Assessment of Body Condition in Long-Distance Sled Dogs: Validation of the Body Condition Score and Its Association with Ultrasonographic, Plicometric, and Anthropometric Measurements †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Dog Population
2.2. Data Collection
2.2.1. Body Weight and Body Condition Score
- Scores 1–2 (Too thin): Indicate a severe or pronounced energy deficit. Dogs in this condition are considered too lean to safely start or continue a race.
- Score 3 (Borderline): Reflects a marginal body condition; dogs require close monitoring to ensure adequate energy intake and avoid further weight loss
- Scores 4–5 (Ideal weight): Represent the optimal condition for most sled dogs, ensuring sufficient energy reserves while avoiding excess body fat.
- Score 6 (Slightly over ideal): Still within the ideal range and considered a favorable starting condition for long-distance races in cold environments, as slightly increased energy stores can be advantageous.
- Scores 7–8 (Overweight): Slightly above the ideal range for racing dogs, this score is still acceptable for the start of endurance events in cold climates. However, it is not desirable for sprint racing or competitions in warmer temperatures.
- Scores 8–9 (Obese): Indicative of excessive body fat and generally considered unsuitable for competitive racing.
2.2.2. Anthropometric Measurements
- Height at withers (HWi): Vertical distance from the ground to the dorsal limit of the scapular region.
