Live Yeast Supplementation Attenuates the Effects of Heat Stress in Dairy Cows
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Housing, and Feeding
2.2. Measurements
2.3. Chemical Analysis
2.4. Statistical Analysis
- µ = overall mean;
- bl = main effect of block;
- di = main effect of diet;
- b = regression coefficients of daily temperature or THI within diet;
- x = daily temperature or THI covariates within diet;
- cow(di) = random effect of cow nested within diet;
- prelim = preliminary period covariables;
- e = random residual error, assuming N ~ (0, σ2e).
- ─
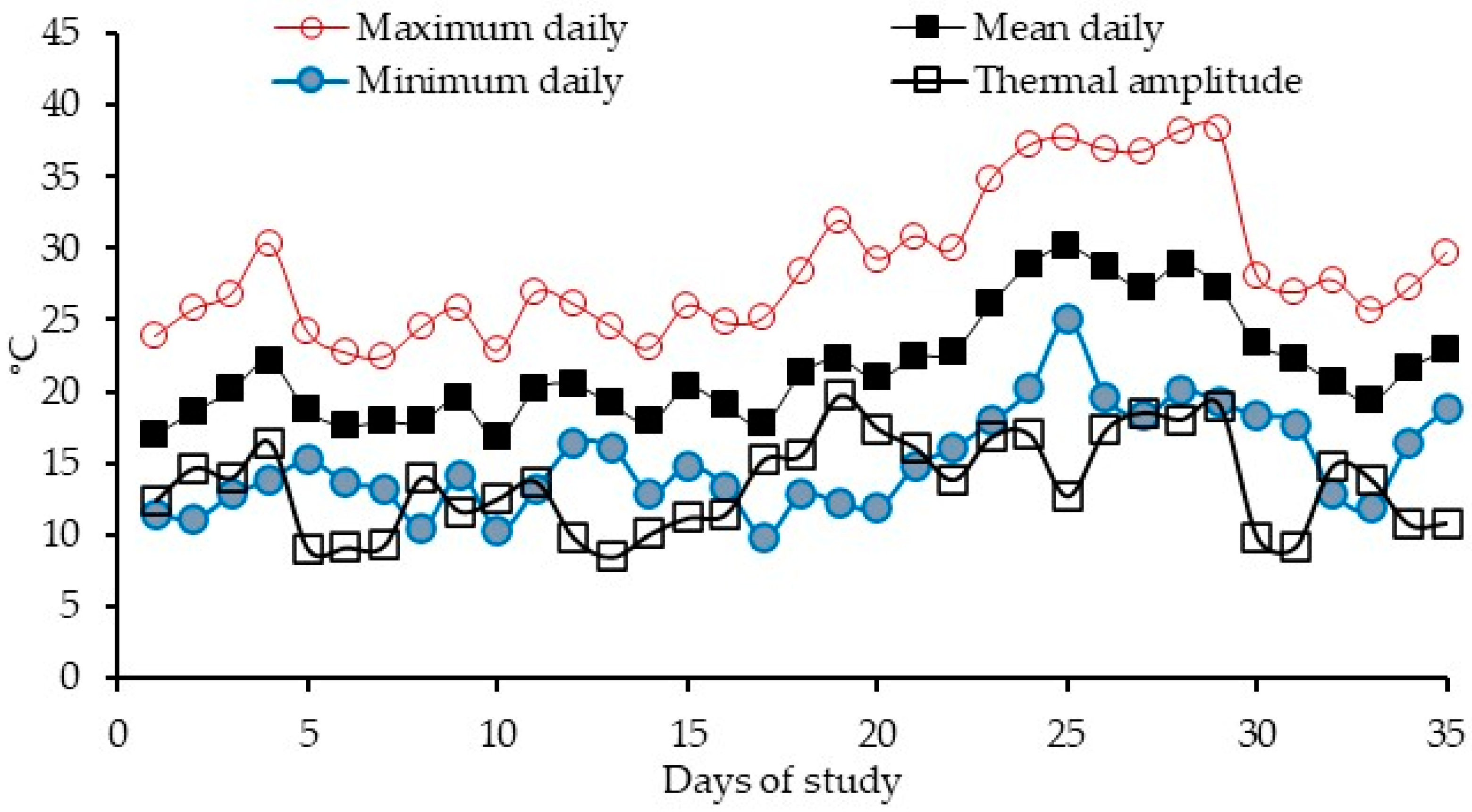
- Minimum: 9–14, 14–19, 19–24, 24–29;
- ─
- Mean: 16–19, 19–22, 22–25, 25–28, 28–31;
- ─
- Maximum: 20–23, 23–26, 26–29, 29–32;
- ─
- Amplitude: 8–10, 10–12, 12–14, 14–16, 16–18, 18–20.
