Inflammation and Necrosis Syndrome in Young Piglets—A Longitudinal Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Housing, Feeding and Zootechnical Measures in Sows
2.3. Housing, Feeding and Zootechnical Measures in Piglets
2.4. Clinical Scoring of SINS Characteristics
2.5. Measurement of Body Temperature
2.6. Statistical Analysis
3. Results
3.1. Prevalence of SINS Characteristics in the Piglets and Body Temperature
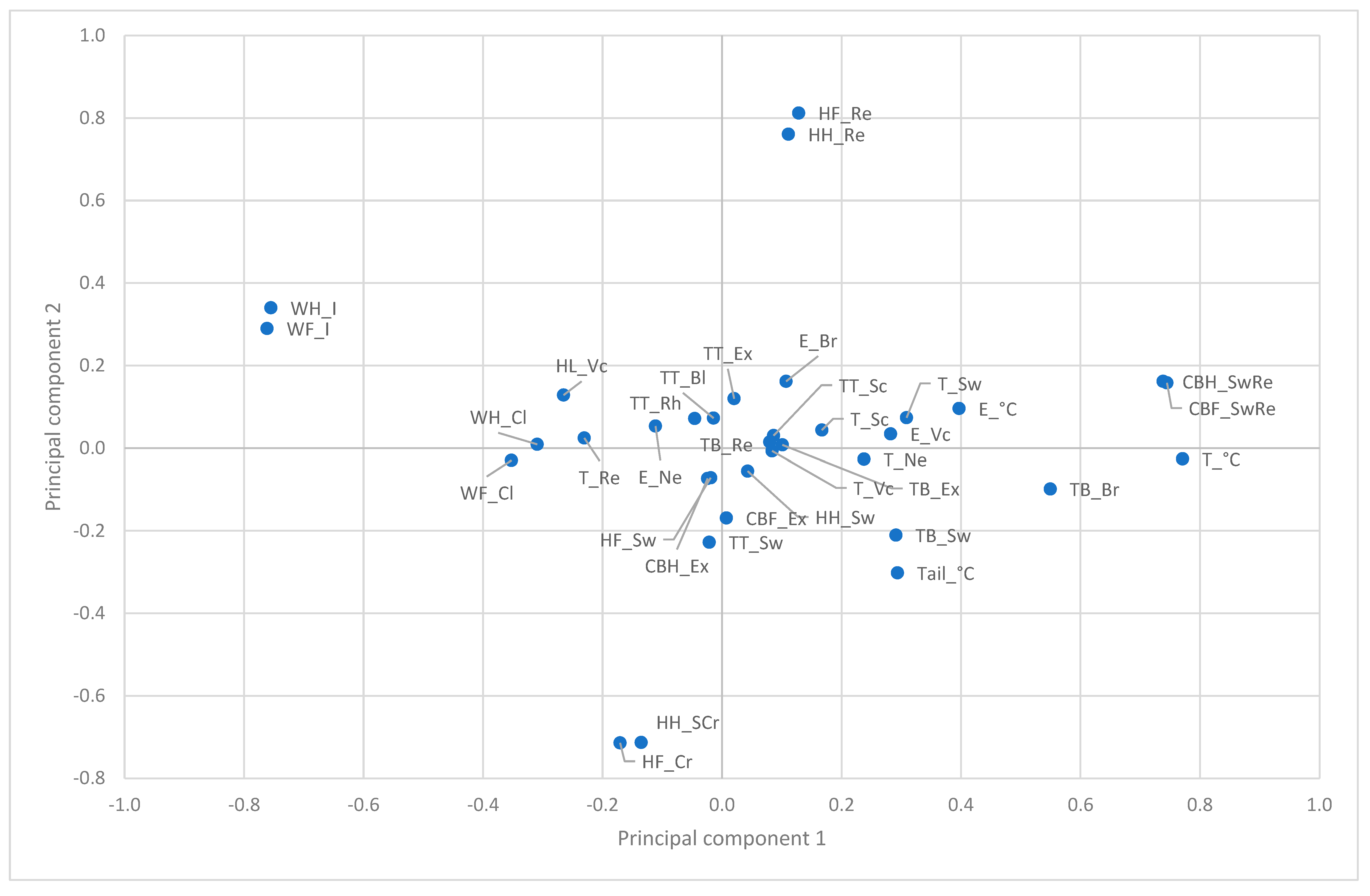
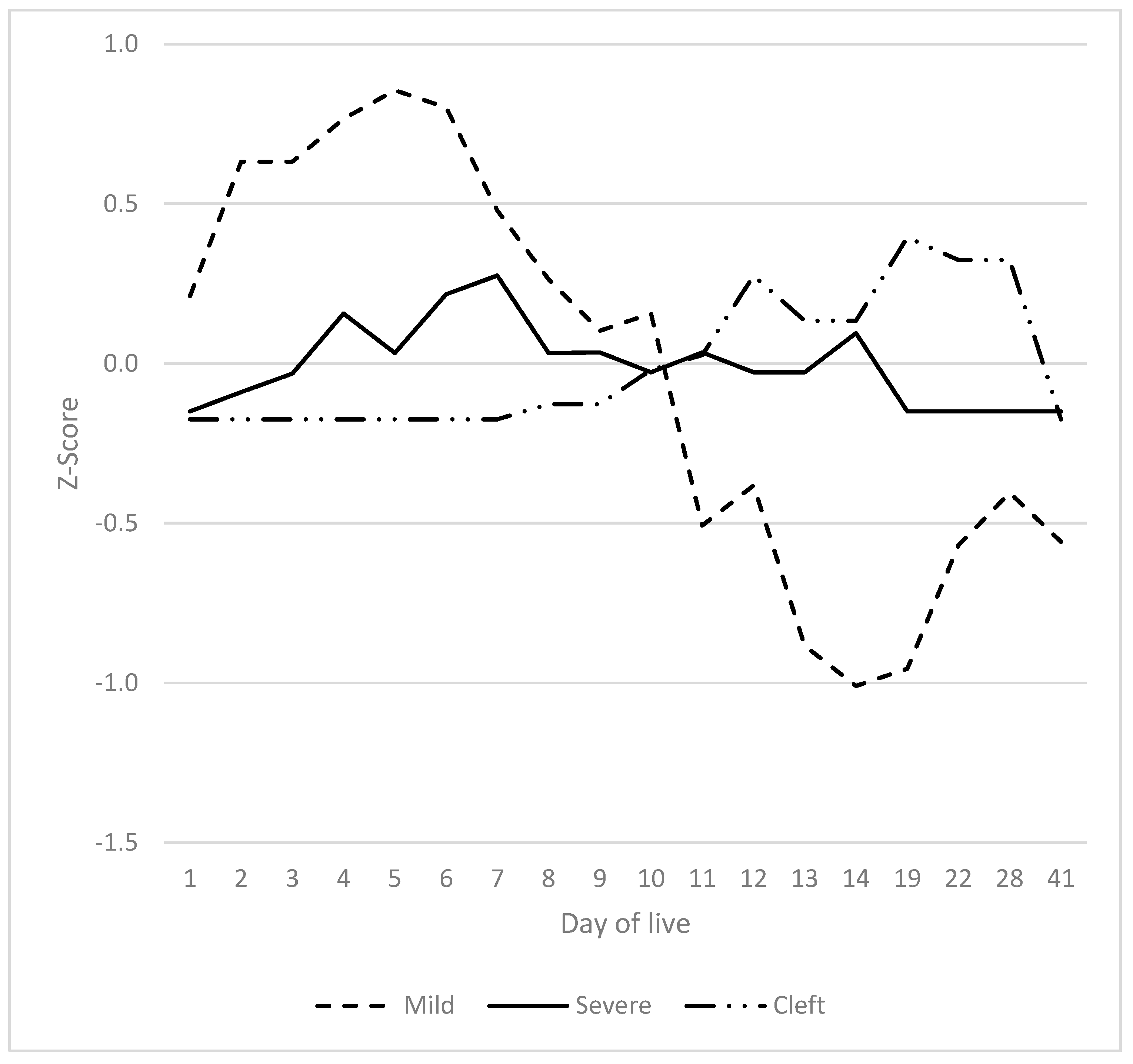
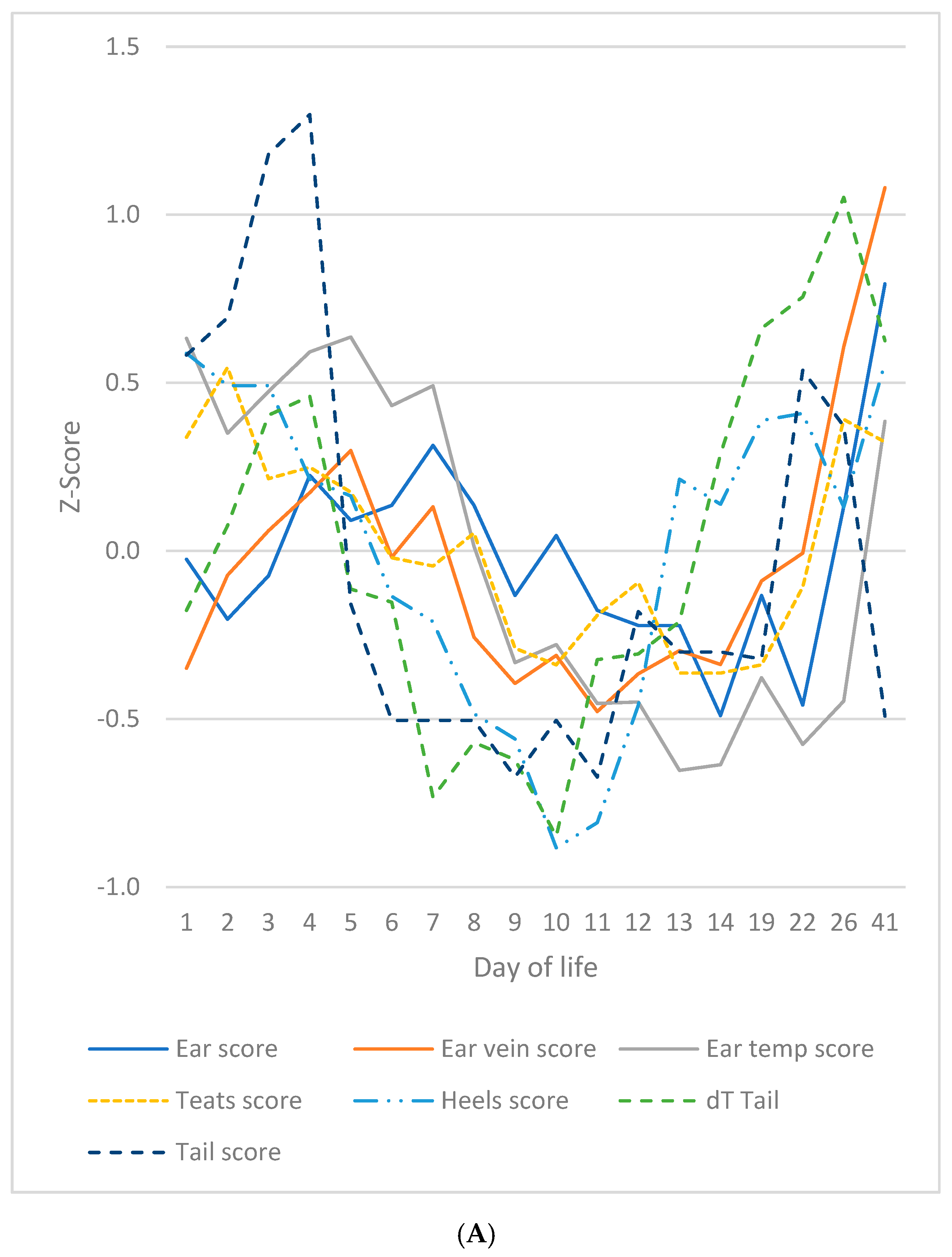
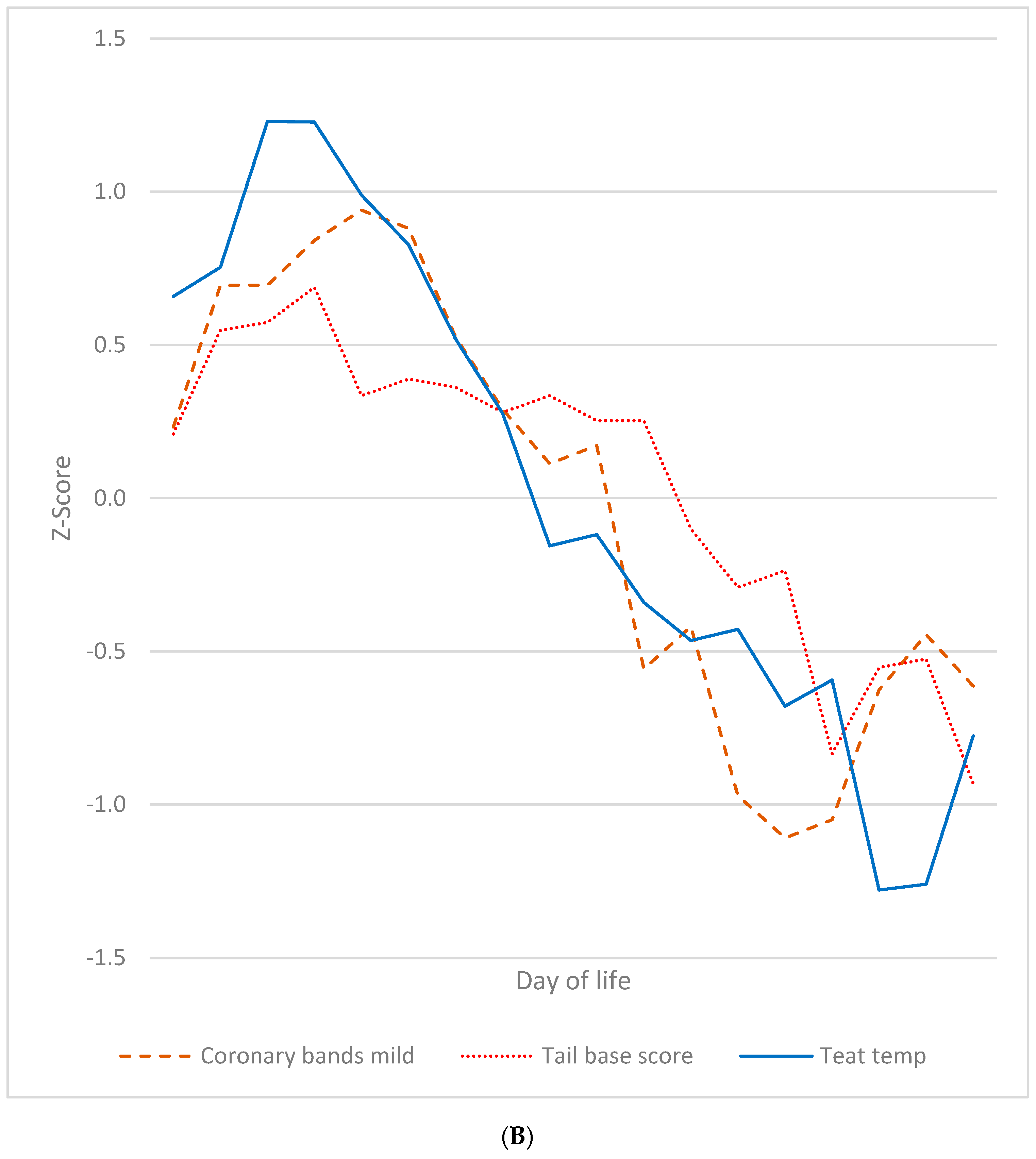
3.2. Principal Components of SINS Characteristics
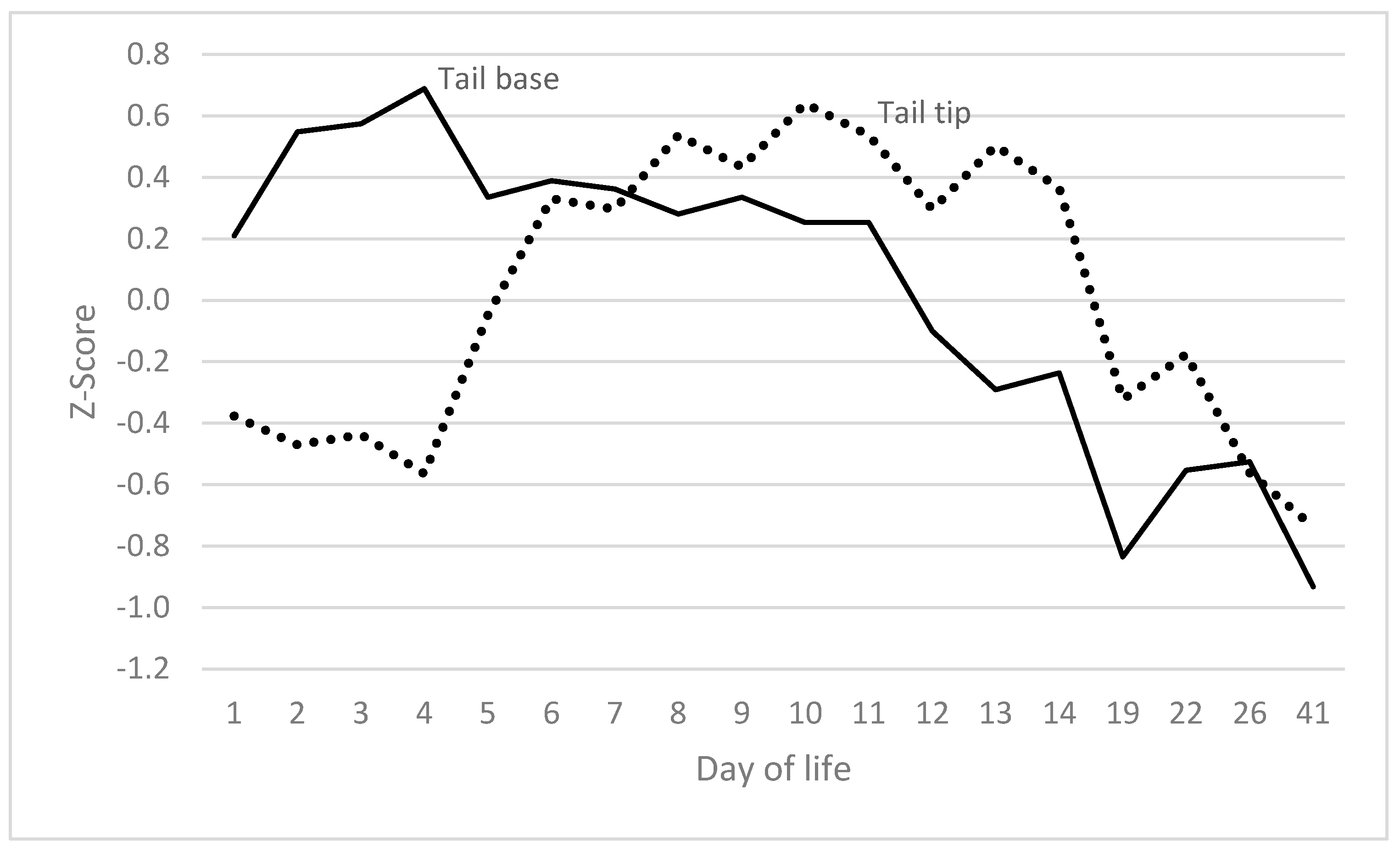
3.3. Tail and Tail Base
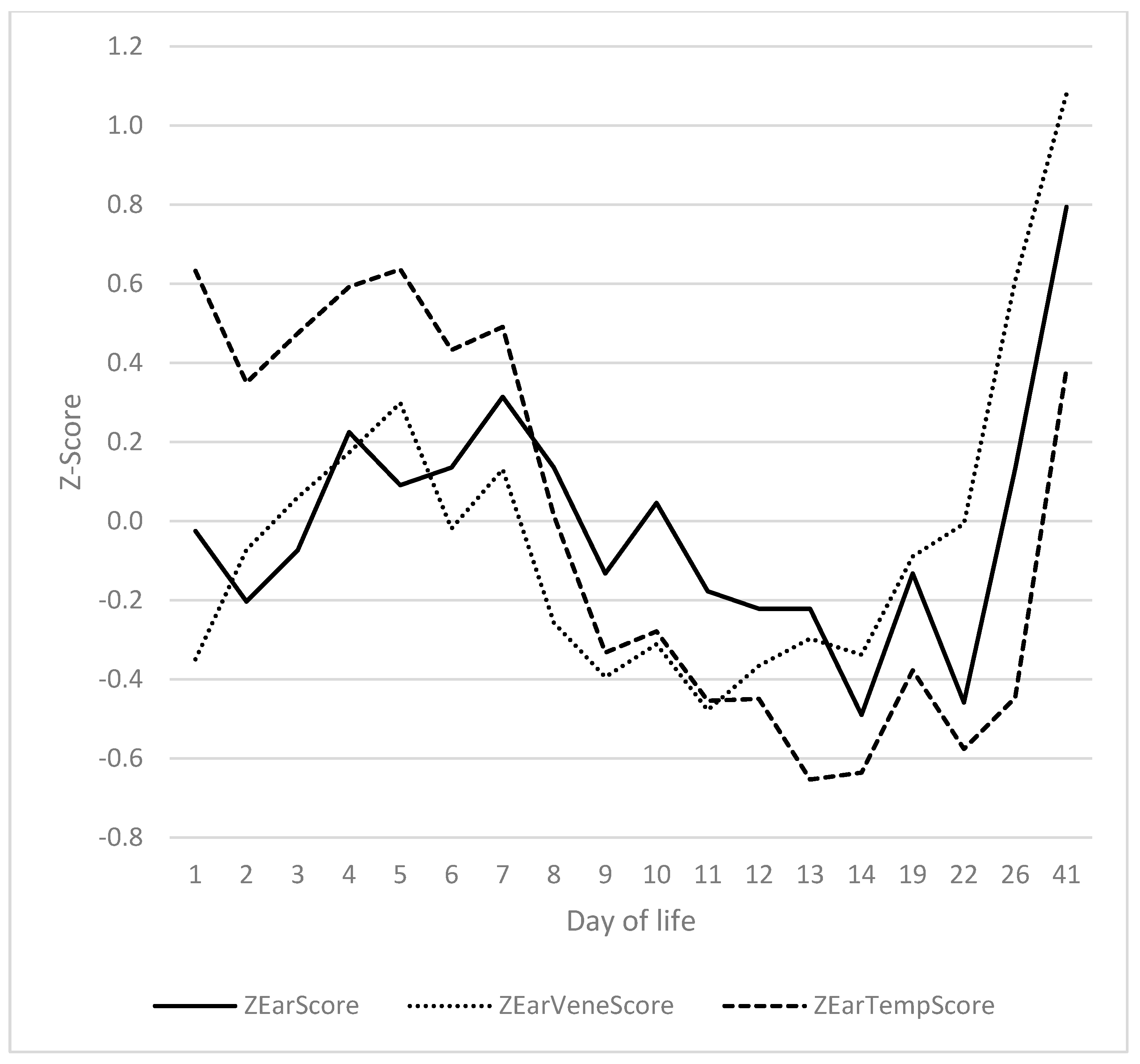
3.4. Ears
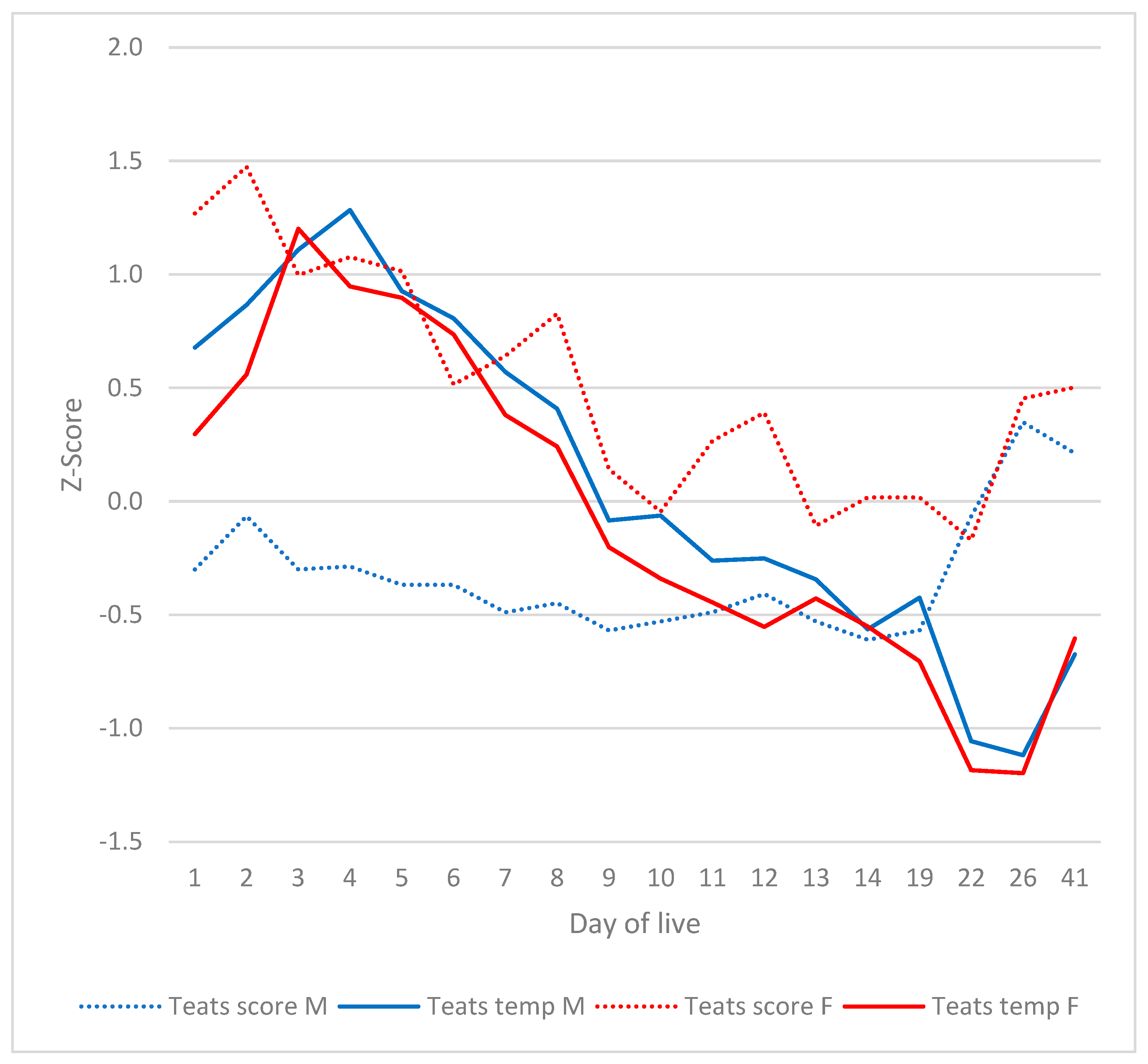
3.5. Teats
3.6. Heels, Coronary Bands and Claws
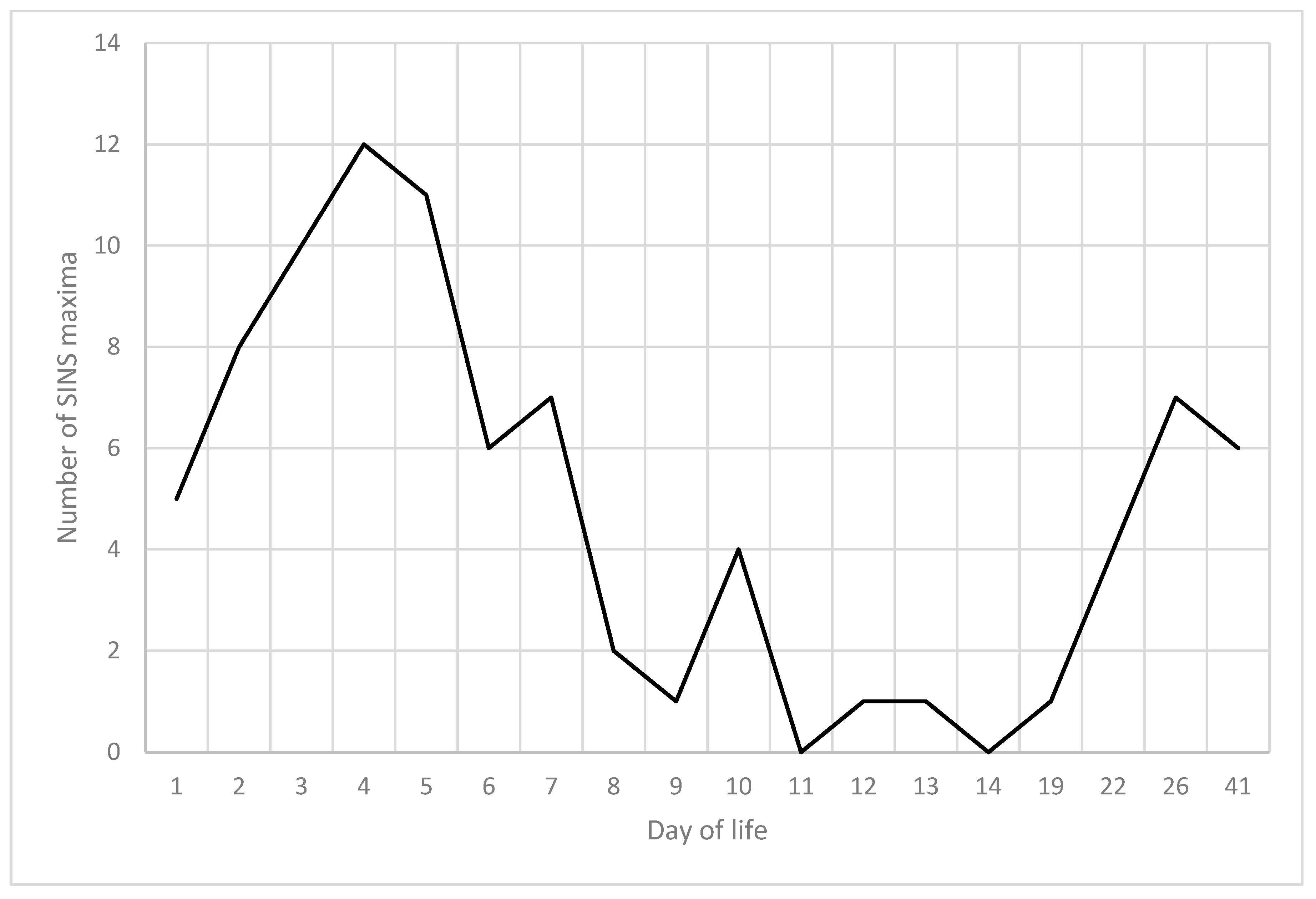
3.7. Summarised Time Patterns
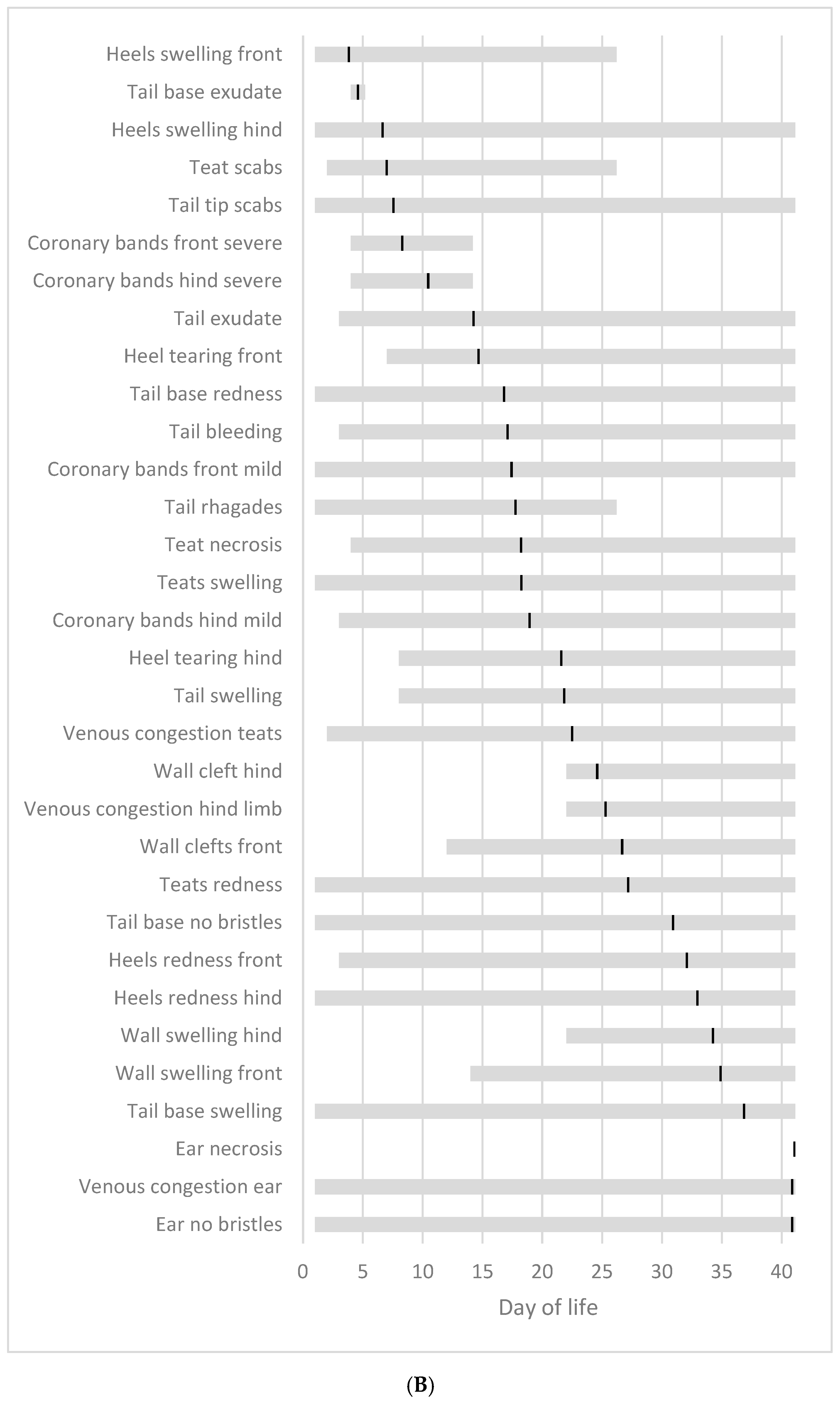
3.8. Chronological Sequence of SINS Characteristics
3.9. Association Between Individual SINS Scores at Different Days of Life
4. Discussion
4.1. Findings Derived from the Longitudinal Design of the Study
4.2. SINS in Young Individual Piglets Can Have One or Two Peaks at Two Distinct Time Points
4.3. Hypothetical Link Between Endogenous Imbalances and SINS Peaks
4.4. SINS Characteristics and Body Temperature
4.5. SINS Characteristics at the Claws
4.6. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| SINS | Swine Inflammation and Necrosis Syndrome |
References
- Koenders-van Gog, K.; Wijnands, T.; Lechner, M.; Reiner, G.; Fink-Gremmels, J. Screening of Piglets for Signs of Inflammation and Necrosis as Early Life Indicators of Animal Health and Welfare Hazards. Animals 2025, 15, 378. [Google Scholar] [CrossRef]
- Leite, N.G.; Knol, E.F.; Nuphaus, S.; Vogelzang, R.; Tsuruta, S.; Wittmann, M.; Lourenco, D. The genetic basis of swine inflammation and necrosis syndrome and its genetic association with post-weaning skin damage and production traits. J. Anim. Sci. 2023, 101, skad067. [Google Scholar] [CrossRef]
- Fortune, H.; Micout, S.; Monjouste, A. Évaluation de la prévalence du syndrome inflammatoire et nécrotique porcin dans les troupeaux français. Journées Rech. Porc. 2024, 56, 301–302. [Google Scholar]
- Reiner, G.; Kuehling, J.; Loewenstein, F.; Lechner, M.; Becker, S. Swine Inflammation and Necrosis Syndrome (SINS). Animals 2021, 11, 1670. [Google Scholar] [CrossRef] [PubMed]
- Kuehling, J.; Loewenstein, F.; Wenisch, S.; Kressin, M.; Herden, C.; Lechner, M.; Reiner, G. An in-depth diagnostic exploration of an inflammation and necrosis syndrome in a population of newborn piglets. Animal 2021, 15, 100078. [Google Scholar] [CrossRef]
- Kuehling, J.; Eisenhofer, K.; Lechner, M.; Becker, S.; Willems, H.; Reiner, G. The effects of boar on susceptibility to swine inflammation and necrosis syndrome in piglets. Porc. Health Manag. 2021, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Reiner, G.; Lechner, M.; Eisenack, A.; Kallenbach, K.; Rau, K.; Müller, S.; Fink-Gremmels, J. Prevalence of an inflammation and necrosis syndrome in suckling piglets. Animal 2019, 13, 2007–2017. [Google Scholar] [CrossRef] [PubMed]
- Reiner, G.; Kuehling, J.; Lechner, M.; Schrade, H.J.; Saltzmann, J.; Muelling, C.; Daenicke, S.; Loewenstein, F. Swine Inflammation and Necrosis Syndrome is influenced by husbandry and quality of sow in suckling piglets, weaners and fattening pigs. Porc. Health Manag. 2020, 6, 32. [Google Scholar] [CrossRef]