- Chest girth (CC): Circumference of the chest measured immediately caudal to the scapula, at the widest part of the ribcage.
- Pelvic circumference (PC): Measured immediately cranial to the inguinal region around the level of the flank.
- Hock to stifle length (HS): Distance from the most caudal aspect of the tuber calcanei (hock) to the midpoint of the patellar ligament (stifle joint).
- Occipital-to-tail length (OT): Length from the occipital protuberance to the base of the tail.
- Skull circumference (SC): Circumference measured around the widest part of the skull.
- Skull length (SL): Distance from the occipital protuberance to the medial canthus of the eye.
- Tarsal pad–heel distance (TH): Distance from the proximal aspect of the tarsal pad to the most caudal point of the calcaneal tuberosity.
- Carpal pad–olecranon distance (CO): Distance from the proximal aspect of the carpal pad to the proximal tip of the olecranon.
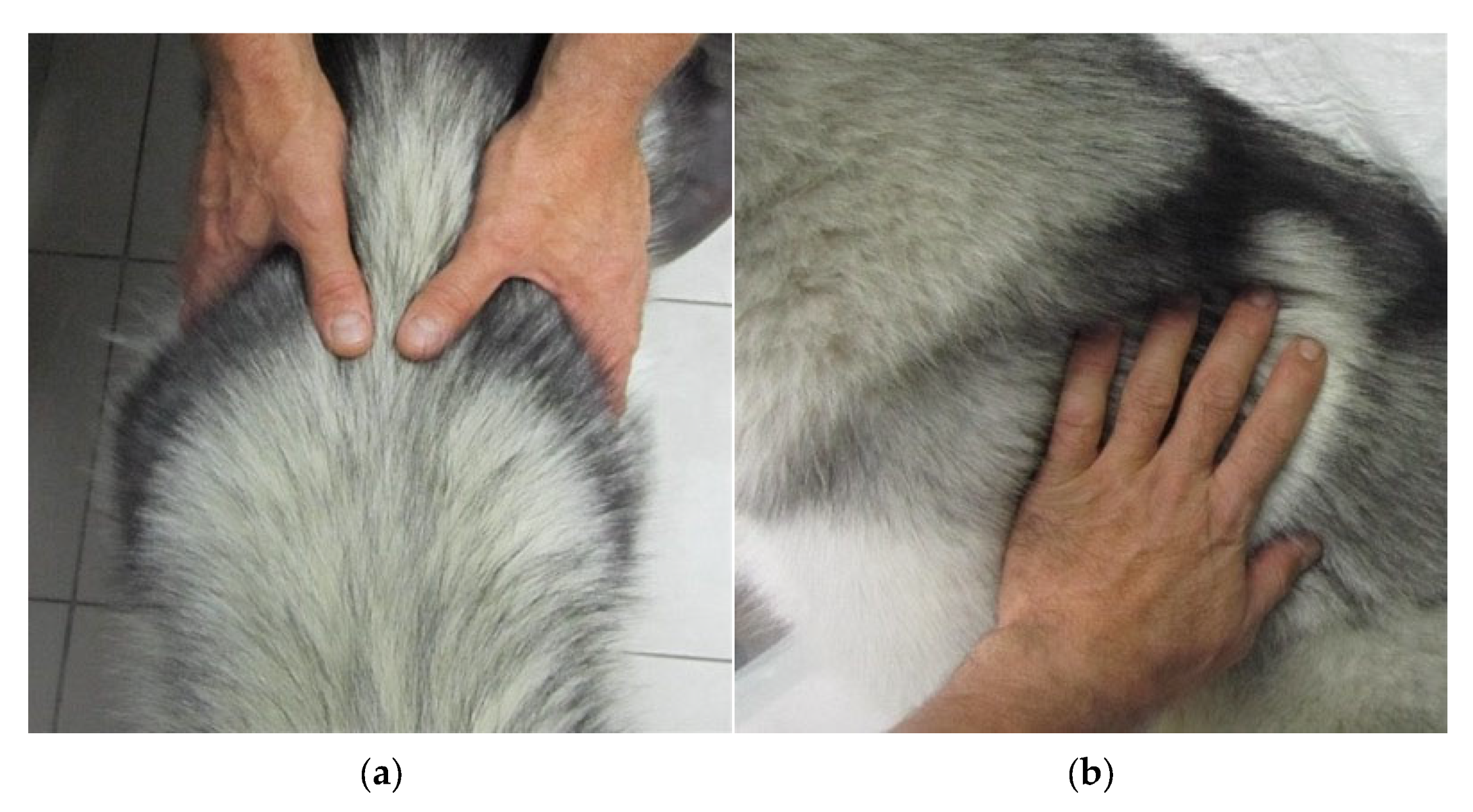
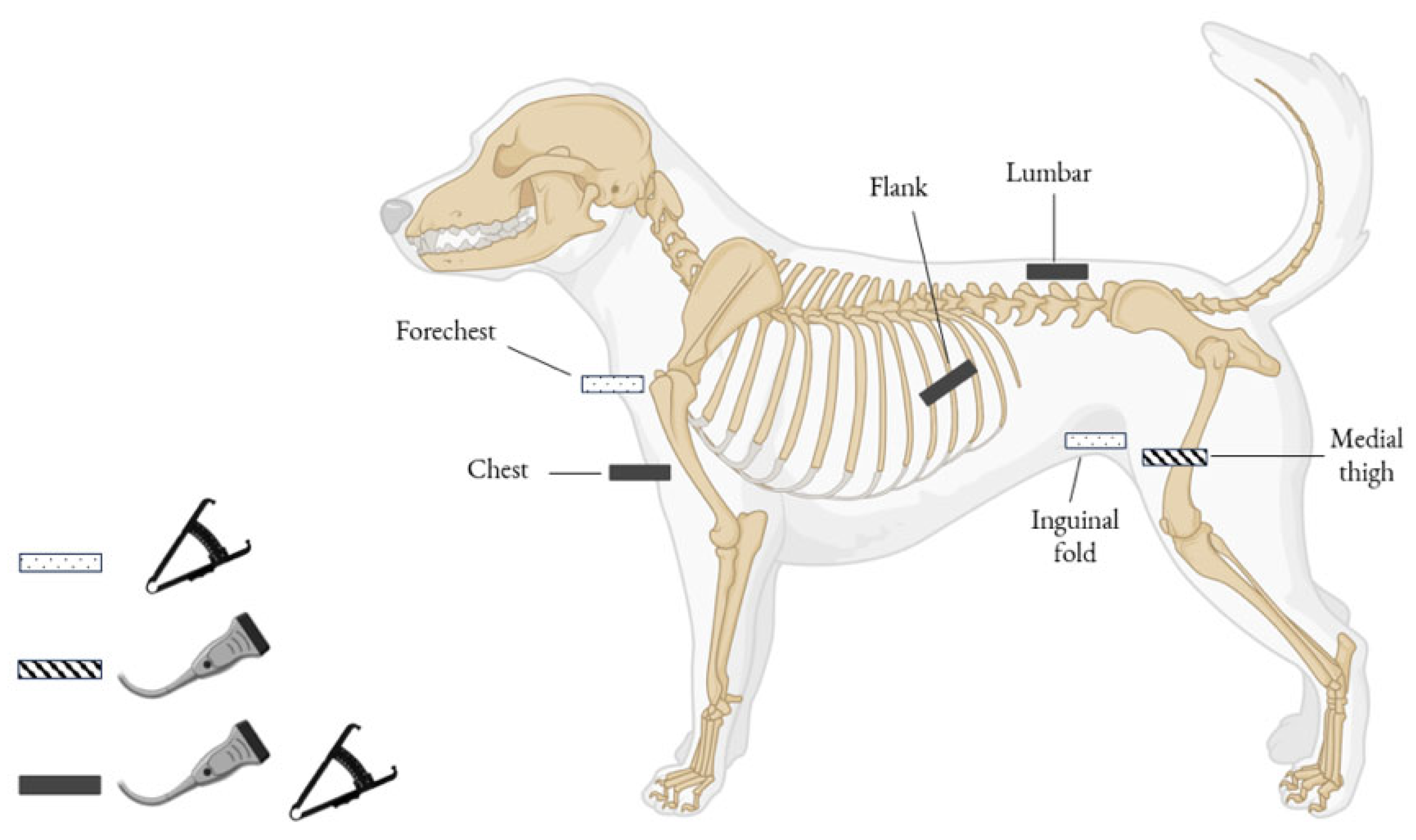
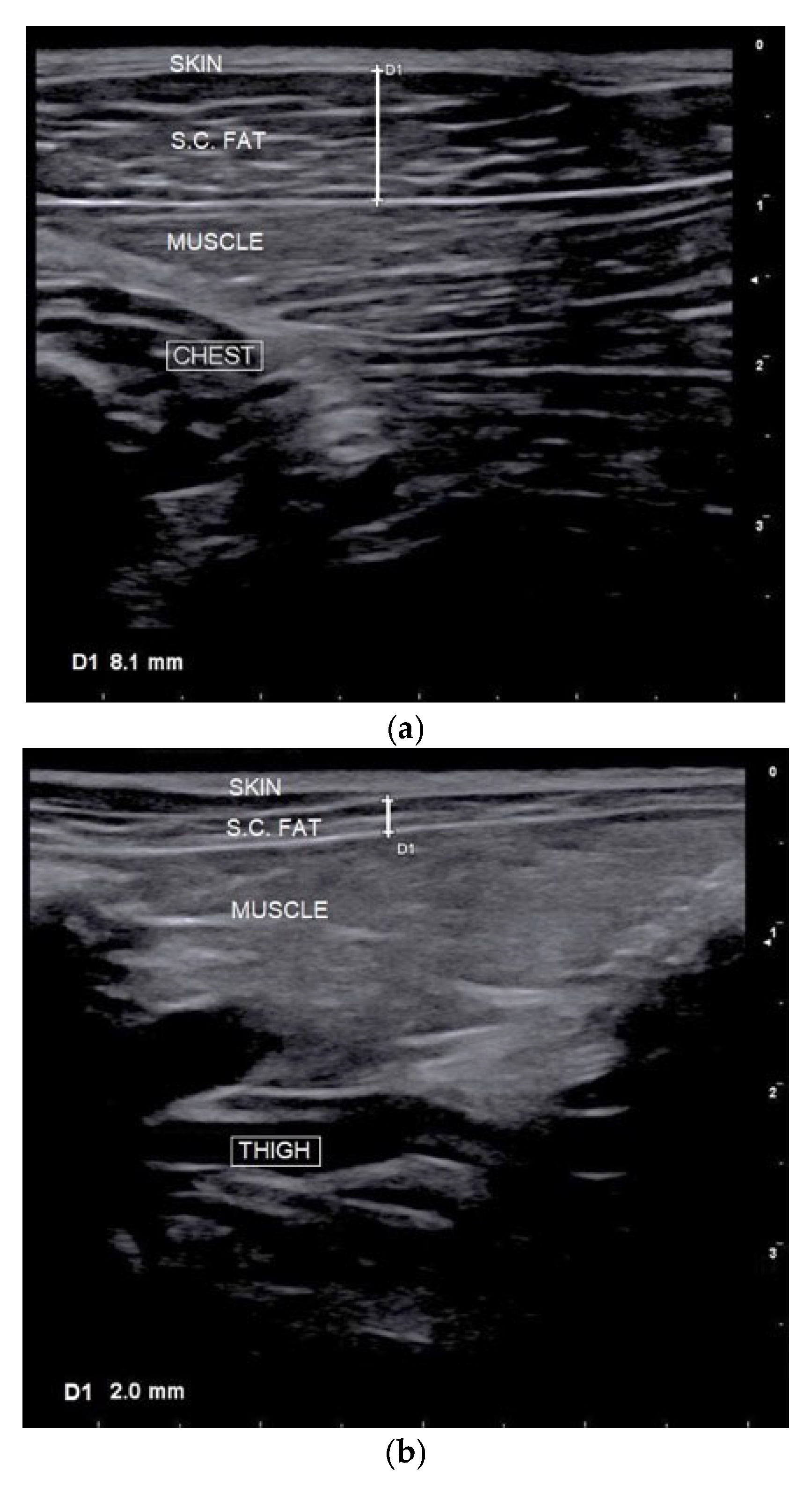
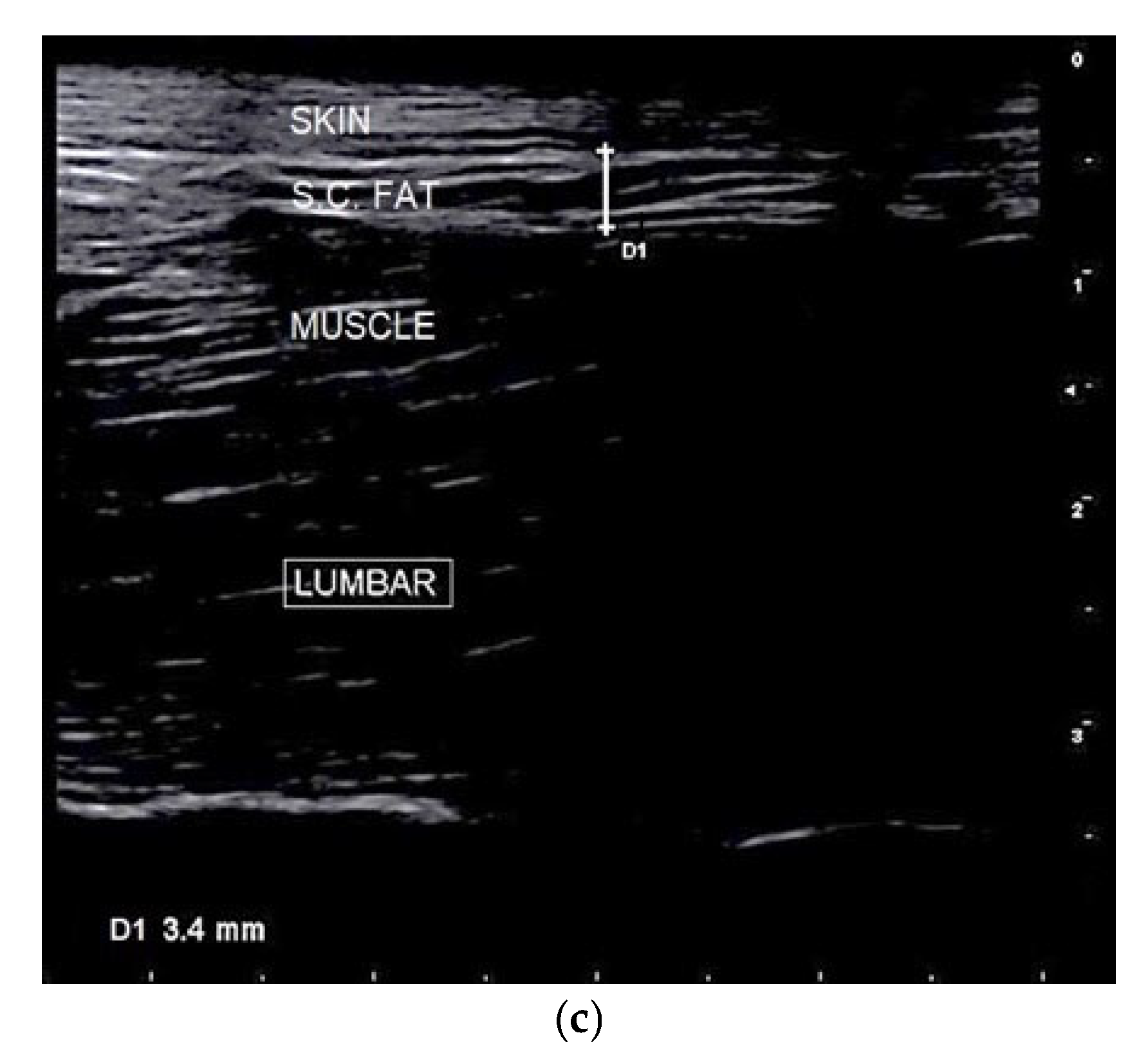
2.2.3. Subcutaneous Fat Thickness (SFT) Measurement
2.3. Data Analysis
3. Results
3.1. Anthropometric Measurements: Descriptive and Sexual Dimorphism
3.2. BCS Validation: Intra and Inter-Observer Agreement
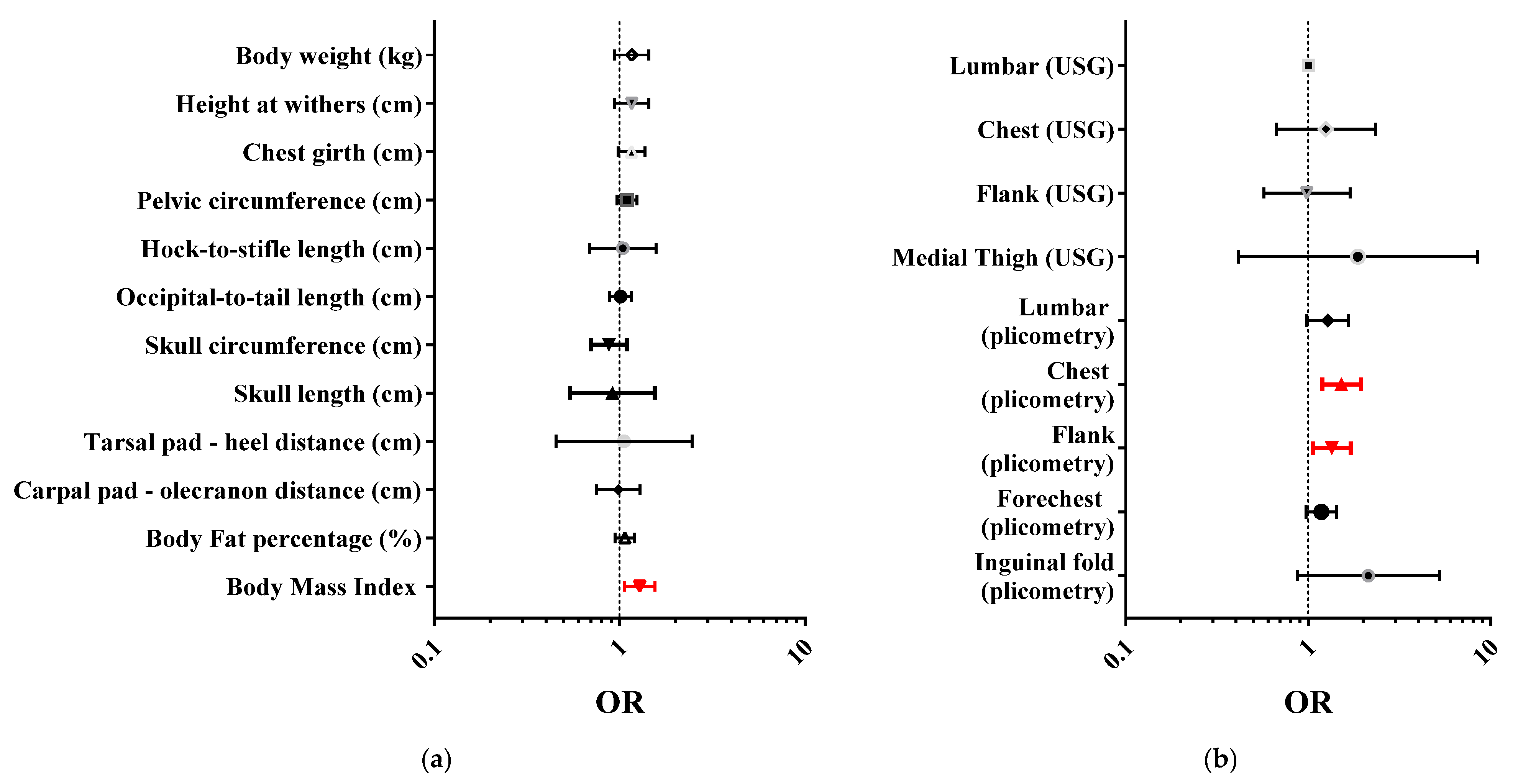
3.3. BCS Validation: Concurrent Validity
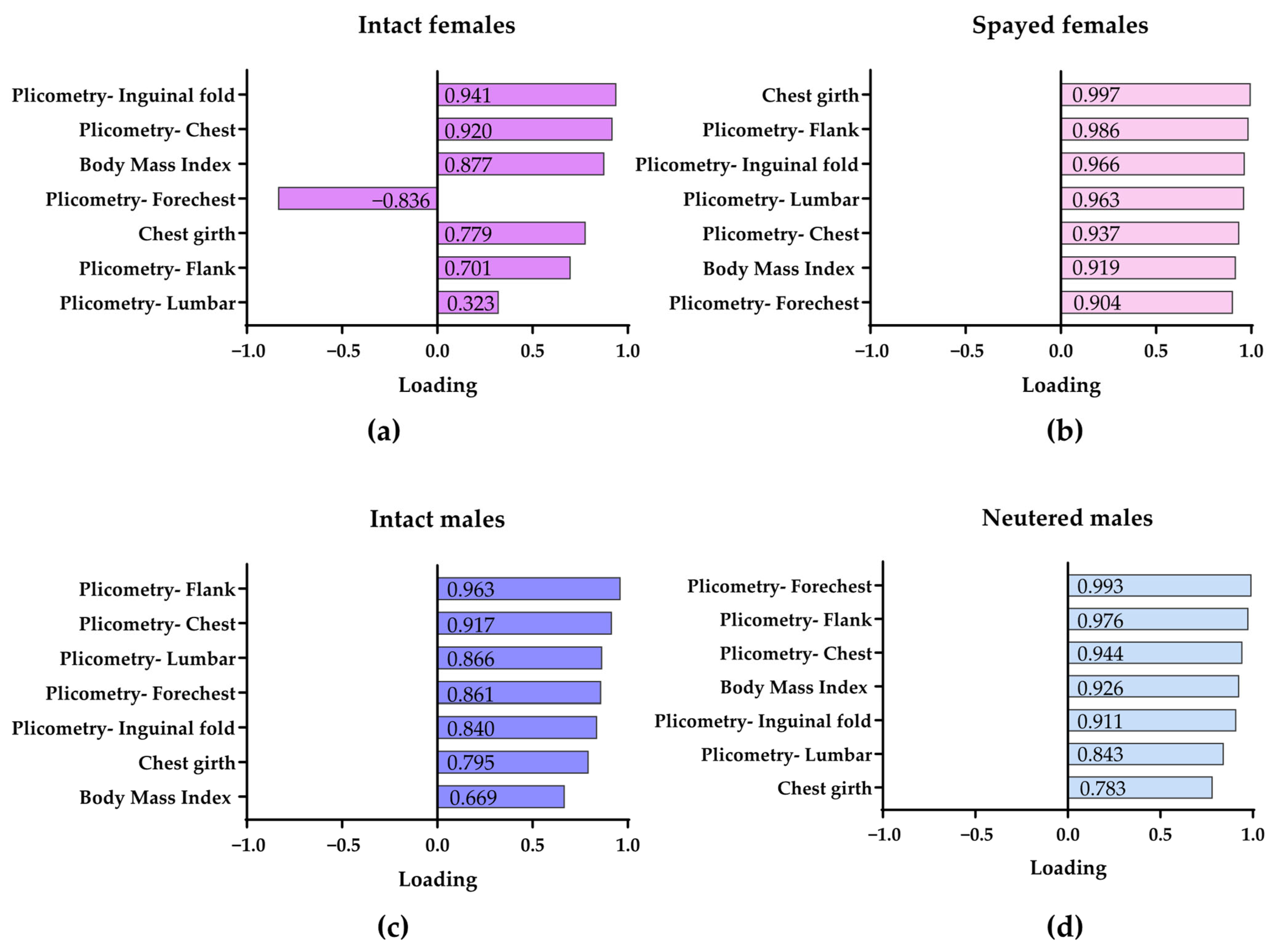