- ─
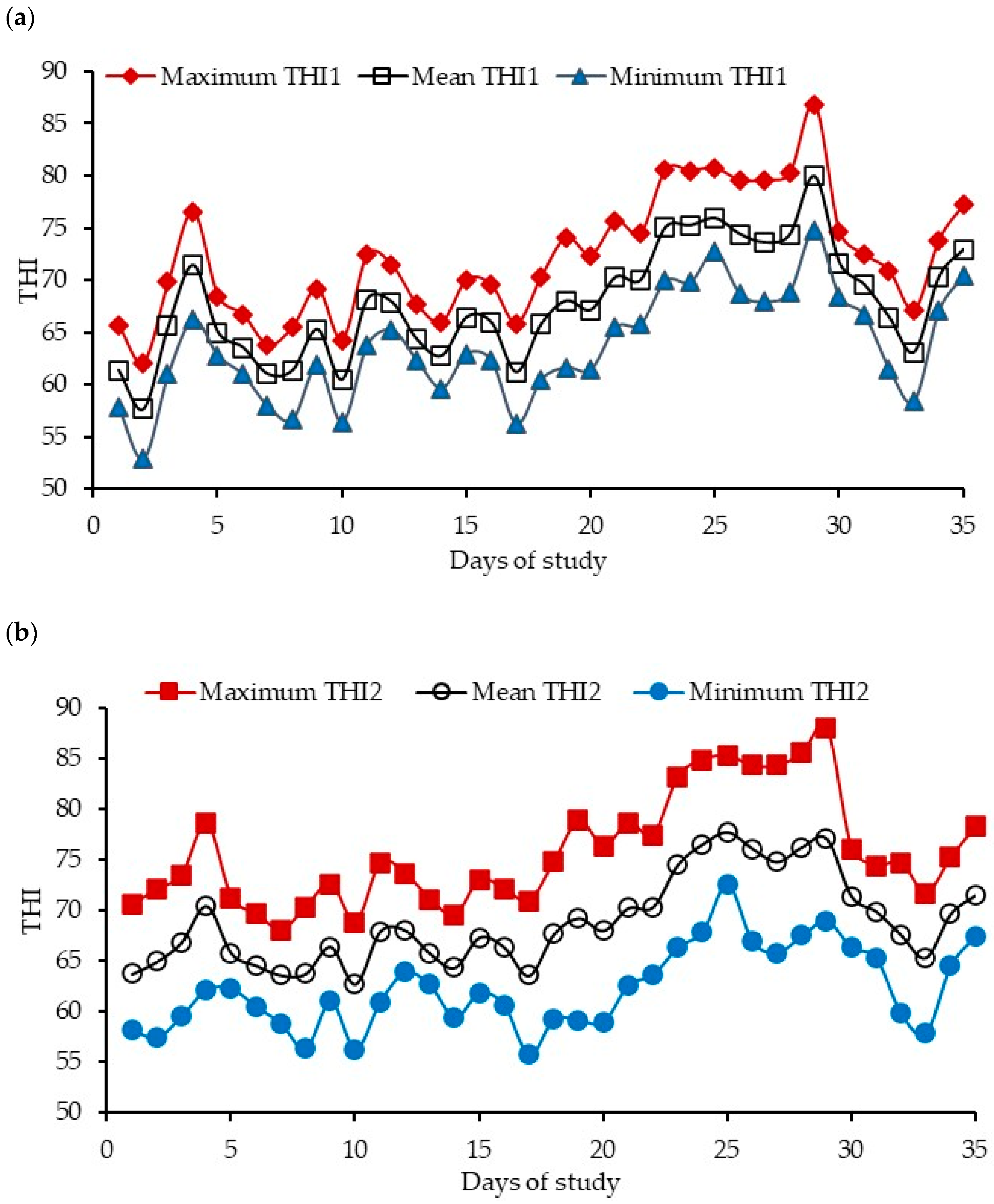
- Minimum: 54–60, 60–66, 66–72, 72–78;
- ─
- Mean: 54–60, 60–66, 66–72, 72–78, 78–84;
- ─
- Maximum: 60–66, 66–72, 72–78, 78–84, 84–90.
- ─
- Minimum: 54–60, 60–66, 66–72, 72–78;
- ─
- Mean: 60–66, 66–72, 72–78;
- ─
- Maximum: 66–72, 72–78, 78–84, 84–90.