- Betz, P. Histological and enzyme histochemical parameters for the age estimation of human skin wounds. Int. J. Leg. Med. 1994, 107, 60–68. [Google Scholar] [CrossRef]
- Loewenstein, F.; Becker, S.; Kuehling, J.; Schrade, H.; Lechner, M.; Ringseis, R.; Eder, K.; Moritz, A.; Reiner, G. Inflammation and necrosis syndrome is associated with alterations in blood and metabolism in pigs. BMC Vet. Res. 2022, 18, 50. [Google Scholar] [CrossRef]
- Ringseis, R.; Gessner, D.K.; Loewenstein, F.; Kuehling, J.; Becker, S.; Willems, H.; Lechner, M.; Eder, K.; Reiner, G. Swine Inflammation and Necrosis Syndrome Is Associated with Plasma Metabolites and Liver Transcriptome in Affected Piglets. Animals 2021, 11, 772. [Google Scholar] [CrossRef]
- Gerhards, K.; Becker, S.; Kuehling, J.; Lechner, M.; Willems, H.; Ringseis, R.; Reiner, G. Screening for transcriptomic associations with Swine Inflammation and Necrosis Syndrome. BMC Vet. Res. 2025, 21, 26. [Google Scholar] [CrossRef]
- Gerß, H. Anwendung der Infrarotthermographie zur nicht-invasiven Detektion fieberhafter Tiere in Schweinegruppen—Einschätzung der Anwendbarkeit im Tierseuchenkrisenfall am Beispiel der Klassischen Schweinepest. Ph.D. Thesis, Tierärztliche Hochschule Hannover, Hannover, Germany, 2014. [Google Scholar]
- Iba, T.; Levy, J.H. Inflammation and thrombosis: Roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J. Thromb. Haemost. 2018, 16, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shan, W.; Zhao, X.; Sun, W. Neutrophils: Linking Inflammation to Thrombosis and Unlocking New Treatment Horizons. Int. J. Mol. Sci. 2025, 26, 1965. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kil, D.Y.; Kim, B.G.; Stein, H.H. Feed energy evaluation for growing pigs. Asian-Australas. J. Anim. Sci. 2013, 26, 1205–1217. [Google Scholar] [CrossRef]
- Zhang, G.F.; Liu, D.W.; Wang, F.L.; Li, D.F. Estimation of the net energy requirements for maintenance in growing and finishing pigs. J. Anim. Sci. 2014, 92, 2987–2995. [Google Scholar] [CrossRef]
- Whittemore, C.; Kyriazakis, I. Growth and body composition changes in pigs. In Whittemore’s Science and Practice of Pig Production, 3rd ed.; Kyriazakis, I., Whittemore, C., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2006; pp. 65–100. [Google Scholar]
- Meyer, E.; Paulick, M. Untersuchungen zum Einfluss des Geburtsgewichtes von Saugferkeln auf nekrotische Veränderungen des Integuments und ihre mögliche Bedeutung für den Kupierverzicht. Zuechtungskunde 2024, 96, 422–431. [Google Scholar]
- Studzinski, T. The post-natal changes in minimal metabolic rate in the pig. J. Physiol. 1972, 224, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Ask, B.; Pedersen, L.V.; Christensen, O.F.; Nielsen, H.M.; Turner, S.P.; Nielsen, B. Selection for social genetic effects in purebreds increases growth in crossbreds. Genet. Sel. Evol. 2021, 53, 15. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zmora, N.; Levy, M.; Elinav, E. The microbiome and innate immunity. Nature 2016, 535, 65–74. [Google Scholar] [CrossRef]
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut Microbiota Dysbiosis in Postweaning Piglets: Understanding the Keys to Health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef]
- Holman, D.B.; Chénier, M.R. Temporal changes and the effect of subtherapeutic concentrations of antibiotics in the gut microbiota of swine. FEMS Microbiol. Ecol. 2014, 90, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Hu, X.; Jin, W.; Liu, G. Dietary nutrition, intestinal microbiota dysbiosis and post-weaning diarrhea in piglets. Anim. Nutr. 2024, 17, 188–207. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Yin, J.; Xu, K.; Li, T.; Yin, Y. What is the impact of diet on nutritional diarrhea associated with gut microbiota in weaning piglets: A system review. Biomed. Res. Int. 2019, 2019, 6916189. [Google Scholar] [CrossRef]
