Multiwave Locked System Laser Treatment Reduces the Bacterial Load in the Gingival Sulcus of Dogs After Plaque Removal
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Laser Therapy
2.2. Mesophilic Bacteria Count and Microbiological Culture
2.3. Statistical Analysis
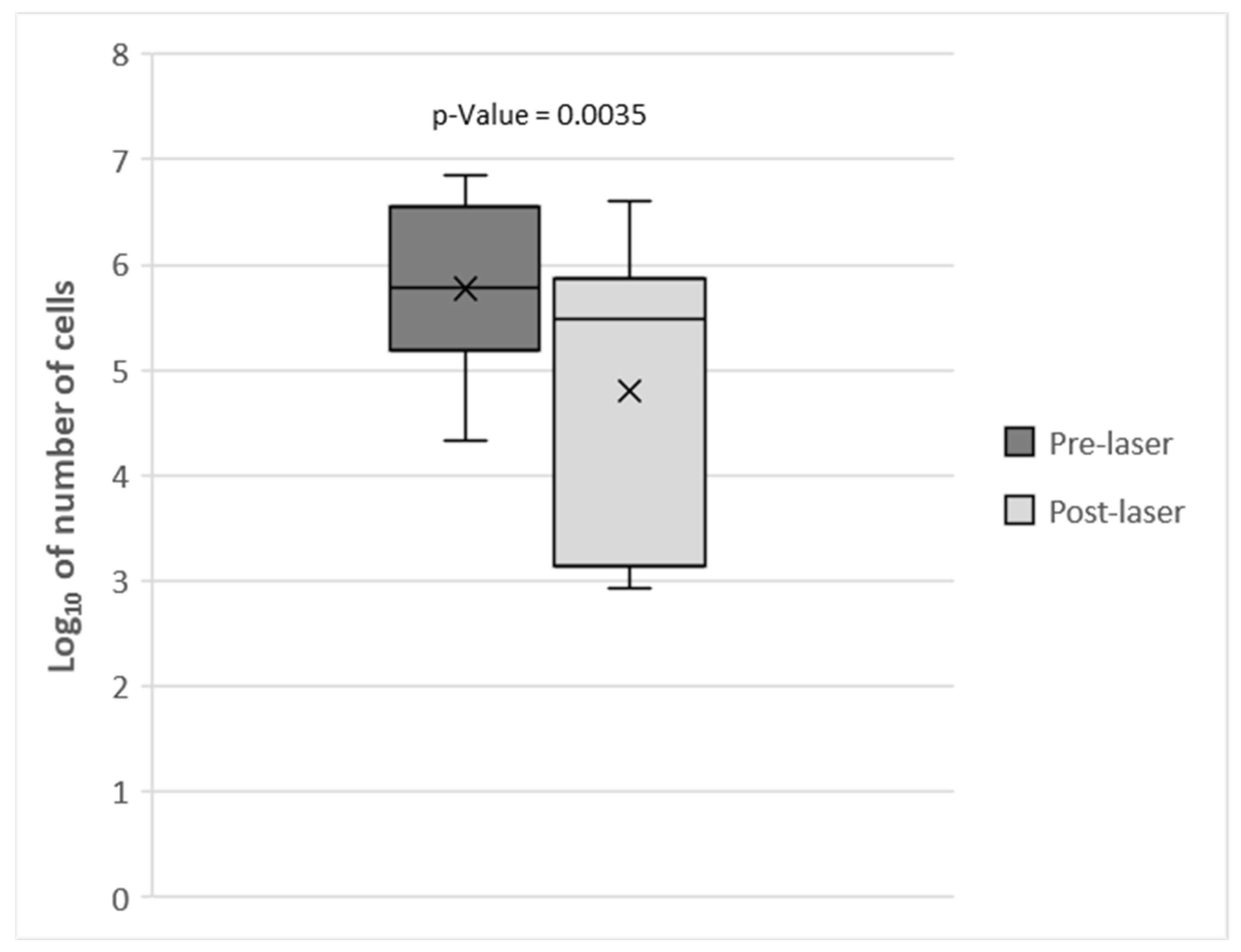
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wallis, C.; Marshall, M.; Colyer, A.; O’Flynn, C.; Deusch, O.; Harris, S. A longitudinal assessment of changes in bacterial community composition associated with the development of periodontal disease in dogs. Vet. Microbiol. 2015, 181, 271–282. [Google Scholar] [CrossRef]
- Wallis, C.; Holcombe, L.J. A review of the frequency and impact of periodontal disease in dogs. J. Small Anim. Pract. 2020, 61, 529–540. [Google Scholar] [CrossRef]
- Niemiec, B.A. Oral pathology. Top. Companion Anim. Med. 2008, 23, 59–71. [Google Scholar] [CrossRef]
- Gorrel, C. Veterinary Dentistry for the General Practitioner, 2nd ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Lund, E. Using data to understand periodontal disease risk. Banfield J. 2008, 15–20. [Google Scholar]
- Burns, K. Below the surface of anesthesia-free dentistry. J. Am. Vet. Med. Assoc. 2016, 248, 242–258. [Google Scholar]
- Bellows, J.; Berg, M.L.; Dennis, S.; Harvey, R.; Lobprise, H.B.; Snyder, C.J.; Stone, A.E.S.; Van de Wetering, A.G. 2019 AAHA Dental Care Guidelines for Dogs and Cats. J. Am. Anim. Hosp. Assoc. 2019, 55, 49–69. [Google Scholar] [CrossRef]
- Harvey, C.E.; Shofer, F.S.; Laster, L. Association of age and body weight with periodontal disease in North American dogs. J. Vet. Dent. 1994, 11, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Hayashi, K.; Kijima, S.; Nonaka, C.; Yamazoe, K. Tooth brushing inhibits oral bacteria in dogs. J. Vet. Med. Sci. 2015, 77, 1323–1325. [Google Scholar] [CrossRef] [PubMed]
- Ingham, K.E.; Gorrel, C. Effect of long-term intermittent periodontal care on canine periodontal disease. J. Small Anim. Pract. 2001, 42, 67–70. [Google Scholar] [CrossRef]
- Tromp, J.A.; Jansen, J.; Pilot, T. Gingival health and frequency of tooth brushing in the beagle dog model. Clin. Find. J. Clin. Periodontol. 1986, 13, 164–168. [Google Scholar] [CrossRef]
- Kačírová, J.; Sondorová, M.; Maďari, A.; Styková, E.; Mucha, R.; Nemcová, R.; Marečáková, N.; Farbáková, J.; Maďar, M. Detection of Periodontal Pathogens from Dental Plaques of Dogs with and without Periodontal Disease. Pathogens 2022, 11, 480. [Google Scholar] [CrossRef] [PubMed]
- Hennet, P. Effectiveness of an enzymatic rawhide dental chew to reduce plaque in beagle dogs. J. Vet. Dent. 2001, 18, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Van Dyke, T.E.; Sheilesh, D. Risk factors for periodontitis. J. Int. Acad. Periodontol. 2005, 7, 3–7. [Google Scholar] [PubMed]