3. Results
3.1. Lactation Performance
3.2. Relationships Between Environmental Parameters and Animal Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADF | Acid detergent fiber |
| ADL | Acid detergent lignin |
| CP | Crude protein |
| DIM | Days in milk |
| DM | Dry matter |
| DMI | Dry matter intake |
| ECM | Energy-corrected milk |
| EE | Ether extract |
| LRT | Likelihood ratio test |
| NDF | Neutral detergent fiber |
| SEM | Standard error of the mean |
| SNFs | Solids not fat |
| Tdp | Dew point temperature |
| THI | Thermal humidity index |
| TMR | Total mixed ration |
| Twb | Wet bulb temperature |
| VFA | Volatile fatty acid |
References
- Oliveira, C.P.; Sousa, F.C.; Silva, A.L.; Schultz, É.B.; Valderrama Londoño, R.I.; Souza, P.A.R. Heat stress in dairy cows: Impacts, identification, and mitigation strategies—A review. Animals 2025, 15, 249. [Google Scholar] [CrossRef]
- Cartwright, S.L.; Schmied, J.; Karrow, N.; Mallard, B.A. Impact of heat stress on dairy cattle and selection strategies for thermotolerance: A review. Front. Vet. Sci. 2023, 10, 1198697. [Google Scholar] [CrossRef] [PubMed]
- Ogundeji, A.A.; Lakew, H.; Tesfuhuney, W.; Lombard, W. Influence of heat stress on milk production and its financial implications in semi-arid areas of South Africa. Heliyon 2021, 7, e06202. [Google Scholar] [CrossRef]
- St-Pierre, N.R.; Cobanov, B.; Schnitkey, G. Economic losses from heat stress by US livestock industries. J. Dairy Sci. 2003, 86, E52–E77. [Google Scholar] [CrossRef]
- Mader, T.L. Environmental stress in confined beef cattle. J. Anim. Sci. 2003, 81 (Suppl. 2), E110–E119. [Google Scholar] [CrossRef]
- Bohmanova, J.; Misztal, I.; Cole, J.B. Temperature-humidity indices as indicators of milk production losses due to heat stress. J. Dairy Sci. 2007, 90, 1947–1956. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.S.; Misztal, I.; Bohmanova, J.; West, J. Utility of on- and off-farm weather records for studies in genetics of heat tolerance. Livest. Sci. 2006, 105, 223–228. [Google Scholar] [CrossRef]
- Ravagnolo, O.; Misztal, I. Studies on genetics of heat tolerance in dairy cattle with reduced weather information via cluster analysis. J. Dairy Sci. 2002, 85, 1586–1589. [Google Scholar] [CrossRef]
- Singaravadivelan, A.; Prasad, A.; Balusami, C.; Harikumar, S.; Beena, V.; Gleeja, V.L.; Sejian, V.; Vijayakumar, P.; Sachin, P.B.; Praveen Kumar, I.; et al. Navigating the Labyrinth of heat stress assessment in dairy cattle: A comprehensive review of methods and emerging technologies. Comput. Electron. Agric. 2025, 237, 110517. [Google Scholar] [CrossRef]
- Armstrong, D.V. Heat stress interaction with shade and cooling. J. Dairy Sci. 1994, 77, 2044–2050. [Google Scholar] [CrossRef]
- Huber, J.T.; Higginbotham, G.; Gomez-Alarcon, R.A.; Taylor, R.B.; Chen, K.H.; Chan, S.C.; Wu, Z. Heat stress interactions with protein, supplemental fat, and fungal cultures. J. Dairy Sci. 1994, 77, 2080–2090. [Google Scholar] [CrossRef]
