, J.M.; Illera, J.C. Establishment and Characterization of a New Cell Line of Canine Inflammatory Mammary Cancer: IPC-366. PLoS ONE 2015, 10, e0122277. [Google Scholar] [CrossRef]
- Mei, C.; Xin, L.; Liu, Y.; Lin, J.; Xian, H.; Zhang, X.; Hu, W.; Xia, Z.; Wang, H.; Lyu, Y. Establishment of a New Cell Line of Canine Mammary Tumor CMT-1026. Front. Vet. Sci. 2021, 8, 744032. [Google Scholar] [CrossRef]
- Li, R.; Wu, H.; Sun, Y.; Zhu, J.; Tang, J.; Kuang, Y.; Li, G. A Novel Canine Mammary Cancer Cell Line: Preliminary Identification and Utilization for Drug Screening Studies. Front. Vet. Sci. 2021, 8, 665906. [Google Scholar] [CrossRef]
- Yeom, J.; Cho, Y.; Ahn, S.; Jeung, S. Anticancer Effects of Alpelisib on PIK3CA-Mutated Canine Mammary Tumor Cell Lines. Front. Vet. Sci. 2023, 10, 1279535. [Google Scholar] [CrossRef]
- Park, S.Y.; Baek, Y.B.; Lee, C.H.; Kim, H.J.; Kim, H.P.; Jeon, Y.J.; Song, J.E.; Jung, S.B.; Kim, H.J.; Moon, K.S.; et al. Establishment of Canine Mammary Gland Tumor Cell Lines Harboring PI3K/Akt Activation as a Therapeutic Target. BMC Vet. Res. 2024, 20, 233. [Google Scholar] [CrossRef]
- Borges, A.; Adega, F.; Chaves, R. Establishment and Characterization of a New Feline Mammary Cancer Cell Line, FkMTp. Cytotechnology 2016, 68, 1529–1543. [Google Scholar] [CrossRef]
- Granados-Soler, J.L.; Junginger, J.; Hewicker-Trautwein, M.; Bornemann-Kolatzki, K.; Beck, J.; Brenig, B.; Betz, D.; Schille, J.T.; Murua Escobar, H.; Nolte, I. TiHo-0906: A New Feline Mammary Cancer Cell Line with Molecular, Morphological, and Immunocytological Characteristics of Epithelial to Mesenchymal Transition. Sci. Rep. 2018, 8, 13231. [Google Scholar] [CrossRef]
- Correia, A.S.; Matos, R.; Gärtner, F.; Amorim, I.; Vale, N. High Drug Resistance in Feline Mammary Carcinoma Cell Line (FMCm) and Comparison with Human Breast Cancer Cell Line (MCF-7). Animals 2021, 11, 2321. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Casper, J.; Bradley, E.; Sandbach, J.; Jones, D.; Makuch, R. Association between Human Tumor Colony-Forming Assay Results and Response of an Individual Patient’s Tumor to Chemotherapy. Am. J. Med. 1981, 70, 1027–1032. [Google Scholar] [CrossRef]
- Geevimaan, K.; Guo, J.-Y.; Shen, C.-N.; Jiang, J.-K.; Fann, C.S.J.; Hwang, M.-J.; Shui, J.-W.; Lin, H.-T.; Wang, M.-J.; Shih, H.-C.; et al. Patient-Derived Organoid Serves as a Platform for Personalized Chemotherapy in Advanced Colorectal Cancer Patients. Front. Oncol. 2022, 12, 883437. [Google Scholar] [CrossRef]
- Maeda, M.; Ochiai, K.; Michishita, M.; Morimatsu, M.; Sakai, H.; Kinoshita, N.; Sakaue, M.; Onozawa, E.; Azakami, D.; Yamamoto, M.; et al. In Vitro Anticancer Effects of Alpelisib against PIK3CA-mutated Canine Hemangiosarcoma Cell Lines. Oncol. Rep. 2022, 47, 84. [Google Scholar] [CrossRef] [PubMed]
- Granados-Soler, J.L.; Bornemann-Kolatzki, K.; Beck, J.; Brenig, B.; Schütz, E.; Betz, D.; Junginger, J.; Hewicker-Trautwein, M.; Escobar, H.M.; Nolte, I. Analysis of Copy-Number Variations and Feline Mammary Carcinoma Survival. Sci. Rep. 2020, 10, 1003. [Google Scholar] [CrossRef]
- Kruczynski, A.; Kiss, R. Evidence of a Direct Relationship between the Increase in the in Vitro Passage Number of Human Non-Small-Cell-Lung Cancer Primocultures and Their Chemosensitivity. Lung Cancer 1994, 10, 418. [Google Scholar] [CrossRef]
- Gameiro, A.; Nascimento, C.; Correia, J.; Ferreira, F. HER2-Targeted Immunotherapy and Combined Protocols Showed Promising Antiproliferative Effects in Feline Mammary Carcinoma Cell-Based Models. Cancers 2021, 13, 2007. [Google Scholar] [CrossRef] [PubMed]
- Gradauskaite, V.; Inglebert, M.; Doench, J.; Scherer, M.; Dettwiler, M.; Wyss, M.; Shrestha, N.; Rottenberg, S.; Plattet, P. LRP6 Is a Functional Receptor for Attenuated Canine Distemper Virus. mBio 2023, 14, e0311422. [Google Scholar] [CrossRef] [PubMed]