- Davis, I.J.; Wallis, C.; Deusch, O.; Colyer, A.; Milella, L.; Loman, N.; Harris, S. A cross-sectional survey of bacterial species in plaque from client owned dogs with healthy gingiva, gingivitis or mild periodontitis. PLoS ONE 2013, 8, e83158. [Google Scholar] [CrossRef] [PubMed]
- Haffajee, A.D.; Japlit, M.; Bogren, A.; Kent, R.L., Jr.; Goodson, J.M.; Socransky, S.S. Differences in the subgingival microbiota of Swedish and USA subjects who were periodontally healthy or exhibited minimal periodontal disease. Ann. Epidemiol. 2016, 26, 306–310. [Google Scholar] [CrossRef]
- Van Dyke, T.E. The etiology and pathogenesis of periodontitis revisited. J. Appl. Oral Sci. 2009, 17, i. [Google Scholar] [CrossRef]
- Holcombe, L.J.; Patel, N.; Colyer, A.; Deusch, O.; O’Flynn, C.; Harris, S. Early canine plaque biofilms: Characterization of key bacterial interactions involved in initial colonization of enamel. PLoS ONE 2014, 9, e113744. [Google Scholar] [CrossRef]
- Sanguansermsri, P.; Nobbs, A.H.; Jenkinson, H.F.; Surarit, R. Interspecies dynamics among bacteria associated with canine periodontal disease. Mol. Oral Microbiol. 2018, 33, 59–67. [Google Scholar] [CrossRef]
- Polkowska, I.; Sobczyńska-Rak, A.; Gołyńska, M. Analysis of gingival pocket microflora and biochemical blood parameters in dogs suffering from periodontal disease. In Vivo 2014, 28, 1085–1090. [Google Scholar]
- Gorrel, C.; Rawlings, J.M. The role of tooth-brushing and diet in the maintenance of periodontal health in dogs. J. Vet. Dent. 1996, 13, 139–143. [Google Scholar] [CrossRef]
- Miller, B.R.; Harvey, C.E. Compliance with oral hygiene recommendations following periodontal treatment in client-owned dogs. J. Vet. Dent. 1994, 11, 18–19. [Google Scholar] [CrossRef]
- Ray, J.D., Jr.; Eubanks, D.L. Dental homecare: Teaching your clients to care for their pet’s teeth. J. Vet. Dent. 2009, 26, 57–60. [Google Scholar] [CrossRef]
- Crespi, R.; Covani, U.; Margarone, J.E.; Andreana, S. Periodontal tissue regeneration in beagle dogs after laser therapy. Lasers Surg. Med. 1997, 21, 395–402. [Google Scholar] [CrossRef]
- Mizutani, K.; Aoki, A.; Takasaki, A.A.; Kinoshita, A.; Hayashi, C.; Oda, S.; Ishikawa, I. Periodontal tissue healing following flap surgery using an Er:YAG laser in dogs. Lasers Surg. Med. 2006, 38, 314–324. [Google Scholar] [CrossRef]