3.4. Multivariate Analyses Defining the Body Condition of Intact and Neutered Male and Female Dogs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, R.W. Dog Remains from Devon Island, N.W.T.: Archaeological and Osteological Evidence for Domestic Dog Use in the Thule Culture. Arctic 1987, 40, 184–190. [Google Scholar] [CrossRef]
- Huson, H.J.; Parker, H.G.; Runstadler, J.; Ostrander, E.A. A Genetic Dissection of Breed Composition and Performance Enhancement in the Alaskan Sled Dog. BMC Genet. 2010, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Li, M.F.; Nagendran, L.; Schroeder, L.; Samson, D.R. The Activity Patterns of Nonworking and Working Sled Dogs. Sci. Rep. 2022, 12, 7999. [Google Scholar] [CrossRef] [PubMed]
- Jæger, K.; Viken, A. Sled Dog Racing and Tourism Development in Finnmark. A Symbiotic Relationship. In Tourism Destination Development; Routledge Milton Park: Oxfordshire, UK, 2014; ISBN 978-1-315-55075-6. [Google Scholar]
- Kemp, S.F. Sled Dog Racing: The Celebration of Co-Operation in a Competitive Sport. Ethnology 1999, 38, 81–95. [Google Scholar] [CrossRef]
- Calogiuri, G.; Weydahl, A. Health Challenges in Long-Distance Dog Sled Racing: A Systematic Review of Literature. Int. J. Circumpolar Health 2017, 76, 1396147. [Google Scholar] [CrossRef]
- Thorsrud, J.A.; Huson, H.J. Description of Breed Ancestry and Genetic Health Traits in Arctic Sled Dog Breeds. Canine Genet. Epidemiol. 2021, 8, 8. [Google Scholar] [CrossRef]
- Davis, M.S. Glucocentric Metabolism in Ultra-Endurance Sled Dogs. Integr. Comp. Biol. 2021, 61, 103–109. [Google Scholar] [CrossRef]