- Merchant, H.A.; McConnell, E.L.; Liu, F.; Ramaswamy, C.; Kulkarni, R.P.; Basit, A.W.; Murdan, S. Assessment of gastrointestinal pH, fluid and lymphoid tissue in the guinea pig, rabbit and pig, and implications for their use in drug development. Eur. J. Pharm. Sci. 2011, 42, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Zhang, L.; He, X.; Smidt, H.; Zhu, W. Dietary fibres modulate the composition and activity of butyrate-producing bacteria in the large intestine of suckling piglets. Antonie Leeuwenhoek 2017, 110, 687–696. [Google Scholar] [CrossRef]
- Klein, K.; Fuchs, G.J.; Kulanpongs, P.; Mertz, G.; Suskind, R.M.; Olson, R.E. Endotoxemia in protein-energy malnutrition. J. Pediatr. Gastroent Nutr. 1988, 7, 225–228. [Google Scholar]
- Kamada, N.; Chen, G.; Inohara, N.; Nunez, G. Control of pathogens and pathobionts by the gut microbiota. Nat. Immunol. 2013, 14, 685–690. [Google Scholar] [CrossRef]
- Henao-Mejia, J.; Elinav, E.; Jin, C.; Hao, L.; Mehal, W.Z.; Strowig, T.; Thaiss, C.A.; Kau, A.L.; Eisenbarth, S.C.; Jurczak, M.J.; et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012, 482, 179–185. [Google Scholar] [CrossRef]
- Hua, X.; Ungaro, R.C.; Petrick, L.M.; Chan, A.T.; Porter, C.K.; Khalili, H.; Nurses’ Health Study; PREDICTS Collaborators. Inflammatory Bowel Disease Is Associated with Prediagnostic Perturbances in Metabolic Pathways. Gastroenterology 2023, 164, 147–150.e2. [Google Scholar] [CrossRef]
- Odenwald, M.A.; Turner, J.R. Intestinal permeability defects: Is it time to treat? Clin. Gastroenterol. Hepatol. 2013, 11, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Moonwiriyakit, A.; Pathomthongtaweechai, N.; Steinhagen, P.R.; Chantawichitwong, P.; Satianrapapong, W.; Pongkorpsakol, P. Tight junctions: From molecules to gastrointestinal diseases. Tissue Barriers 2023, 11, 2077620. [Google Scholar] [CrossRef] [PubMed]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut microbiota, intestinal permeability, and systemic inflammation: A narrative review. Intern. Emerg. Med. 2024, 19, 275–293. [Google Scholar] [CrossRef] [PubMed]
- Mendes, B.G.; Schnabl, B. From intestinal dysbiosis to alcohol-associated liver disease. Clin. Mol. Hepatol. 2020, 26, 595–605. [Google Scholar] [CrossRef]
- Peña-Durán, E.; García-Galindo, J.J.; López-Murillo, L.D.; Huerta-Huerta, A.; Balleza-Alejandri, L.R.; Beltrán-Ramírez, A.; Anaya-Ambriz, E.J.; Suárez-Rico, D.O. Microbiota and Inflammatory Markers: A Review of Their Interplay, Clinical Implications, and Metabolic Disorders. Int. J. Mol. Sci. 2025, 26, 1773. [Google Scholar] [CrossRef]
- Alexander, C.; Rietschel, E. Invited review: Bacterial lipopolysaccharides and innate immunity. J. Endotoxin Res. 2001, 7, 167–202. [Google Scholar] [CrossRef]
- Cao, S.; Zhang, Q.; Wang, C.; Wu, H.; Jiao, L.; Hong, Q.; Hu, C. LPS challenge increased intestinal permeability, dis-rupted mitochondrial function and triggered mitophagy of piglets. Innate Immun. 2018, 24, 221–230. [Google Scholar] [CrossRef]