- Saglam, M.; Kantarci, A.; Dundar, N.; Hakki, S.S. Clinical and biochemical effects of diode laser as an adjunct to nonsurgical treatment of chronic periodontitis: A randomized, controlled clinical trial. Lasers Med. Sci. 2014, 29, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Fontana, C.R.; Kurachi, C.; Mendonça, C.R.; Bagnato, V.S. Microbial reduction in periodontal pockets under exposition of a medium power diode laser: An experimental study in rats. Lasers Surg. Med. 2004, 35, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Khadra, M.; Rønold, H.J.; Lyngstadaas, S.P.; Ellingsen, J.E.; Haanaes, H.R. Low-level laser therapy stimulates bone-implant interaction: An experimental study in rabbits. Clin. Oral Implant. Res. 2004, 15, 325–332. [Google Scholar] [CrossRef]
- Matys, J.; Świder, K.; Grzech-Leśniak, K.; Dominiak, M.; Romeo, U. Photobiomodulation by a 635nm Diode Laser on Peri-Implant Bone: Primary and Secondary Stability and Bone Density Analysis-A Randomized Clinical Trial. BioMed Res. Int. 2019, 2019, 2785302. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, T.; Hosoya, A.; Nakamura, H.; Sano, K.; Nishisaka, T.; Ozawa, H. Increase of bone volume by a nanosecond pulsed laser irradiation is caused by a decreased osteoclast number and an activated osteoblasts. Bone 2007, 40, 140–148. [Google Scholar] [CrossRef]
- Previti, A.; Pugliese, M.; Meggiolaro, S.; Passantino, A. A systematic review via text mining approaches of human and veterinary applications of photobiomodulation: Focus on multiwave locked system laser therapy. Lasers Med. Sci. 2025, 40, 321. [Google Scholar] [CrossRef]
- Yasuda, J.; Yasuda, H.; Nomura, R.; Matayoshi, S.; Inaba, H.; Gongora, E.; Iwashita, N.; Shirahata, S.; Kaji, N.; Akitomo, T.; et al. Investigation of periodontal disease development and Porphyromonas gulae FimA genotype distribution in small dogs. Sci. Rep. 2024, 14, 5360. [Google Scholar] [CrossRef]
- Kortegaard, H.E.; Eriksen, T.; Baelum, V. Periodontal disease in research beagle dogs—An epidemiological study. J. Small Anim. Pract. 2008, 49, 610–616. [Google Scholar] [CrossRef]
- Hamp, S.E.; Olsson, S.E.; Farsø-Madsen, K.; Viklands, P.; Fornell, J. A macroscopic and radiologic investigation of dental diseases of the dog. Vet. Radiol. 1984, 25, 86–92. [Google Scholar] [CrossRef]
- Isogai, H.; Isogai, E.; Okamoto, H.; Shirakawa, H.; Nakamura, F.; Matsumoto, T.; Watanabe, T.; Miura, H.; Aoi, Y.; Kagota, W.; et al. Epidemiological study on periodontal diseases and some other dental disorders in dogs. Nihon Juigaku Zasshi 1989, 51, 1151–1162. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, W.P.; Löe, H.; Ramfjord, S.P. Periodontal disease in the beagle dog. A cross sectional clinical study. J. Periodontal Res. 1980, 15, 380–389. [Google Scholar] [CrossRef]
- Lindhe, J.; Rylander, H. Experimental gingivitis in young dogs. Scand. J. Dent. Res. 1975, 83, 314–326. [Google Scholar] [CrossRef]
- ISO 4833-1:2013; 2013-Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms. Part 1: Colony Count at 30 °C by the Pour Plate technique. International Organization for Standardization: Geneva, Switzerland, 2013.