- West, J.W. Effects of heat-stress on production in dairy cattle. J. Dairy Sci. 2003, 86, 2131–2144. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Ramos, S.C.; Valencia, R.A.; Cho, Y.I.; Lee, S.S. Heat stress: Effects on rumen microbes and host physiology, and strategies to alleviate the negative impacts on lactating dairy cows. Front. Microbiol. 2022, 13, 804562. [Google Scholar] [CrossRef]
- Dann, H.M.; Drackley, J.K.; McCoy, G.C.; Hutjens, M.F.; Garrett, J.E. Effects of yeast culture (Saccharomyces cerevisiae) on prepartum intake and postpartum intake and milk production of Jersey cows. J. Dairy Sci. 2000, 83, 123–127. [Google Scholar] [CrossRef]
- Moallem, U.; Lehrer, H.; Livshitz, L.; Zachut, M.; Yakoby, S. The effects of live yeast supplementation to dairy cows during the hot season on production, feed efficiency, and digestibility. J. Dairy Sci. 2009, 92, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Salvati, G.G.S.; Morais Júnior, N.N.; Melo, A.C.S.; Vilela, R.R.; Cardoso, F.F.; Aronovich, M.; Pereira, R.A.N.; Pereira, M.N. Response of lactating cows to live yeast supplementation during summer. J. Dairy Sci. 2015, 98, 4062–4073. [Google Scholar] [CrossRef]
- Bruno, R.G.S.; Rutigliano, H.M.; Cerri, R.L.; Robinson, P.H.; Santos, J.E.P. Effect of feeding Saccharomyces Cerevisiae on performance of dairy cows during summer heat stress. Anim. Feed Sci. Technol. 2009, 150, 175–186. [Google Scholar] [CrossRef]
- Takiya, C.S.; Chesini, R.G.; de Freitas, A.C.; Grigoletto, N.T.S.; Vieira, D.J.C.; Poletti, G.; Martins, N.P.; Sbaralho, O.P.; Roth, N.; Acedo, T.; et al. Dietary supplementation with live or autolyzed yeast: Effects on performance, nutrient digestibility, and ruminal fermentation in dairy cows. J. Dairy Sci. 2024, 107, 4495–4508. [Google Scholar] [CrossRef]
- Muñoz, C.; Wills, D.A.; Yan, T. Effects of dietary active dried yeast (Saccharomyces cerevisiae) supply at two levels of concentrate on energy and nitrogen utilisation and methane emissions of lactating dairy cows. Anim. Prod. Sci. 2017, 57, 656–664. [Google Scholar] [CrossRef]
- Marden, J.P.; Julien, C.; Monteils, V.; Auclair, E.; Moncoulon, R.; Bayourthe, C. How Does Live Yeast Differ from Sodium Bicarbonate to Stabilize Ruminal pH in High-Yielding Dairy Cows? J. Dairy Sci. 2008, 91, 3528–3535. [Google Scholar] [CrossRef]
- Jiang, Y.; Ogunade, I.M.; Qi, S.; Hackmann, T.J.; Staples, C.R.; Adesogan, A.T. Effects of the dose and viability of Saccharomyces cerevisiae. 1. Diversity of ruminal microbes as analyzed by Illumina MiSeq sequencing and quantitative PCR. J. Dairy Sci. 2017, 100, 325–342. [Google Scholar] [CrossRef]
- Perdomo, M.C.; Marsola, R.S.; Favoreto, M.G.; Adesogan, A.; Staples, C.R.; Santos, J.E.P. Effects of feeding live yeast at 2 dosages on performance and feeding behavior of dairy cows under heat stress. J. Dairy Sci. 2020, 103, 325–339. [Google Scholar] [CrossRef]