- Andreasen, A.S.; Krabbe, K.S.; Krogh-Madsen, R.; Taudorf, S.; Pedersen, B.K.; Møller, K. Human endotoxemia as a model of systemic inflammation. Curr. Med. Chem. 2008, 15, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.P.; Texeira, T.F.; Ferreira, A.B.; Peluzio Mdo, C.; Alfenas Rde, C. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br. J. Nutr. 2012, 108, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Rainone, V.; Schneider, L.; Saulle, I.; Ricci, C.; Biasin, M.; Al-Daghri, N.M.; Giani, E.; Zuccotti, G.V.; Clerici, M.; Trabattoni, D. Upregulation of inflammasome activity and increased gut permeability are associated with obesity in children and adolescents. Int. J. Obes. 2016, 40, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Munsterhjelm, C.; Nordgreen, J.; Aae, F.; Heinonen, M.; Valros, A.; Janczak, A.M. Sick and grumpy: Changes in social behaviour after a controlled immune stimulation in group-housed gilts. Physiol. Behav. 2019, 198, 76–83. [Google Scholar] [CrossRef]
- Nordgreen, J.; Edwards, S.A.; Boyle, L.A.; Bolhuis, J.E.; Veit, C.; Sayyari, A.; Marin, D.E.; Dimitrov, I.; Janczak, A.M.; Valros, A. A Proposed Role for Pro-Inflammatory Cytokines in Damaging Behavior in Pigs. Front. Vet. Sci. 2020, 7, 646. [Google Scholar] [CrossRef]
- Pearce, C.S.; Mani, V.; Boddicker, R.L.; Johnson, J.S.; Weber, T.E.; Ross, J.W.; Rhoads, R.P.; Baumgard, L.H.; Gabler, N.K. Heat stress reduces intestinal barrier integrity and favors intestinal glucose transport in growing pigs. PLoS ONE 2013, 8, e70215. [Google Scholar] [CrossRef]
- Pearce, S.C.; Mani, V.; Boddicker, R.L.; Johnson, J.S.; Weber, T.E.; Ross, J.W.; Baumgard, L.H.; Gabler, N.K. Heat stress reduces barrier function and alters intestinal metabolism in growing pigs. J. Anim. Sci. 2012, 90, 257–259. [Google Scholar] [CrossRef]
- Pearce, S.C.; Sanz-Fernandez, M.V.; Hollis, J.H.; Baumgard, L.H.; Gabler, N.K. Short-term exposure to heat stress attenuates appetite and intestinal integrity in growing pigs. J. Anim. Sci. 2014, 92, 5444–5454. [Google Scholar] [CrossRef] [PubMed]
- Ortega, A.D.S.V.; Szabó, C. Adverse Effects of Heat Stress on the Intestinal Integrity and Function of Pigs and the Mitigation Capacity of Dietary Antioxidants: A Review. Animals 2021, 11, 1135. [Google Scholar] [CrossRef]
- Ayman, M. Mahmoud AM, Wilkinson FL, Sandhu MA, Lightfoot AP. The Interplay of Oxidative Stress and Inflammation: Mechanistic Insights and Therapeutic Potential of Antioxidants. Oxidative Med. Cell. Longev. 2021, 2021, 9851914. [Google Scholar] [CrossRef]
- Liu, B.; Zhu, X.; Cui, Y.; Wang, W.; Liu, H.; Li, Z.; Guo, Z.; Ma, S.; Li, D.; Wang, C.; et al. Consumption of Dietary Fiber from Different Sources during Pregnancy Alters Sow Gut Microbiota and Improves Performance and Reduces Inflammation in Sows and Piglets. mSystems 2021, 6, e00591-20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shang, Q.; Liu, H.; Liu, S.; He, T.; Piao, X. Effects of dietary fiber sources during late gestation and lactation on sow performance, milk quality, and intestinal health in piglets1. J. Anim. Sci. 2019, 97, 4922–4933. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Chen, J.; Liu, J.; Chen, F.; Guan, W.; Zhang, S. Dietary fiber and microbiota interaction regulates sow metabolism and reproductive performance. Anim. Nutr. 2020, 6, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.e21. [Google Scholar] [CrossRef]
- Sanz Fernandez, M.V.; Stoakes, S.K.; Abuajamieh, M.; Seibert, J.T.; Johnson, J.S.; Horst, E.A.; Rhoads, R.P.; Baumgard, L.H. Heat stress increases insulin sensitivity in pigs. Physiol. Repetit. 2015, 3, e1247. [Google Scholar] [CrossRef]
- Sahasrabudhe, N.; Beukema, M.; Tian, L.; Troost, B.; Scholte, J.; Bruininx, E.; Bruggeman, G.; van den Berg, M.; Scheurink, A.; Schols, H.; et al. Dietary fiber pectin directly blocks Toll-like receptor 2-1 and prevents doxorubicin-induced ileitis. Front. Immunol. 2018, 9, 383. [Google Scholar] [CrossRef]