- Kuhl, G. Human-Sled Dog Relations: What Can We Learn from the Stories and Experiences of Mushers? Soc. Anim. 2011, 19, 22–37. [Google Scholar] [CrossRef]
- Dorsten, C.M.; Cooper, D.M. Use of Body Condition Scoring to Manage Body Weight in Dogs. Contemp. Top. Lab. Anim. Sci. 2004, 43, 34–37. [Google Scholar]
- Payan-Carreira, R.; Martins, L.; Miranda, S.; Olivério, P.; Silva, S.R. In Vivo Assessment of Subcutaneous Fat in Dogs by Real-Time Ultrasonography and Image Analysis. Acta Vet. Scand. 2016, 58, 58. [Google Scholar] [CrossRef]
- Wilkinson, M.J.A.; McEwan, N.A. Use of Ultrasound in the Measurement of Subcutaneous Fat and Prediction of Total Body Fat in Dogs. J. Nutr. 1991, 121, S47–S50. [Google Scholar] [CrossRef] [PubMed]
- Burkholder, W.J.; Burkholder, W.J. Precision and Practicality of Methods Assessing Body Composition of Dogs and Cats. Compend. Contin. Educ. Pract. Vet. 2001, 23, 1–10. [Google Scholar]
- German, A.J.; Morgan, L.E. How Often Do Veterinarians Assess the Bodyweight and Body Condition of Dogs? Vet. Rec. 2008, 163, 503–505. [Google Scholar] [CrossRef] [PubMed]
- Hand, M.S. Small Animal Clinical Nutrition; Mark Morris Institute: Topeka, KS, USA, 2000; ISBN 978-0-945837-05-3. [Google Scholar]
- Laflamme-1997-Development and Validation of a Body Condition Score System for Dogs (1)|PDF. Available online: https://www.scribd.com/document/846233127/Laflamme-1997-Development-and-Validation-of-a-Body-Condition-Score-System-for-Dogs-1 (accessed on 14 April 2025).