- Dias, A.L.G.; Freitas, J.A.; Micai, B.; Azevedo, R.A.; Greco, L.F.; Santos, J.E.P. Effect of supplemental yeast culture and dietary starch content on rumen fermentation and digestion in dairy cows. J. Dairy Sci. 2018, 101, 201–221. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.S.; Ying, Y. Effects of Saccharomyces cerevisiae fermentation product on ruminal starch digestion are dependent upon dry matter intake for lactating cows. J. Dairy Sci. 2012, 95, 6591–6605. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.L.G.; Freitas, J.A.; Micai, B.; Azevedo, R.A.; Greco, L.F.; Santos, J.E.P. Effects of supplementing yeast culture to diets differing in starch content on performance and feeding behavior of dairy cows. J. Dairy Sci. 2018, 101, 186–200. [Google Scholar] [CrossRef]
- Bach, A.; Iglesias, C.; Devant, M. Daily rumen pH pattern of loose-housed dairy cattle as affected by feeding pattern and live yeast supplementation. Anim. Feed Sci. Technol. 2007, 136, 146–153. [Google Scholar] [CrossRef]
- DeVries, T.J.; Chevaux, E. Modification of the feeding behavior of dairy cows through live yeast supplementation. J. Dairy Sci. 2014, 97, 6499–6510. [Google Scholar] [CrossRef]
- Wohlt, J.E.; Corcione, T.T.; Zajac, P.K. Effect of yeast on feed intake and performance of cows fed diets based on corn silage during early lactation. J. Dairy Sci. 1998, 81, 1345–1352. [Google Scholar] [CrossRef]
- Wallace, R.J. Ruminal microbiology, biotechnology, and ruminant nutrition: Progress and problems. J. Anim. Sci. 1994, 72, 2992–3003. [Google Scholar] [CrossRef]
- Piva, G.; Belladonna, S.; Fusconi, G.; Sicbaldi, F. Effects of yeast on dairy cow performance, ruminal fermentation, blood components, and milk manufacturing properties. J. Dairy Sci. 1993, 76, 2717–2722. [Google Scholar] [CrossRef]
- Chaucheyras-Durand, F.; Walker, N.D.; Bach, A. Effects of active dry yeasts on the rumen microbial ecosystem: Past, present and future. Anim. Feed Sci. Technol. 2008, 145, 5–26. [Google Scholar] [CrossRef]
- Charan, J.; Kantharia, N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International; Horwitz, W., Ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Bianca, W. Relative Importance of Dry- and Wet-Bulb Temperatures in Causing Heat Stress in Cattle. Nature 1962, 195, 251–252. [Google Scholar] [CrossRef] [PubMed]
- Yousef, M.K. Stress Physiology in Livestock. Volume I. Basic Principles; CRC Press: Boca Raton, FL, USA, 1985; p. 217. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.; Van Soest, P. The detergent system of analysis. In The Analysis of Dietary Fiber in Food; James, W., Teander, O., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1981; pp. 123–158. [Google Scholar]
- Norma Portuguesa. Norma Portuguesa 873: Alimentos para Animais, Determinação do teor de Fósforo, Método Gravimétrico; Diário da República III Série de 04/05 No. 103; Norma Portuguesa: Caparica, Portugal, 1997. [Google Scholar]
- Norma Portuguesa. Norma Portuguesa 1786: Alimentos para Animais, Determinação do teor de Cálcio, Método Volumétrico, Processo Corrente; Diário Da República III Série De 09/07 No. 155; Norma Portuguesa: Caparica, Portugal, 1985. [Google Scholar]
- Salomonsson, A.C.; Theander, O.; Westerlund, E. Chemical characterization of some swedish cereal whole meal and bran fractions. Swed. J. Agric. Res. 1984, 14, 111–117. [Google Scholar]