- Luu, M.; Monning, H.; Visekruna, A. Exploring the molecular mechanisms underlying the protective effects of microbial SCFAs on intestinal tolerance and food allergy. Front. Immunol. 2020, 11, 1225. [Google Scholar] [CrossRef]
- Berchieri-Ronchi, C.B.; Kim, S.W.; Zhao, Y.; Correa, C.R.; Yeum, K.J.; Ferreira, A.L. Oxidative stress status of highly prolific sows during gestation and lactation. Animal 2011, 5, 1774–1779. [Google Scholar] [CrossRef]
- Jabbour, H.N.; Sales, K.J.; Catalano, R.D.; Norman, J.E. Inflammatory pathways in female reproductive health and disease. Reproduction 2009, 138, 903–919. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Wei, H.; Xu, C.; Xie, X.; Jiang, S.; Peng, J. Maternal soluble fiber diet during pregnancy changes the intestinal microbiota, improves growth performance, and reduces intestinal permeability in piglets. Appl. Environ. Microbiol. 2018, 84, e01047-18. [Google Scholar] [CrossRef] [PubMed]
- Costermans, N.; Teerds, K.; Keijer, J.; Knol, E.; Koopmanschap, R.; Kemp, B.; Soede, N. Follicular development of sows at weaning in relation to estimated breeding value for within-litter variation in piglet birth weight. Animal 2019, 13, 554–563. [Google Scholar] [CrossRef]
- Sawicki, C.M.; Livingston, K.A.; Obin, M.; Roberts, S.B.; Chung, M.; McKeown, N.M. Dietary fiber and the human gut microbiota: Application of evidence mapping methodology. Nutrients 2017, 9, 125. [Google Scholar] [CrossRef]
- Huang, C.; Song, P.; Fan, P.; Hou, C.; Thacker, P.; Ma, X. Dietary sodium butyrate decreases postweaning diarrhea by modulating intestinal permeability and changing the bacterial communities in weaned piglets. J. Nutr. 2015, 145, 2774–2780. [Google Scholar] [CrossRef]
- Martens, E.; Neumann, M.; Desai, M. Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nat. Rev. Microbiol. 2018, 16, 457–470. [Google Scholar] [CrossRef]
- Gerhards, K.; Becker, S.; Kuehling, J.; Lechner, M.; Bathke, J.; Willems, H.; Reiner, G. GWAS reveals genomic associations with swine inflammation and necrosis syndrome. Mamm. Genome 2023, 34, 586–601. [Google Scholar] [CrossRef]
- Gerhards, K.; Becker, S.; Kühling, J.; Mickan, J.; Lechner, M.; Willems, H.; Reiner, G. Fine Mapping Identifies Candidate Genes Associated with Swine Inflammation and Necrosis Syndrome. Vet. Sci. 2025, 12, 508. [Google Scholar] [CrossRef]
- Wick-Urban, B. Störfall in der Chemiefabrik. 2011. Available online: https://www.pharmazeutische-zeitung.de/ausgabe-112011/stoerfall-in-der-chemiefabrik/ (accessed on 1 August 2025).
- He, G.; Ouyang, Q.; Chen, D.; Li, F.; Zhou, J. The Microvacular Thrombi of Colonic Tissue in Ulcerative Colitis. Dig. Dis. Sci. 2007, 52, 2236–2240. [Google Scholar] [CrossRef]
- Pierron, A.; Alassane-Kpembi, I.; Oswald, I.P. Impact of two mycotoxins deoxynivalenol and fumonisin on pig intestinal health. Porc. Health Manag. 2016, 2, 21. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J.; Zhou, H.R.; Moon, Y.; Chung, Y.J. Cellular and molecular mechanisms for immune modulation by deoxynivalenol and other trichothecenes: Unravelling a paradoxon. Toxicol. Lett. 2004, 153, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Pinton, P.; Nougayrede, J.P.; Del Rio, J.C.; Moreno, C.; Marin, D.E.; Ferrier, L.; Bracarense, A.P.; Kolf-Clauw, M.; Oswald, J.P. The food contaminant deoxynivalenol decreases intestinal barrier permeability and reduces claudin expression. Toxicol. Appl. Pharmacol. 2009, 237, 41–48. [Google Scholar] [CrossRef]