- Santarossa, A.; Parr, J.M.; Verbrugghe, A. The Importance of Assessing Body Composition of Dogs and Cats and Methods Available for Use in Clinical Practice. J. Am. Vet. Med. Assoc. 2017, 251, 521–529. [Google Scholar] [CrossRef]
- Michel, K.E.; Anderson, W.; Cupp, C.; Laflamme, D.P. Correlation of a Feline Muscle Mass Score with Body Composition Determined by Dual-Energy X-Ray Absorptiometry. Br. J. Nutr. 2011, 106 (Suppl. S1), S57–S59. [Google Scholar] [CrossRef]
- Mawby, D.I.; Bartges, J.W.; d’Avignon, A.; Laflamme, D.P.; Moyers, T.D.; Cottrell, T. Comparison of Various Methods for Estimating Body Fat in Dogs. J. Am. Anim. Hosp. Assoc. 2004, 40, 109–114. [Google Scholar] [CrossRef]
- Ricci, R.; Gottardo, F.; Ferlito, J.C.; Stefani, A.; Andrighetto, I. Body Condition Score (BCS) and Metabolic Status of Shelter Dogs. Ital. J. Anim. Sci. 2007, 6, 859–861. Available online: https://www.tandfonline.com/doi/abs/10.4081/ijas.2007.1s.859 (accessed on 20 June 2025). [CrossRef]
- Reynolds, A.; Fuhrer, L.; Dunlap, H.; Finke, M.; Kallfelz, F. Lipid Metabolite Responses to Diet and Training in Sled Dogs. J. Nutr. 1995, 124, 2754S–2759S. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, J.-H. The Dog as an Exercise Science Animal Model: A Review of Physiological and Hematological Effects of Exercise Conditions. Phys. Act. Nutr. 2020, 24, 1–6. [Google Scholar] [CrossRef]
- Kluess, H.A.; Jones, R.L. A Comparison of Owner Perceived and Measured Body Condition, Feeding and Exercise in Sport and Pet Dogs. Front. Vet. Sci. 2023, 10, 1211996. [Google Scholar] [CrossRef]
- de Souza Buzo, R.; Tremea, J.F.; Bispo, G.A.; Oliveira, B.S.; Bizi, J.; Ferreira, W.L.; Pinoti, L.D.R. Radiography, Ultrasound, and Anthropometry in Dog Nutrition Evaluation. Ciênc. Anim. Bras. 2023, 24, e75686. [Google Scholar] [CrossRef]
- Witzel, A.L.; Kirk, C.A.; Henry, G.A.; Toll, P.W.; Brejda, J.J.; Paetau-Robinson, I. Use of a Novel Morphometric Method and Body Fat Index System for Estimation of Body Composition in Overweight and Obese Dogs. J. Am. Vet. Med. Assoc. 2014, 244, 1279–1284. [Google Scholar] [CrossRef] [PubMed]
- Nösslinger, H.; Mair, E.; Toplak, H.; Hörmann-Wallner, M. Measuring Subcutaneous Fat Thickness Using Skinfold Calipers vs. High-Resolution B-Scan Ultrasonography in Healthy Volunteers: A Pilot Study. Clin. Nutr. Open Sci. 2022, 41, 19–32. [Google Scholar] [CrossRef]
- Loftus, J.P.; Yazwinski, M.; Milizio, J.G.; Wakshlag, J.J. Energy Requirements for Racing Endurance Sled Dogs. J. Nutr. Sci. 2014, 3, e34. [Google Scholar] [CrossRef] [PubMed]
- International Sled Dog Veterinary Medical Association. In Dog Care Information; International SledDog Veterinary Medical Association: St. Augustine, FL, USA, 1 August 2019; Available online: https://isdvma.org/home/ (accessed on 1 August 2025).
- Nutrition Guidelines—WSAVA. Available online: https://wsava.org/global-guidelines/global-nutrition-guidelines/ (accessed on 18 July 2025).
- International Sled Dog Veterinary Medical Association. Available online: https://isdvma.org/ (accessed on 19 June 2025).