- Norma Portuguesa. Norma Portuguesa 3255: Determinação do teor de Ureia. Método Espectrofotométrico; Diário Da República III Série De 30/05 No. 123; Norma Portuguesa: Caparica, Portugal, 1986. [Google Scholar]
- Polsky, L.; von Keyserlingk, M.A.G. Invited review: Effects of heat stress on dairy cattle welfare. J. Dairy Sci. 2017, 100, 8645–8657. [Google Scholar] [CrossRef] [PubMed]
- De Rensis, F.; Garcia-Ispierto, I.; López-Gatius, F. Seasonal heat stress: Clinical implications and hormone treatments for the fertility of dairy cows. Theriogenology 2015, 84, 659–666. [Google Scholar] [CrossRef]
- Kabuga, J.D.; Sarpong, K. Influence of weather conditions on milk production and rectal temperature of Holsteins fed two levels of concentrate. Int. J. Biometeorol. 1991, 34, 226–230. [Google Scholar] [CrossRef]
- Brobeck, J.R. Neural regulation of food intake. Ann. N. Y. Acad. Sci. 1955, 63, 44–55. [Google Scholar] [CrossRef]
- Oliver, J.C.; Hellman, H.M.; Bishop, S.E.; Pelissier, C.L.; Bennett, L.F. Heat stress survey. Calif. Agric. 1979, 33, 6–8. [Google Scholar]
- West, J.W. Nutritional strategies for managing the heat-stressed dairy cow. J. Anim. Sci. 1999, 77 (Suppl. 2), 21–35. [Google Scholar] [CrossRef] [PubMed]
- Maust, L.E.; McDowell, R.E.; Hooven, N.W. Effect of summer weather on performance of Holstein cows in three stages of lactation. J. Dairy Sci. 1972, 55, 1133–1139. [Google Scholar] [CrossRef]
- Mitra, R.; Christison, G.I.; Johnson, H.D. Effect of prolonged thermal exposure on growth hormone (GH) secretion in cattle. J. Anim. Sci. 1972, 34, 776–779. [Google Scholar] [CrossRef]
- Rhoads, M.L.; Rhoads, R.P.; VanBaale, M.J.; Collier, R.J.; Sanders, S.R.; Weber, W.J.; Crooker, B.A.; Baumgard, L.H. Effects of heat stress and plane of nutrition on lactating Holstein cows: I. Production, metabolism, and aspects of circulating somatotropin. J. Dairy Sci. 2009, 92, 1986–1997. [Google Scholar] [CrossRef]
- Wheelock, J.B.; Rhoads, R.P.; VanBaale, M.J.; Sanders, S.R.; Baumgard, L.H. Effects of heat stress on energetic metabolism in lactating Holstein cows. J. Dairy Sci. 2010, 93, 644–655. [Google Scholar] [CrossRef]
- Morrison, S.R. Ruminant heat stress: Effect on production and means of alleviation. J. Anim. Sci. 1983, 57, 1594–1600. [Google Scholar] [CrossRef]
- Fox, D.G.; Tylutki, T.P. Accounting for the effects of environment on the nutrient requirements of dairy cattle. J. Dairy Sci. 1998, 81, 3085–3095. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Banhazi, T.; Ghahramani, A.; Bowtell, L.; Wang, C.; Li, B. Modelling of heat stress in a robotic dairy farm. Part 2: Identifying the specific thresholds with production factors. Biosyst. Eng. 2020, 199, 43–57. [Google Scholar] [CrossRef]
- Neethirajan, S. The role of sensors, big data and machine learning in modern animal farming. Sens. Bio-Sens. Res. 2020, 29, 100367. [Google Scholar] [CrossRef]
- Sejian, V.; Shashank, C.G.; Silpa, M.V.; Madhusoodan, A.P.; Devaraj, C.; Koenig, S. Non-invasive methods of quantifying heat stress response in farm animals with special reference to dairy cattle. Atmosphere 2022, 13, 1642. [Google Scholar] [CrossRef]

| Diet 1 | ||
|---|---|---|