- Kullik, K.; Brosig, B.; Kersten, S.; Valenta, H.; Diesing, A.K.; Panther, P.; Reinhardt, N.; Kluess, J.; Rothkötter, H.J.; Breves, G.; et al. Interactions between the Fusarium toxin deoxynivaleno and lipopolysaccharides on the in vivo protein synthesis of acute phase proteins, cytokines and metabolic activity of peripheral blood mononuclear cells in pigs. Food Chem. Toxicol. 2013, 57, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Klunker, I.R.; Kahlert, S.; Panther, P.; Diesing, A.K.; Reinhardt, N.; Brosig, B.; Kersten, S.; Dänicke, S.; Rothkötter, H.J.; Kluess, J.W. DON and LPS alter epithelial proliferation and spatial distribution of apical junction proteins along the small intestinal axis. J. Anim. Sci. 2012, 90, 276–285. [Google Scholar]
- Dänicke, S.; Valenta, H.; Ganter, M.; Brosig, B.; Kersten, S.; Diesing, A.K.; Kahlert, S.; Kluess, J.; Rothkötter, H.J. Lipopolysaccharides (LPS) modulate the metabolism of deoxynivalenol (DON) in the pig. Mycotox Res. 2014, 30, 161–170. [Google Scholar] [CrossRef]
- Ravin, H.A.; Rowley, D.; Jenkins, C.; Fine, J. On the absorption of bacterial endotoxin from the gastro-intestinal tract of the normal and shocked animal. J. Exp. Med. 1960, 112, 783–792. [Google Scholar] [CrossRef]
- Ganey, P.E.; Roth, R.A. Concurrent inflammation as a determinant of susceptibility to toxicity from xenobiotic agents. Toxicology 2001, 169, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Schrauwen, E.; Thoonen, H.; Hoorens, J.; Houvenaghel, A. Pathophysiological effects of endotoxin infusion in young piglets. Br. Vet. J. 1986, 142, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Guillou, D.; Demey, V.; Chacheyras-Durand, F.; Le Treut, Y. Mise en evidence du transfer des endotoxines de la truie vers sa portée dans le context du syndrome de dysgalactie post-partum. J. Rech. Porc. 2013, 45, 269–270. [Google Scholar]
- Jadamus, A.; Schneider, D. Long-term effect of fusariotoxins on the reproduction performance of sows testing the effectiveness of detoxifying feed additives 700. FEED Mag. 2002, 10, 396–405. [Google Scholar]
- Busch, M.E.; Jensen, I.M.; Korsgaard, J. Development and consequences of ear necrosis in a weaner herd and two growingfinishing herds. In Proceedings of the 21st International Pig Veterinary Society Congress, Vancouver, BC, Canada, 18–21 July 2010; p. 45. [Google Scholar]
- Nolan, J.P. The role of intestinal endotoxins in gastrointestinal and liver diseases. Prog. Clin. Biol. Res. 1988, 272, 147–159. [Google Scholar]
- Knolle, P.A.; Gerken, G. Local control of the immune response in the liver. Immunol. Rev. 2000, 174, 21–34. [Google Scholar] [CrossRef]
- Soerensen, D.D.; Pedersen, L.J. Infrared skin temperature measurements for monitoring health in pigs: A review. Acta Vet. Scand. 2015, 57, 5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gómez-Prado, J.; Pereira, A.M.F.; Wang, D.; Villanueva-García, D.; Domínguez-Oliva, A.; Mora-Medina, P.; Hernández-Avalos, I.; Martínez-Burnes, J.; Casas-Alvarado, A.; Olmos-Hernández, A.; et al. Thermoregulation mechanisms and perspectives for validating thermal windows in pigs with hypothermia and hyperthermia: An overview. Front. Vet. Sci. 2022, 9, 1023294. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Falotico, J.M.; Shinozaki, K.; Saeki, K.; Becker, L.B. Advances in the Approaches Using Peripheral Perfusion for Monitoring Hemodynamic Status. Front. Med. 2020, 7, 614326. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Granger, D.N.; Senchenkova, E. Inflammation and the Microcirculation. San Rafael (CA): Morgan and Claypool Life Sciences. 2010. Available online: https://www.ncbi.nlm.nih.gov/books/NBK53373/ (accessed on 1 August 2025).