- Inglebert, M.; Dettwiler, M.; He, C.; Markkanen, E.; Opitz, L.; Naguleswaran, A.; Rottenberg, S. Individualized Pooled CRISPR/Cas9 Screenings Identify CDK2 as a Druggable Vulnerability in a Canine Mammary Carcinoma Patient. Vet. Sci. 2025, 12, 183. [Google Scholar] [CrossRef]
- Cocco, S.; Piezzo, M.; Calabrese, A.; Cianniello, D.; Caputo, R.; Di Lauro, V.; Fusco, G.; di Gioia, G.; Licenziato, M.; de Laurentiis, M. Biomarkers in Triple-Negative Breast Cancer: State-of-the-Art and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 4579. [Google Scholar] [CrossRef]
- Wiese, D.A.; Thaiwong, T.; Yuzbasiyan-Gurkan, V.; Kiupel, M. Feline Mammary Basal-like Adenocarcinomas: A Potential Model for Human Triple-Negative Breast Cancer (TNBC) with Basal-like Subtype. BMC Cancer 2013, 13, 403. [Google Scholar] [CrossRef]
- Govoni, V.M.; Da Silva, T.C.; Guerra, J.M.; Pereira, I.V.A.; Queiroga, F.L.; Cogliati, B. Genetic Variants of BRCA1 and BRCA2 Genes in Cats with Mammary Gland Carcinoma. Vet. Comp. Oncol. 2021, 19, 404–408. [Google Scholar] [CrossRef]
- Meijer, T.G.; Nguyen, L.; Van Hoeck, A.; Sieuwerts, A.M.; Verkaik, N.S.; Ladan, M.M.; Ruigrok-Ritstier, K.; van Deurzen, C.H.M.; van de Werken, H.J.G.; Lips, E.H.; et al. Functional RECAP (REpair CAPacity) Assay Identifies Homologous Recombination Deficiency Undetected by DNA-Based BRCAness Tests. Oncogene 2022, 41, 3498–3506. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.A.; Gogola, E.; Sachs, N.; Barazas, M.; Annunziato, S.; De Ruiter, J.R.; Velds, A.; Blatter, S.; Houthuijzen, J.M.; Van De Ven, M.; et al. BRCA-Deficient Mouse Mammary Tumor Organoids to Study Cancer-Drug Resistance. Nat. Methods 2018, 15, 134–140. [Google Scholar] [CrossRef]
- Mcmillin, D.W.; Negri, J.M.; Mitsiades, C.S. The Role of Tumour-Stromal Interactions in Modifying Drug Response: Challenges and Opportunities. Nat. Rev. Drug Discov. 2013, 12, 217–228. [Google Scholar] [CrossRef]
- Pulz, L.H.; Cordeiro, Y.G.; Huete, G.C.; Cadrobbi, K.G.; Rochetti, A.L.; Xavier, P.L.P.; Nishiya, A.T.; de Freitas, S.H.; Fukumasu, H.; Strefezzi, R.F. Intercellular Interactions between Mast Cells and Stromal Fibroblasts Obtained from Canine Cutaneous Mast Cell Tumours. Sci. Rep. 2021, 11, 23881. [Google Scholar] [CrossRef]
- Majety, M.; Pradel, L.P.; Gies, M.; Ries, C.H. Fibroblasts Influence Survival and Therapeutic Response in a 3D Co-Culture Model. PLoS ONE 2015, 10, e0127948. [Google Scholar] [CrossRef]
- Richter, A.; Feßler, A.T.; Böttner, A.; Köper, L.M.; Wallmann, J.; Schwarz, S. Reasons for Antimicrobial Treatment Failures and Predictive Value of In-Vitro Susceptibility Testing in Veterinary Practice: An Overview. Vet. Microbiol. 2020, 245, 108694. [Google Scholar] [CrossRef] [PubMed]
- Lorian, V.; Burns, L. Predictive Value of Susceptibility Tests for the Outcome of Antibacterial Therapy. J. Antimicrob. Chemother. 1990, 25, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Chu, H.; Jin, Z.; Long, H.; Zhu, B. High-Throughput Single-Cell Sequencing in Cancer Research. Signal Transduct. Target. Ther. 2022, 7, 145. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, C.; Rottenberg, S. Advancing In Vitro Tools for Oncologic Research in Cats and Dogs. Vet. Sci. 2025, 12, 815. https://doi.org/10.3390/vetsci12090815
He C, Rottenberg S. Advancing In Vitro Tools for Oncologic Research in Cats and Dogs. Veterinary Sciences. 2025; 12(9):815. https://doi.org/10.3390/vetsci12090815
Chicago/Turabian StyleHe, Chang, and Sven Rottenberg. 2025. "Advancing In Vitro Tools for Oncologic Research in Cats and Dogs" Veterinary Sciences 12, no. 9: 815. https://doi.org/10.3390/vetsci12090815
APA StyleHe, C., & Rottenberg, S. (2025). Advancing In Vitro Tools for Oncologic Research in Cats and Dogs. Veterinary Sciences, 12(9), 815. https://doi.org/10.3390/vetsci12090815