- Cradic, D.W.; Aulakh, K.S.; Hymel, P.; Barnes, K.; Gines, J.A.; Rademacher, N.; Aulakh, H.K.; Liu, C.-C. Morphometric Measurements to Predict Meniscal Size in Skeletally Mature Dogs for Meniscal Transplantation. Vet. Surg. 2020, 49, 172–179. [Google Scholar] [CrossRef]
- Mugnier, A.; Cellard, F.; Morin, A.; Delmas, M.; Grellet, A.; Chastant, S. Association between Birth Weight and Subcutaneous Fat Thickness at Adulthood in Dogs. Vet. Sci. 2023, 10, 208. [Google Scholar] [CrossRef]
- Garson, G.D. Testing Statistical Assumptions; Statistical Associates Publishing: Asheboro, NC, USA, 2012. [Google Scholar]
- Field, A. Discovering Statistics Using SPSS; SAGE Publications: Thousand Oaks, CA, USA, 2009; ISBN 978-1-84787-907-3. [Google Scholar]
- Meagher, R.K. Observer Ratings: Validity and Value as a Tool for Animal Welfare Research. Appl. Anim. Behav. Sci. 2009, 119, 1–14. [Google Scholar] [CrossRef]
- Nannarone, S.; Ortolani, F.; Scilimati, N.; Gialletti, R.; Menchetti, L. Refinement and Revalidation of the Equine Ophthalmic Pain Scale: R-EOPS a New Scale for Ocular Pain Assessment in Horses. Vet. J. 2024, 304, 106079. [Google Scholar] [CrossRef]
- Ortolani, F.; Scilimati, N.; Gialletti, R.; Menchetti, L.; Nannarone, S. Development and Preliminary Validation of a Pain Scale for Ophthalmic Pain in Horses: The Equine Ophthalmic Pain Scale (EOPS). Vet. J. 2021, 278, 105774. [Google Scholar] [CrossRef]
- Fleiss, J.L. The Design and Analysis of Clinical Experiments, 1st ed.; Wiley Classics Library: Chichester, UK, 1999; ISBN 978-0-471-82047-5. [Google Scholar]
- Boateng, G.O.; Neilands, T.B.; Frongillo, E.A.; Melgar-Quiñonez, H.R.; Young, S.L. Best Practices for Developing and Validating Scales for Health, Social, and Behavioral Research: A Primer. Front. Public Health 2018, 6, 149. [Google Scholar] [CrossRef]
- Menchetti, L.; Calipari, S.; Guelfi, G.; Catanzaro, A.; Diverio, S. My Dog Is Not My Cat: Owner Perception of the Personalities of Dogs and Cats Living in the Same Household. Animals 2018, 8, 80. [Google Scholar] [CrossRef]
- Cartoni Mancinelli, A.; Mattioli, S.; Menchetti, L.; Dal Bosco, A.; Ciarelli, C.; Guarino Amato, M.; Castellini, C. The Assessment of a Multifactorial Score for the Adaptability Evaluation of Six Poultry Genotypes to the Organic System. Animals 2021, 11, 2992. [Google Scholar] [CrossRef]
- Christie, K.M.; Barnhard, J.A.; Otto, C.M.; Mallikarjun, A.; Wilson, C.; Levine, D.; Tringali, A.A.; Payne, C.E.; Langenbach, A.; Brunke, M.W. Interobserver Variability of Assessing Body Condition Scores and Muscle Condition Scores in a Population of 43 Active Working Explosive Detection Dogs. Front. Vet. Sci. 2024, 11, 1431855. [Google Scholar] [CrossRef]
- Arena, L.; Menchetti, L.; Diverio, S.; Guardini, G.; Gazzano, A.; Mariti, C. Overweight in Domestic Cats Living in Urban Areas of Italy: Risk Factors for an Emerging Welfare Issue. Animals 2021, 11, 2246. [Google Scholar] [CrossRef] [PubMed]
- Gille, S.; Fischer, H.; Lindåse, S.; Palmqvist, L.; Lärka, J.; Wolf, S.; Penell, J.; Söder, J. Dog Owners’ Perceptions of Canine Body Composition and Effect of Standardized Education for Dog Owners on Body Condition Assessment of Their Own Dogs. Vet. Sci. 2023, 10, 447. [Google Scholar] [CrossRef] [PubMed]
- German, A.J.; Holden, S.L.; Morris, P.J.; Biourge, V. Comparison of a Bioimpedance Monitor with Dual-Energy x-Ray Absorptiometry for Noninvasive Estimation of Percentage Body Fat in Dogs. Am. J. Vet. Res. 2010, 71, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Lee, P.; Mori, N.; Yamamoto, I.; Kawasumi, K.; Tanabe, H. Supplementing Five-Point Body Condition Score with Body Fat Percentage Increases the Sensitivity for Assessing Overweight Status of Small to Medium Sized Dogs. VMRR 2012, 3, 71–78. [Google Scholar] [CrossRef]
- Jeusette, I.; Greco, D.; Aquino, F.; Detilleux, J.; Peterson, M.; Romano, V.; Torre, C. Effect of Breed on Body Composition and Comparison between Various Methods to Estimate Body Composition in Dogs. Res. Vet. Sci. 2010, 88, 227–232. [Google Scholar] [CrossRef]
- da Conceição Gomes, A.G.; de Souza Cruz Morais, K.M.A.; da Silva Lima, N.D.; da, S.; Umigi, R.T.; de Paiva, J.T.; Fagundes, G.M. Canine Obesity: Contributing Factors and Body Condition Evaluation. Pets 2025, 2, 22. [Google Scholar] [CrossRef]
- Salt, C.; Morris, P.J.; Wilson, D.; Lund, E.M.; German, A.J. Association between Life Span and Body Condition in Neutered Client-Owned Dogs. J. Vet. Intern. Med. 2019, 33, 89–99. [Google Scholar] [CrossRef]
- Horoszewicz, E.; Król, M.; Niedziółka, R.; Sweklej, E. Preliminary Analysis of Biometrics of Siberian Husky Dogs. Acta Sci. Pol. Zootech. 2017, 14, 109–120. [Google Scholar]
- Losey, R.J.; Osipov, B.; Sivakumaran, R.; Nomokonova, T.; Kovychev, E.V.; Diatchina, N.G. Estimating Body Mass in Dogs and Wolves Using Cranial and Mandibular Dimensions: Application to Siberian Canids: Body Mass in Dogs and Wolves Using Cranial and Mandibular Dimensions. Int. J. Osteoarchaeol. 2015, 25, 946–959. [Google Scholar] [CrossRef]