- Furniss, S.J.; Edwards, S.A.; Lightfoot, A.L.; Spechters, H.H. The effect of floor type in farrowing pens on pig injury. I. Leg and teat damage of suckling piglets. Br. Vet. J. 1986, 142, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Pritts, T.; Hungness, E.; Wang, Q.; Robb, B.; Hershko, D.; Hasselgren, P.O. Mucosal and enterocyte IL-6 production during sepsis and endotoxemia--role of transcription factors and regulation by the stress response. Am. J. Surg. 2002, 183, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.W. Longitudinal Studies 1: Determinants of Risk. Methods Mol. Biol. 2021, 2249, 83–101. [Google Scholar] [CrossRef]
- Bodden, C.; Siestrup, S.; Palme, R.; Kaiser, S.; Sachser, N.; Richter, S.H. Evidence-based severity assessment: Impact of repeated versus single open-field testing on welfare in C57BL/6J mice. Behav. Brain Res. 2018, 336, 261–268. [Google Scholar] [CrossRef]
- Cnops, V.; Iyer, V.R.; Parathy, N.; Wong, P.; Dawe, G.S. Test, rinse, repeat: A review of carryover effects in rodent behavioral assays. Neurosci. Biobehav. Rev. 2022, 135, 104560. [Google Scholar] [CrossRef]






| Day of Life | Tail Base | Tail/Tail Tip | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No Bristles | Swelling | Redness | Exudation | Necrosis | Swelling | Scabs | Rhagades | Exudation | Necrosis | Ring Constriction | Bleeding | |
| 1 | 100.0 | 83.1 | 3.4 | 0.0 | 0.0 | 5.1 | 11.9 | 1.7 | 0.0 | 0.0 | 0.0 | 0.0 |
| 2 | 100.0 | 94.8 | 13.8 | 0.0 | 0.0 | 3.4 | 10.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 3 | 98.3 | 98.3 | 13.8 | 0.0 | 0.0 | 1.7 | 6.9 | 0.0 | 5.2 | 0.0 | 0.0 | 1.7 |
| 4 | 100.0 | 100.0 | 14.3 | 3.6 | 0.0 | 1.8 | 3.6 | 0.0 | 1.8 | 0.0 | 0.0 | 1.8 |
| 5 | 92.9 | 100.0 | 0.0 | 1.8 | 0.0 | 33.9 | 0.0 | 0.0 | 1.8 | 0.0 | 0.0 | 0.0 |
| 6 | 94.6 | 100.0 | 3.6 | 0.0 | 0.0 | 55.4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 7 | 92.9 | 100.0 | 3.6 | 0.0 | 0.0 | 53.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 8 | 89.3 | 98.2 | 3.6 | 0.0 | 0.0 | 66.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 9 | 94.6 | 100.0 | 0.0 | 0.0 | 0.0 | 60.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 10 | 85.7 | 100.0 | 3.6 | 0.0 | 0.0 | 71.4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 11 | 91.1 | 98.2 | 0.0 | 0.0 | 0.0 | 66.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 12 | 73.2 | 91.1 | 1.8 | 0.0 | 0.0 | 50.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 3.6 |
| 13 | 64.3 | 85.7 | 3.6 | 0.0 | 0.0 | 62.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.8 |
| 14 | 71.4 | 82.1 | 3.6 | 0.0 | 0.0 | 55.4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.8 |
| 19 | 33.9 | 80.4 | 3.6 | 0.0 | 0.0 | 19.6 | 0.0 | 0.0 | 1.8 | 0.0 | 0.0 | 0.0 |
| 22 | 45.5 | 89.1 | 1.8 | 0.0 | 0.0 | 18.2 | 3.6 | 1.8 | 3.6 | 0.0 | 0.0 | 1.8 |
| 26 | 56.4 | 80.0 | 1.8 | 0.0 | 0.0 | 1.8 | 0.0 | 3.6 | 3.6 | 0.0 | 0.0 | 0.0 |
| 41 | 38.5 | 69.2 | 3.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Day of Life | Ears | Teats | ||||||
|---|---|---|---|---|---|---|---|---|
| No Bristles | Venous Congestion | Necrosis | Venous Congestion | Swelling | Redness | Scabs | Necrosis | |
| 1 | 100.0 | 86.4 | 0.0 | 0.0 | 40.7 | 32.2 | 0.0 | 5.1 |
| 2 | 100.0 | 79.3 | 0.0 | 5.2 | 39.7 | 29.3 | 1.7 | 17.2 |
| 3 | 100.0 | 84.5 | 0.0 | 1.7 | 34.5 | 6.9 | 6.9 | 19 |
| 4 | 100.0 | 96.4 | 0.0 | 1.8 | 33.9 | 3.6 | 10.7 | 21.4 |
| 5 | 100.0 | 91.1 | 0.0 | 1.8 | 30.4 | 7.1 | 7.1 | 19.6 |
| 6 | 100.0 | 92.9 | 0.0 | 1.8 | 26.8 | 7.1 | 0.0 | 16.1 |
| 7 | 100.0 | 100.0 | 0.0 | 1.8 | 23.2 | 7.1 | 1.8 | 16.1 |
| 8 | 100.0 | 92.9 | 0.0 | 0.0 | 26.8 | 16.1 | 1.8 | 12.5 |
| 9 | 100.0 | 82.1 | 0.0 | 0.0 | 12.5 | 8.9 | 0.0 | 10.7 |
| 10 | 100.0 | 89.3 | 0.0 | 0.0 | 8.9 | 5.4 | 1.8 | 12.5 |
| 11 | 100.0 | 80.4 | 0.0 | 0.0 | 12.5 | 17.9 | 0.0 | 8.9 |
| 12 | 96.4 | 82.1 | 0.0 | 0.0 | 14.3 | 21.4 | 0.0 | 10.7 |
| 13 | 100.0 | 78.6 | 0.0 | 0.0 | 12.5 | 8.9 | 0.0 | 5.4 |
| 14 | 100.0 | 67.9 | 0.0 | 0.0 | 8.9 | 14.3 | 0.0 | 3.6 |
| 19 | 98.2 | 83.9 | 0.0 | 1.8 | 8.9 | 14.3 | 0.0 | 3.6 |
| 22 | 100.0 | 69.1 | 0.0 | 0.0 | 9.1 | 29.1 | 0.0 | 7.3 |
| 26 | 100.0 | 90.9 | 1.8 | 1.8 | 7.3 | 63.6 | 1.8 | 7.3 |
| 41 | 100.0 | 88.5 | 30.8 | 7.7 | 23.1 | 46.2 | 0.0 | 0.0 |
| Day of Life | Coronary Bands | Heels | Claw Wall | Hind Limb | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Swelling/Redness | Exudation | Cleft | Swelling | Redness | Tearing | Venous Congestion | |||||||||
| Front | Hind | Front | Hind | Front | Hind | Front | Hind | Front | Hind | Front | Hind | Front | Hind | ||
| 1 | 62.7 | 67.8 | 0.0 | 0.0 | 0.0 | 0.0 | 30.5 | 39.0 | 69.5 | 59.3 | 0.0 | 0.0 | 1.7 | 1.7 | 0.0 |
| 2 | 84.5 | 87.9 | 0.0 | 1.7 | 0.0 | 0.0 | 6.9 | 10.3 | 91.4 | 82.8 | 1.7 | 0.0 | 5.2 | 8.6 | 0.0 |
| 3 | 87.9 | 84.5 | 1.7 | 1.7 | 0.0 | 0.0 | 1.7 | 10.3 | 96.6 | 82.8 | 0.0 | 1.7 | 0.0 | 3.4 | 0.0 |
| 4 | 94.6 | 91.1 | 3.6 | 5.4 | 0.0 | 0.0 | 0.0 | 7.1 | 89.3 | 75.0 | 10.7 | 12.5 | 0.0 | 0.0 | 0.0 |