| Ingredient, g/kg DM | Control | Yeast |
| Maize silage | 420.0 | 420.0 |
| Ryegrass hay | 80.0 | 80.0 |
| Concentrate mixture 2 | ||
| Wheat grain | 2.0 | 2.0 |
| Maize grain | 105.0 | 105.0 |
| Wheat bran | 7.5 | 7.5 |
| Cassava meal | 8.7 | 8.7 |
| Soybean meal | 175.0 | 174.8 |
| Corn gluten feed | 113.8 | 113.6 |
| Palm kernel meal | 11.3 | 11.2 |
| Sunflower meal | 11.7 | 11.7 |
| Molasses | 2.0 | 2.0 |
| Calcium soaps 3 | 12.5 | 12.5 |
| Cottonseed | 25.0 | 25.0 |
| Sodium bicarbonate | 5.0 | 5.0 |
| Calcium carbonate | 11.0 | 11.0 |
| Magnesium oxide | 3.0 | 3.0 |
| Salt | 2.5 | 2.5 |
| Mineral–vitamin premix 4 | 1.5 | 1.5 |
| Urea | 2.5 | 2.5 |
| Yeast culture 5 | - | 0.5 |
| Forage | Concentrate | Diet 1 | ||||
|---|---|---|---|---|---|---|
| Item | Maize Silage | Ryegrass Hay | Control | Yeast | Control | Yeast |
| DM, g/kg | 350 | 870 | 900 | 910 | 667 | 672 |
| Ash | 33 | 60 | 119 | 107 | 78 | 72 |
| CP | 75 | 53 | 239 | 238 | 154 | 155 |
| EE | 27 | 10 | 34 | 36 | 29 | 30 |
| NDF | 392 | 565 | 252 | 264 | 336 | 342 |
| ADF | 229 | 338 | 109 | 117 | 178 | 182 |
| ADL | 42 | 42 | 34 | 45 | 38 | 43 |
| Starch | 381 | ND 2 | 188 | 180 | 254 | 250 |
| Urea | ND | ND | 6.7 | 7.0 | 3.4 | 3.5 |
| Ca | 2.3 | 4.1 | 19.5 | 18.7 | 11.0 | 10.6 |
| P | 1.5 | 1.9 | 5.6 | 6.1 | 3.6 | 3.8 |
| Diet 1 | SEM | p | ||
|---|---|---|---|---|
| Control | Yeast | |||
| DMI, kg/d | 19.5 | 20.5 | 0.23 | 0.038 |
| Milk | ||||
| Yield, kg/d | 30.7 | 33.4 | 0.27 | 0.002 |
| ECM 2, kg/d | 31.7 | 33.1 | 0.53 | 0.148 |
| Protein, % | 2.66 | 2.76 | 0.029 | 0.062 |
| Protein, kg/d | 0.82 | 0.88 | 0.020 | 0.136 |
| Fat, % | 4.76 | 4.46 | 0.094 | 0.119 |
| Fat, kg/d | 1.44 | 1.46 | 0.044 | 0.867 |
| Lactose, % | 4.80 | 4.73 | 0.023 | 0.080 |
| Lactose, kg/d | 1.48 | 1.56 | 0.037 | 0.213 |
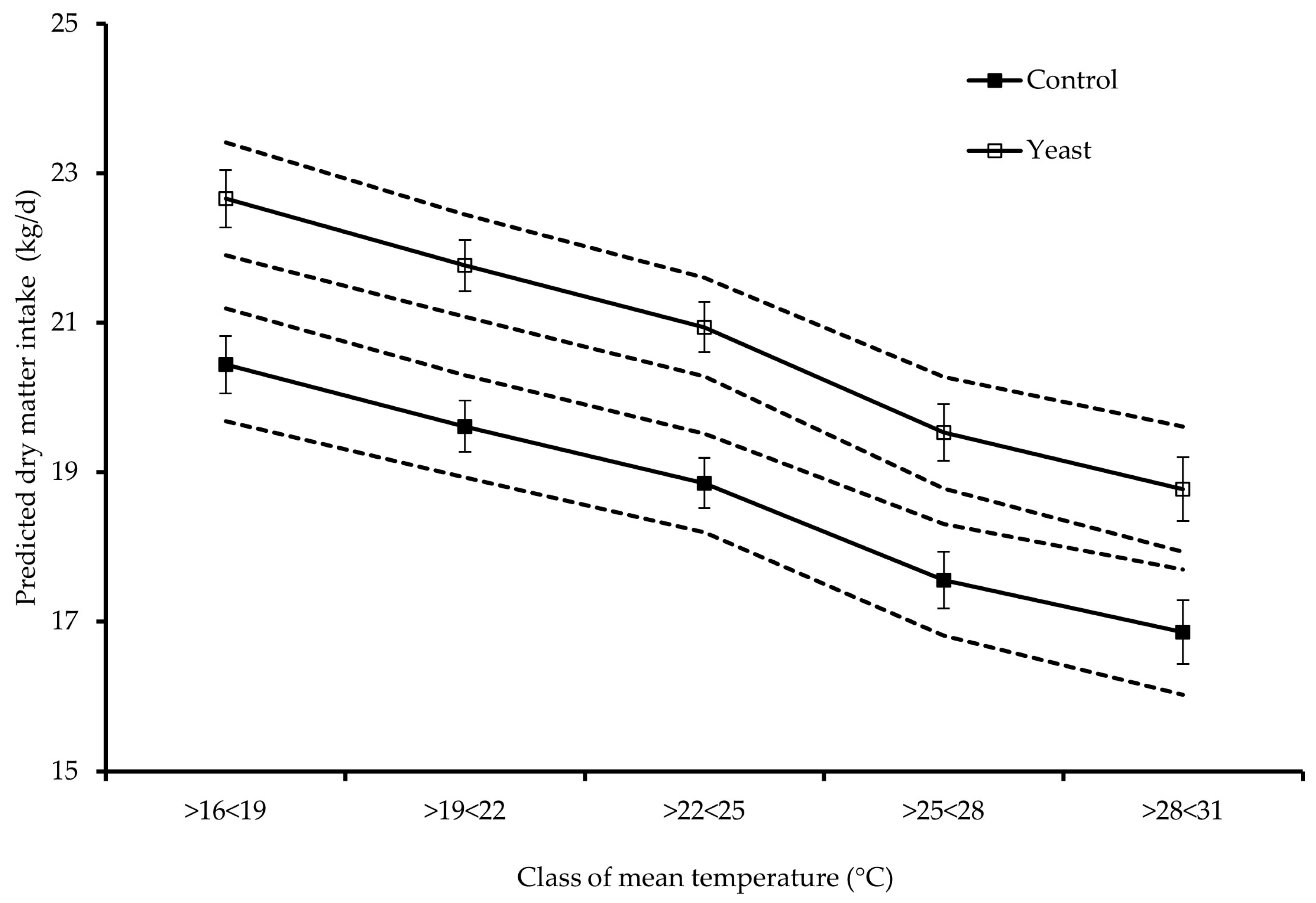
| Dependent Variable | Covariable in the Model 1 | r2 | p-Value | Treatment 2 | Linear | Quadratic | Cubic |
|---|---|---|---|---|---|---|---|
| DMI, kg/d | AMP | 0.66 | <0.001 | Control | 10.02 | −0.70 | 0.02 |
| Yeast | 15.37 | −1.08 | 0.02 | ||||
| MAX | 0.71 | <0.001 | Control | 0.89 | −0.02 | ||
| Yeast | 0.99 | −0.02 | |||||
| MIN | 0.70 | <0.001 | Control | 3.26 | −0.22 | 0.01 | |
| Yeast | 4.44 | −0.29 | 0.01 | ||||
| MEAN | 0.72 | <0.001 | Control | −0.31 | |||
| Yeast | −0.34 | ||||||
| MAX THI1 | 0.69 | <0.001 | Control | −0.18 | |||
| Yeast | −0.21 | ||||||
| MEAN THI1 | 0.69 | <0.001 | Control | 0.88 | −0.01 | ||
| Yeast | 1.44 | −0.01 | |||||
| MIN THI1 | 0.69 | <0.001 | Control | −0.21 | |||
| Yeast | −0.23 | ||||||
| MAX THI2 | 0.70 | <0.001 | Control | 1.63 | −0.01 | ||
| Yeast | 2.06 | −0.01 | |||||
| MEAN THI2 | 0.72 | <0.001 | Control | 1.51 | −0.01 | ||
| Yeast | 2.22 | −0.02 | |||||
| MIN THI2 | 0.70 | <0.001 | Control | 31.2 | −0.49 | 0.01 | |
| Yeast | 35.7 | −0.56 | 0.01 | ||||
| Milk yield, Kg/d | AMP | 0.83 | <0.001 | Control | 1.58 | −0.06 | |
| Yeast | 1.98 | −0.08 | |||||
| MAX | 0.87 | <0.001 | Control | −32.1 | 1.10 | −0.01 | |
| Yeast | −29.1 | 1.00 | −0.01 | ||||
| MIN | 0.87 | <0.001 | Control | 9.25 | −0.58 | 0.01 | |