- Ishioka, K.; Hosoya, K.; Kitagawa, H.; Shibata, H.; Honjoh, T.; Kimura, K.; Saito, M. Plasma Leptin Concentration in Dogs: Effects of Body Condition Score, Age, Gender and Breeds. Res. Vet. Sci. 2007, 82, 11–15. [Google Scholar] [CrossRef]
- Benka, V.A.; Sahrmann, J.M.; Rieke, K.; Briggs, J.R.; Spofford, N.; Zawistowski, S.; Ruple, A.; Romagnoli, S.; Morrison, J.A. Gonadectomy Status and Age Are Associated with Variable Risk of Overweight or Obese Outcomes in 15 Dog Breeds: A Retrospective Cohort Study Using Data from Primary Care Veterinary Clinics. J. Am. Vet. Med. Assoc. 2025, 263, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bastan, I. Possible Relationship between Long-Term Post Neutering Complications in Dogs and Caregiver Burden in the Owners. Front. Vet. Sci. 2025, 12, 1532039. [Google Scholar] [CrossRef] [PubMed]
- Graves, J.L.; McKenzie, B.A.; Koch, Z.; Naka, A.; Spofford, N.; Morrison, J. Body Weight, Gonadectomy, and Other Risk Factors for Diagnosis of Osteoarthritis in Companion Dogs. Front. Vet. Sci. 2023, 10, 1275964. [Google Scholar] [CrossRef]
- Poole, D.C.; Erickson, H.H. Highly Athletic Terrestrial Mammals: Horses and Dogs. Compr. Physiol. 2011, 1, 1–37. [Google Scholar] [CrossRef]

| Type | Definition | Statistic Used | Criteria |
|---|---|---|---|
| Inter-observer reliability | Agreement between multiple expert people (n = 3) and between the main observer and the musher * Independently rating the same dog [35]. | Krippendorff’s alpha (Kα; using 1000 bootstrap samples to estimate the 95% confidence interval) [36]. | Krippendorff’s alpha (Kα) coefficient was interpreted as none to slight (0.01 ≤ Kα < 0.20), fair (0.21 ≤ Kα < 0.40), moderate (0.41 ≤ Kα < 0.60), substantial (0.61 ≤ Kα < 0.80), and almost perfect (Kα ≥ 0.81) agreement [36,38]. |
| Intra-observer reliability | Agreement between ratings by the same individual (the most experienced evaluator) on multiple evaluations [35]. | ||
| Concurrent validity | The extent to which BCS scores have a stronger relationship with the criterion (gold standard) measurement [39]. | Generalized linear models (GLM) with multinomial as probability distribution and cumulative logit as link function. The BCS was included as the dependent variable, and objective measurements as independent variables. The three models (one model for the measurements taken with centimeters, one for those of ultrasound and one for those taken by plicometry) evaluated the main effect of the measurements. | The average of the two assessments of the most experienced evaluator was considered the most reliable estimate of the BCS. The objective measurements taken with centimeters, ultrasound, and plicometry were used as the gold standard. The strength of the association between BCS and gold standard measures was expressed as the odds ratio (OR) with a 95% confidence interval (CI) and the p value [33]. |
| Measurement | Sex and Neuter Status | p Value | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female Intact | Female Spayed | Male Intact | Male Neutered | Sex | Neutering | Interaction | ||||||||||||||||||
| Technique | Parameter | M | SE | Md | Min | Max | M | SE | Md | Min | Max | M | SE | Md | Min | Max | M | SE | Md | Min | Max | |||
| Anthropometric measurements | BW (kg) | 21.5 | 1.2 | 21.8 | 17.3 | 24.4 | 20.6 | 1.4 | 19.0 | 17.8 | 25.5 | 22.3 | 1.0 | 21.6 | 17.8 | 27.9 | 24.5 | 1.4 | 25.9 | 20.2 | 26.0 | 0.087 | 0.618 | 0.217 |
| Hwi † (cm) | 54.4 | 0.9 | 55.0 | 51.0 | 56.0 | 54.7 | 1.3 | 54.0 | 51.0 | 60.0 | 58.1 | 0.6 | 58.5 | 52.0 | 60.0 | 59.1 | 1.0 | 59.5 | 56.5 | 61.0 | 0.001 | 0.863 | 0.009 | |
| CC (cm) | 65.2 | 0.9 | 66.0 | 62.0 | 67.0 | 65.3 | 1.9 | 63.5 | 61.0 | 72.0 | 66.6 | 1.0 | 65.5 | 61.0 | 72.0 | 71.0 | 2.5 | 72.0 | 64.0 | 76.0 | 0.034 | 0.160 | 0.185 | |
| PC (cm) | 50.0 | 0.8 | 51.0 | 47.0 | 51.0 | 46.7 | 2.3 | 45.5 | 40.0 | 54.0 | 48.8 | 1.6 | 46.0 | 42.0 | 58.0 | 53.3 | 3.0 | 51.5 | 48.0 | 62.0 | 0.233 | 0.809 | 0.093 | |
| HS ○ (cm) | 20.8 | 0.4 | 21.0 | 20.0 | 22.0 | 20.8 | 0.8 | 20.5 | 18.0 | 24.0 | 22.0 | 0.4 | 22.5 | 20.0 | 24.0 | 23.0 | 0.8 | 23.0 | 21.0 | 25.0 | 0.013 | 0.472 | 0.437 | |
| OT (cm) | 75.8 | 1.7 | 76.0 | 70.0 | 79.0 | 73.2 | 1.0 | 73.0 | 70.0 | 76.0 | 76.9 | 1.5 | 77.0 | 69.0 | 88.0 | 79.5 | 3.0 | 81.5 | 71.0 | 84.0 | 0.066 | 0.990 | 0.190 | |
| SC ○ (cm) | 39.0 | 1.5 | 38.0 | 36.0 | 44.0 | 39.3 | 1.1 | 39.5 | 35.0 | 43.0 | 41.5 | 1.0 | 41.5 | 37.0 | 47.0 | 40.8 | 0.8 | 40.0 | 40.0 | 43.0 | 0.310 | 0.528 | 0.474 | |
| SL (cm) | 13.0 | 0.0 | 13.0 | 13.0 | 13.0 | 12.3 | 0.3 | 12.0 | 12.0 | 14.0 | 13.3 | 0.5 | 13.0 | 11.0 | 16.0 | 13.3 | 0.6 | 13.0 | 12.0 | 15.0 | 0.279 | 0.532 | 0.532 | |
| TH ○ (cm) | 14.2 | 0.2 | 14.0 | 14.0 | 15.0 | 14.7 | 0.3 | 14.5 | 14.0 | 16.0 | 15.0 | 0.2 | 15.0 | 14.0 | 16.0 | 15.3 | 0.5 | 15.5 | 14.0 | 16.0 | 0.052 | 0.298 | 0.743 | |