- Grzech-Leśniak, K.; Matys, J.; Dominiak, M. Comparison of the clinical and microbiological effects of antibiotic therapy in periodontal pockets following laser treatment: An in vivo study. Adv. Clin. Exp. Med. 2018, 27, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Świder, K.; Dominiak, M.; Grzech-Leśniak, K.; Matys, J. Effect of Different Laser Wavelengths on Periodontopathogens in Peri-Implantitis: A Review of In Vivo Studies. Microorganisms 2019, 7, 189. [Google Scholar] [CrossRef]
- Lazăr, L.; Dako, T.; Mârțu, M.A.; Bica, C.I.; Bud, A.; Suciu, M.; Păcurar, M.; Lazăr, A.P. Effects of Laser Therapy on Periodontal Status in Adult Patients Undergoing Orthodontic Treatment. Diagnostics 2022, 12, 2672. [Google Scholar] [CrossRef]
- Lund, E.M.; Armstrong, P.J.; Kirk, C.A.; Kolar, L.M.; Klausner, J.S. Health status and population characteristics of dogs and cats examined at private veterinary practices in the United States. J. Am. Vet. Med. Assoc. 1999, 214, 1336–1341. [Google Scholar] [CrossRef]
- Robinson, N.J.; Dean, R.S.; Cobb, M.; Brennan, M.L. Factors influencing common diagnoses made during first-opinion small-animal consultations in the United Kingdom. Prev. Vet. Med. 2016, 131, 87–94. [Google Scholar] [CrossRef]
| Subject ID | Race | Sex | Age (Years Old) | Diet | Severity of Periodontitis |
|---|---|---|---|---|---|
| 1 | Toy Poodle | M | 5.5 | Dry Food + Wet Food | Mild |
| 2 | Miniature Poodle | F | 11 | Dry Food + Wet Food | Moderate |
| 3 | Miniature Poodle | F | 5.5 | Dry Food + Wet Food | Moderate–Severe |
| 4 | Miniature Poodle | M | 8 | Dry Food + Wet Food | Mild–Moderate |
| 5 | Toy Poodle | F | 3.5 | Dry Food + Wet Food + Home-cooked food | Moderate |
| 6 | Chihuahua | M | 3.5 | Dry Food + Wet Food | Moderate–Severe |
| 7 | Toy Poodle | F | 2.5 | Dry Food + Wet Food + Home-cooked food | Moderate |
| 8 | Miniature Poodle | M | 7 | Wet Food + Home-cooked food | Severe |
| 9 | Toy Poodle | M | 4.7 | Dry Food + Wet Food + Home-cooked food | Moderate–Severe |
| 10 | German Shepherd | M | 2.5 | Dry Food | Moderate |
| 11 | Border Collie | M | 0.58 | Dry Food | Absent |
| 12 | Czechoslovakian Wolfdog | F | 4 | Wet Food | Moderate |
| 13 | Jack Russell Terrier | F | 1 | Dry Food | Mild–Moderate |
| 14 | Mixed breed | F | 11 | Dry Food + Wet Food + Home-cooked food | Moderate |
| 15 | Mixed breed | F | 0.5 | Dry Food | Moderate |
| 16 | Shih Tzu | M | 4.5 | Home-cooked food | Moderate–Severe |
| Subject ID | Isolated Microorganisms Pre-Treatment | Isolated Microorganisms Post-Treatment |
|---|---|---|
| 1 | Polymicrobism | Polymicrobism |
| 2 | Polymicrobism | Polymicrobism |
| 3 | Polymicrobism | Polymicrobism |
| 4 | Escherichia coli | Escherichia coli |
| 5 | Polymicrobism | Polymicrobism |
| 6 | Escherichia coli | Enterobacteriaceae |
| 7 | Pseudomonas aeruginosa | Pseudomonas aeruginosa |
| 8 | Pseudomonas spp. | Pseudomonas spp. |
| 9 | Pseudomonas spp. | Pseudomonas spp. |
| 10 | Polymicrobism | Polymicrobism |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pallante, I.; Squarzoni, P.; Mazzotta, E.; Pozzato, N.; Monici, M. Multiwave Locked System Laser Treatment Reduces the Bacterial Load in the Gingival Sulcus of Dogs After Plaque Removal. Vet. Sci. 2025, 12, 767. https://doi.org/10.3390/vetsci12080767
Pallante I, Squarzoni P, Mazzotta E, Pozzato N, Monici M. Multiwave Locked System Laser Treatment Reduces the Bacterial Load in the Gingival Sulcus of Dogs After Plaque Removal. Veterinary Sciences. 2025; 12(8):767. https://doi.org/10.3390/vetsci12080767
Chicago/Turabian StylePallante, Ivana, Paolo Squarzoni, Elisa Mazzotta, Nicola Pozzato, and Monica Monici. 2025. "Multiwave Locked System Laser Treatment Reduces the Bacterial Load in the Gingival Sulcus of Dogs After Plaque Removal" Veterinary Sciences 12, no. 8: 767. https://doi.org/10.3390/vetsci12080767
APA StylePallante, I., Squarzoni, P., Mazzotta, E., Pozzato, N., & Monici, M. (2025). Multiwave Locked System Laser Treatment Reduces the Bacterial Load in the Gingival Sulcus of Dogs After Plaque Removal. Veterinary Sciences, 12(8), 767. https://doi.org/10.3390/vetsci12080767