| Yeast | 10.96 | −0.69 | 0.01 | ||||
| MEAN | 0.87 | <0.001 | Control | 12.79 | −0.57 | 0.01 | |
| Yeast | 20.99 | −0.93 | 0.01 | ||||
| MAX THI1 | 0.86 | <0.001 | Control | 1.91 | −0.01 | ||
| Yeast | 2.55 | −0.02 | |||||
| MEAN THI1 | 0.87 | <0.001 | Control | 2.72 | −0.02 | ||
| Yeast | 3.67 | −0.03 | |||||
| MIN THI1 | 0.86 | <0.001 | Control | 2.68 | −0.02 | ||
| Yeast | 3.54 | −0.03 | |||||
| MAX THI2 | 0.87 | <0.001 | Control | −61.98 | 0.82 | −0.01 | |
| Yeast | −60.68 | 0.81 | −0.01 | ||||
| MEAN THI2 | 0.87 | <0.001 | Control | 3.33 | −0.03 | ||
| Yeast | 4.48 | −0.04 | |||||
| MIN THI2 | 0.86 | <0.001 | Control | 82.46 | −1.28 | 0.01 | |
| Yeast | 95.97 | −1.49 | 0.01 | ||||
| ECM 3, Kg/d | AMP | 0.83 | <0.001 | Control | 8.08 | −0.29 | |
| Yeast | 9.12 | −0.34 | |||||
| MAX | 0.80 | <0.001 | Control | −38.09 | 1.30 | −0.01 | |
| Yeast | −58.77 | 2.01 | −0.02 | ||||
| MIN | 0.80 | <0.001 | Control | −0.59 | |||
| Yeast | −0.64 | ||||||
| MEAN | 0.79 | <0.001 | Control | −0.45 | |||
| Yeast | −0.57 | ||||||
| MEAN THI1 | 0.79 | <0.001 | Control | −0.33 | |||
| Yeast | −0.36 | ||||||
| MEAN THI2 | 0.79 | <0.001 | Control | −0.38 | |||
| Yeast | −0.47 | ||||||
| Milk protein, kg/d | AMP | 0.80 | <0.001 | Control | 0.23 | −0.01 | |
| Yeast | 0.24 | −0.01 | |||||
| MAX | 0.79 | <0.001 | Control | −0.95 | 0.03 | −0.01 | |
| Yeast | −1.55 | 0.05 | −0.01 | ||||
| MIN | 0.77 | <0.001 | Control | 0.05 | −0.01 | ||
| Yeast | 0.07 | −0.01 | |||||
| MEAN | 0.76 | <0.001 | Control | −0.02 | |||
| Yeast | −0.02 | ||||||
| MEAN THI1 | 0.75 | <0.001 | Control | −0.01 | |||
| Yeast | −0.01 | ||||||
| MEAN THI2 | 0.77 | <0.001 | Control | 0.13 | −0.01 | ||
| Yeast | 0.18 | −0.01 | |||||
| Milk fat, kg/d | AMP | 0.70 | <0.001 | Control | 0.34 | −0.01 | |
| Yeast | 0.42 | −0.02 | |||||
| MAX | 0.68 | <0.001 | Control | −1.43 | 0.05 | −0.01 | |
| Yeast | −3.14 | 0.11 | −0.01 | ||||
| MIN | 0.67 | <0.001 | Control | −0.02 | |||
| Yeast | −0.03 | ||||||
| MEAN | 0.66 | <0.001 | Control | −0.01 | |||
| Yeast | −0.02 | ||||||
| MEAN THI1 | 0.66 | <0.001 | Control | −0.01 | |||
| Yeast | −0.01 | ||||||
| MEAN THI2 | 0.66 | <0.001 | Control | −0.01 | |||
| Yeast | −0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabrita, A.R.J.; Carvalheira, J.; Fonseca, A.J.M. Live Yeast Supplementation Attenuates the Effects of Heat Stress in Dairy Cows. Vet. Sci. 2025, 12, 791. https://doi.org/10.3390/vetsci12090791
Cabrita ARJ, Carvalheira J, Fonseca AJM. Live Yeast Supplementation Attenuates the Effects of Heat Stress in Dairy Cows. Veterinary Sciences. 2025; 12(9):791. https://doi.org/10.3390/vetsci12090791
Chicago/Turabian StyleCabrita, Ana R. J., Júlio Carvalheira, and António J. M. Fonseca. 2025. "Live Yeast Supplementation Attenuates the Effects of Heat Stress in Dairy Cows" Veterinary Sciences 12, no. 9: 791. https://doi.org/10.3390/vetsci12090791
APA StyleCabrita, A. R. J., Carvalheira, J., & Fonseca, A. J. M. (2025). Live Yeast Supplementation Attenuates the Effects of Heat Stress in Dairy Cows. Veterinary Sciences, 12(9), 791. https://doi.org/10.3390/vetsci12090791