| CO (cm) | 27.2 | 0.4 | 27.0 | 26.0 | 28.0 | 27.0 | 1.2 | 27.5 | 22.0 | 30.0 | 29.3 | 0.6 | 29.5 | 25.0 | 32.0 | 30.8 | 0.9 | 31.5 | 28.0 | 32.0 | 0.002 | 0.484 | 0.355 | |
| Calculated index | % BF | 15.9 | 1.2 | 15.4 | 12.2 | 19.4 | 12.0 | 1.9 | 12.7 | 4.3 | 17.1 | 13.1 | 1.9 | 11.3 | 6.1 | 27.5 | 16.3 | 3.2 | 14.0 | 11.5 | 25.7 | 0.750 | 0.876 | 0.141 |
| % BF ‡ | 16.1 | 1.0 | 15.8 | 13.0 | 18.4 | 13.0 | 1.8 | 13.6 | 4.8 | 18.6 | 10.8 | 1.4 | 9.8 | 5.5 | 20.7 | 12.8 | 2.4 | 11.7 | 8.3 | 19.5 | 0.150 | 0.758 | 0.177 | |
| BMI | 51.9 | 1.9 | 52.5 | 45.2 | 56.2 | 51.4 | 2.2 | 50.6 | 44.7 | 57.6 | 49.9 | 1.4 | 48.4 | 43.6 | 58.8 | 52.0 | 1.1 | 51.3 | 50.4 | 55.1 | 0.717 | 0.689 | 0.496 | |
| Real-time ultrasonography (mm) | Chest ○ | 3.5 | 0.5 | 3.5 | 2.2 | 4.7 | 2.5 | 0.6 | 2.1 | 1.4 | 5.0 | 2.5 | 0.3 | 2.2 | 1.5 | 5.0 | 2.2 | 0.3 | 2.0 | 1.7 | 2.9 | 0.081 | 0.050 | 0.148 |
| Flank ○ | 4.5 | 0.9 | 4.4 | 2.4 | 7.6 | 2.7 | 0.5 | 2.2 | 1.7 | 4.6 | 3.3 | 0.3 | 3.0 | 1.7 | 6.3 | 3.7 | 0.1 | 3.7 | 3.6 | 3.9 | 0.775 | 0.716 | 0.209 | |
| Medial thigh | 2.6 | 0.3 | 2.6 | 1.7 | 3.3 | 2.1 | 0.2 | 2.1 | 1.5 | 2.9 | 2.2 | 0.1 | 2.2 | 1.6 | 2.8 | 2.3 | 0.2 | 2.5 | 1.9 | 2.5 | 0.595 | 0.279 | 0.095 | |
| Lumbar | 4.9 | 0.7 | 4.8 | 3.1 | 7.2 | 4.1 | 0.9 | 3.6 | 1.7 | 7.5 | 5.4 | 1.2 | 3.7 | 1.2 | 13.0 | 8.2 | 1.5 | 8.0 | 5.2 | 11.7 | 0.092 | 0.458 | 0.186 | |
| Plicometry (mm) | Chest ○ | 10.6 | 1.2 | 9.5 | 8.0 | 15.0 | 10.3 | 2.2 | 8.3 | 5.0 | 19.0 | 8.0 | 0.7 | 7.3 | 5.5 | 13.0 | 12.9 | 2.1 | 14.0 | 7.0 | 16.5 | 0.996 | 0.255 | 0.061 |
| Flank | 11.6 | 0.8 | 11.0 | 9.5 | 14.0 | 8.0 | 1.5 | 6.8 | 4.0 | 13.0 | 7.2 | 0.7 | 6.3 | 4.0 | 13.0 | 9.1 | 2.2 | 8.3 | 5.0 | 15.0 | 0.200 | 0.519 | 0.037 | |
| Lumbar | 12.2 | 1.3 | 12.0 | 8.5 | 16.0 | 8.4 | 1.0 | 7.9 | 5.5 | 12.5 | 8.7 | 0.5 | 8.3 | 6.0 | 12.0 | 10.0 | 1.3 | 8.8 | 8.5 | 14.0 | 0.338 | 0.215 | 0.015 | |
| Forechest | 13.0 | 1.2 | 14.0 | 8.5 | 15.0 | 9.1 | 1.9 | 8.5 | 4.5 | 14.0 | 7.3 | 0.8 | 6.5 | 3.5 | 12.0 | 8.1 | 1.9 | 7.8 | 4.0 | 13.0 | 0.028 | 0.297 | 0.106 | |
| Inguinal fold | 6.7 | 0.4 | 6.5 | 5.5 | 8.0 | 5.5 | 1.0 | 5.5 | 3.5 | 7.5 | 4.6 | 0.4 | 4.5 | 3.5 | 6.5 | 4.3 | 0.4 | 4.5 | 3.0 | 5.0 | 0.011 | 0.194 | 0.515 | |
| Sex and Neuter Status | Total | |||||
|---|---|---|---|---|---|---|
| Intact Female | Spayed Female | Intact Male | Neutered Male | |||
| BCS | 4 | 1 a,b,c (20.0%) | 0 c (0.0%) | 7 b (58.3%) | 0 a,c (0.0%) | 8 (29.6%) |
| 5 | 3 a (60.0%) | 3 a (50.0%) | 5 a (41.7%) | 0 a (0.0%) | 11 (40.7%) | |
| 6 | 0 a (0.0%) | 1 a (16.7%) | 0 a (0.0%) | 4 b (100.0%) | 5 (18.5%) | |
| 7 | 1 a,b (20.0%) | 2 b (33.3%) | 0 a (0.0%) | 0 a,b (0.0%) | 3 (11.1%) | |
| Total | 5 (100.0%) | 6 (100.0%) | 12 (100.0%) | 4 (100.0%) | 27 (100.0%) | |
| Parameter | Intra-Observer Agreement | Inter-Observer Agreement | ||||||
|---|---|---|---|---|---|---|---|---|
| ICC | 95% Confidence Interval | p Value | ICC | 95% Confidence Interval | p Value | |||
| Lower Bound | Upper Bound | Lower Bound | Upper Bound | |||||
| Chest | 0.782 | 0.557 | 0.897 | <0.001 | 0.765 | 0.329 | 0.905 | <0.001 |
| Flank | 0.614 | 0.311 | 0.804 | <0.001 | 0.713 | 0.373 | 0.869 | <0.001 |
| Lumbar | 0.695 | 0.440 | 0.848 | <0.001 | 0.614 | 0.182 | 0.821 | <0.001 |
| Forechest | 0.782 | 0.577 | 0.894 | <0.001 | 0.339 | −0.356 | 0.689 | 0.133 |
| Inguinal fold | 0.674 | 0.299 | 0.862 | <0.001 | 0.270 | −0.208 | 0.651 | 0.036 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maffi, S.; Bonometti, A.; Chiaffredo, C.; Galimberti, A.; Barletta, C.; Morselli, K.; Menchetti, L.; Quattrone, A. Assessment of Body Condition in Long-Distance Sled Dogs: Validation of the Body Condition Score and Its Association with Ultrasonographic, Plicometric, and Anthropometric Measurements. Vet. Sci. 2025, 12, 766. https://doi.org/10.3390/vetsci12080766
Maffi S, Bonometti A, Chiaffredo C, Galimberti A, Barletta C, Morselli K, Menchetti L, Quattrone A. Assessment of Body Condition in Long-Distance Sled Dogs: Validation of the Body Condition Score and Its Association with Ultrasonographic, Plicometric, and Anthropometric Measurements. Veterinary Sciences. 2025; 12(8):766. https://doi.org/10.3390/vetsci12080766
Chicago/Turabian StyleMaffi, Sergio, Alice Bonometti, Chiara Chiaffredo, Andrea Galimberti, Chiara Barletta, Katia Morselli, Laura Menchetti, and Alda Quattrone. 2025. "Assessment of Body Condition in Long-Distance Sled Dogs: Validation of the Body Condition Score and Its Association with Ultrasonographic, Plicometric, and Anthropometric Measurements" Veterinary Sciences 12, no. 8: 766. https://doi.org/10.3390/vetsci12080766
APA StyleMaffi, S., Bonometti, A., Chiaffredo, C., Galimberti, A., Barletta, C., Morselli, K., Menchetti, L., & Quattrone, A. (2025). Assessment of Body Condition in Long-Distance Sled Dogs: Validation of the Body Condition Score and Its Association with Ultrasonographic, Plicometric, and Anthropometric Measurements. Veterinary Sciences, 12(8), 766. https://doi.org/10.3390/vetsci12080766