| 5 | 96.4 | 98.2 | 3.6 | 1.8 | 0.0 | 0.0 | 0.0 | 3.6 | 91.1 | 73.2 | 3.6 | 16.1 | 0.0 | 0.0 | 0.0 |
| 6 | 94.6 | 94.6 | 5.4 | 5.4 | 0.0 | 0.0 | 0.0 | 5.4 | 85.7 | 55.4 | 7.1 | 26.8 | 0.0 | 0.0 | 0.0 |
| 7 | 76.8 | 80.4 | 8.9 | 3.6 | 0.0 | 0.0 | 0.0 | 3.6 | 80.4 | 57.1 | 8.9 | 26.8 | 0.0 | 0.0 | 0.0 |
| 8 | 71.4 | 64.3 | 3.6 | 1.8 | 1.8 | 0.0 | 0.0 | 3.6 | 67.9 | 50.0 | 12.5 | 32.1 | 0.0 | 0.0 | 0.0 |
| 9 | 58.9 | 60.7 | 1.8 | 3.6 | 1.8 | 0.0 | 0.0 | 5.4 | 64.3 | 46.4 | 14.3 | 30.4 | 1.8 | 7.1 | 0.0 |
| 10 | 64.3 | 60.7 | 1.8 | 1.8 | 3.6 | 1.8 | 0.0 | 5.4 | 48.2 | 39.3 | 35.7 | 37.5 | 7.1 | 8.9 | 0.0 |
| 11 | 26.8 | 32.1 | 1.8 | 3.6 | 5.4 | 1.8 | 0.0 | 5.4 | 48.2 | 44.6 | 48.2 | 39.3 | 17.9 | 17.9 | 0.0 |
| 12 | 35.7 | 35.7 | 1.8 | 1.8 | 12.5 | 3.6 | 0.0 | 1.8 | 62.5 | 58.9 | 37.5 | 37.5 | 53.6 | 44.6 | 0.0 |
| 13 | 10.7 | 10.7 | 1.8 | 1.8 | 7.1 | 3.6 | 0.0 | 5.4 | 91.1 | 75.0 | 8.9 | 14.3 | 100.0 | 100.0 | 0.0 |
| 14 | 8.9 | 0.0 | 3.6 | 3.6 | 7.1 | 3.6 | 3.6 | 8.9 | 87.5 | 66.1 | 7.1 | 17.9 | 100.0 | 100.0 | 0.0 |
| 19 | 7.1 | 7.1 | 0.0 | 0.0 | 12.5 | 7.1 | 1.8 | 1.8 | 96.4 | 83.9 | 1.8 | 8.9 | 87.5 | 98.2 | 1.8 |
| 22 | 25.5 | 27.3 | 0.0 | 0.0 | 7.3 | 9.1 | 0.0 | 1.8 | 96.4 | 87.3 | 3.6 | 7.3 | 78.2 | 94.5 | 25.5 |
| 26 | 32.7 | 36.4 | 0.0 | 0.0 | 7.3 | 9.1 | 3.6 | 7.3 | 90.9 | 63.6 | 3.6 | 20.0 | 61.8 | 70.9 | 36.4 |
| 41 | 30.8 | 23.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 3.8 | 96.2 | 96.2 | 3.8 | 0.0 | 96.2 | 100.0 | 0.0 |
| Day of Life | Tail Base (°C) | Tail Tip (°C) | Delta T (°C) | Ears (°C) | Teats (°C) |
|---|---|---|---|---|---|
| 1 | 35.7 ± 0.9 | 31.5 ± 2.4 | 4.2 | 31.5 ± 1.8 | 36.7 ± 1.2 |
| 2 | 35.8 ± 1.4 | 31 ± 2.6 | 4.9 | 31.6 ± 1.8 | 37.0 ± 1.2 |
| 3 | 36.3 ± 1.4 | 30.5 ± 3.1 | 5.8 | 32.6 ± 1.8 | 37.6 ± 0.6 |
| 4 | 36.6 ± 0.8 | 30.6 ± 2.3 | 5.9 | 33.0 ± 1.9 | 37.5 ± 0.6 |
| 5 | 36.5 ± 0.6 | 32.1 ± 2.3 | 4.4 | 33.0 ± 2.0 | 37.3 ± 0.6 |
| 6 | 36.3 ± 0.8 | 32.1 ± 2.3 | 4.3 | 32.2 ± 2.2 | 37.1 ± 0.6 |
| 7 | 36.3 ± 0.8 | 33.7 ± 2.6 | 2.7 | 32.3 ± 2.8 | 36.8 ± 0.5 |
| 8 | 35.5 ± 1.3 | 32.4 ± 3.4 | 3.1 | 30.3 ± 3.9 | 36.6 ± 0.8 |
| 9 | 35.0 ± 1.0 | 32.1 ± 2.7 | 3.0 | 28.7 ± 2.9 | 36.1 ± 0.7 |
| 10 | 35.2 ± 1.0 | 32.8 ± 2.9 | 2.3 | 28.2 ± 3.4 | 36.0 ± 0.8 |
| 11 | 34.6 ± 1.3 | 30.8 ± 3.9 | 3.8 | 27.9 ± 3.4 | 35.8 ± 0.7 |
| 12 | 34.8 ± 1.2 | 31.0 ± 3.7 | 3.8 | 27.9 ± 3.6 | 35.8 ± 0.8 |
| 13 | 34.7 ± 1.0 | 30.6 ± 3.2 | 4.1 | 26.7 ± 2.0 | 35.7 ± 0.7 |
| 14 | 34.3 ± 1.1 | 28.8 ± 3.2 | 5.5 | 26.9 ± 2.0 | 35.5 ± 0.6 |
| 19 | 34.7 ± 1.2 | 28.2 ± 3.2 | 6.5 | 27.7 ± 3.0 | 35.6 ± 0.6 |
| 22 | 34.1 ± 1.3 | 27.3 ± 2.4 | 6.8 | 26.9 ± 2.5 | 34.9 ± 0.7 |
| 26 | 34.6 ± 0.8 | 27.0 ± 2.7 | 7.6 | 27.0 ± 2.4 | 34.6 ± 0.9 |
| 41 | 35.1 ± 1.0 | 28.7 ± 2.4 | 6.4 | 29.9 ± 3.1 | 35.3 ± 0.7 |
| Day of Life | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 19 | 22 | 26 | 41 | |
| 1 | 100.0 | |||||||||||||||||
| 2 | 10.7 | 100.0 | ||||||||||||||||
| 3 | 16.9 | 23.4 | 100.0 | |||||||||||||||
| 4 | 12.3 | 29.1 | 39.6 | 100.0 | ||||||||||||||
| 5 | 7.9 | 5.0 | 24.0 | 44.3 | 100.0 | |||||||||||||
| 6 | 0.9 | 3.0 | 1.1 | 10.8 | 12.4 | 100.0 | ||||||||||||
| 7 | 2.1 | 2.2 | 1.2 | 8.3 | 9.3 | 28.8 | 100.0 | |||||||||||
| 8 | 4.1 | 3.6 | 4.9 | 3.3 | 2.6 | 14.9 | 62.2 | 100.0 | ||||||||||
| 9 | 5.5 | 2.3 | 9.6 | 0.3 | 0.1 | 12.5 | 43.5 | 54.0 | 100.0 | |||||||||
| 10 | 10.0 | 0.5 | 9.2 | 0.4 | 0.9 | 15.5 | 46.1 | 57.5 | 54.7 | 100.0 | ||||||||
| 11 | 8.6 | 3.3 | 4.0 | 1.8 | 1.6 | 16.0 | 34.0 | 43.9 | 39.1 | 60.2 | 100.0 | |||||||
| 12 | 7.3 | 1.5 | 6.2 | 2.4 | 3.0 | 10.6 | 45.5 | 48.3 | 45.5 | 58.4 | 47.4 | 100.0 | ||||||
| 13 | 0.0 | 0.2 | 15.5 | 0.5 | 0.9 | 0.3 | 7.1 | 15.5 | 13.1 | 23.6 | 16.9 | 34.1 | 100.0 | |||||
| 14 | 1.5 | 3.8 | 2.0 | 0.1 | 1.9 | 6.0 | 19.9 | 21.2 | 13.5 | 29.5 | 21.9 | 26.5 | 17.2 | 100.0 | ||||
| 19 | 8.3 | 12.8 | 6.2 | 7.4 | 5.1 | 15.0 | 17.2 | 13.0 | 9.7 | 14.2 | 10.6 | 12.0 | 13.8 | 14.3 | 100.0 | |||
| 22 | 9.7 | 0.1 | 10.2 | 0.6 | 0.1 | 3.8 | 17.7 | 29.2 | 26.9 | 34.1 | 18.5 | 32.8 | 26.9 | 6.2 | 5.8 | 100.0 | ||
| 26 | 2.9 | 1.4 | 6.8 | 0.7 | 1.3 | 5.7 | 20.1 | 22.9 | 38.3 | 43.7 | 31.4 | 48.7 | 38.6 | 22.1 | 11.3 | 23.6 | 100.0 | |
| 41 | 0.0 | 5.1 | 12.9 | 0.7 | 2.3 | 0.0 | 2.2 | 12.0 | 10.1 | 1.7 | 0.4 | 0.0 | 0.0 | 0.0 | 18.4 | 3.9 | 14.6 | 100.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becker, S.; Hindenlang, K.; Kuehling, J.; Lechner, M.; Reiner, G. Inflammation and Necrosis Syndrome in Young Piglets—A Longitudinal Study. Vet. Sci. 2025, 12, 752. https://doi.org/10.3390/vetsci12080752
Becker S, Hindenlang K, Kuehling J, Lechner M, Reiner G. Inflammation and Necrosis Syndrome in Young Piglets—A Longitudinal Study. Veterinary Sciences. 2025; 12(8):752. https://doi.org/10.3390/vetsci12080752
Chicago/Turabian StyleBecker, Sabrina, Katrin Hindenlang, Josef Kuehling, Mirjam Lechner, and Gerald Reiner. 2025. "Inflammation and Necrosis Syndrome in Young Piglets—A Longitudinal Study" Veterinary Sciences 12, no. 8: 752. https://doi.org/10.3390/vetsci12080752
APA StyleBecker, S., Hindenlang, K., Kuehling, J., Lechner, M., & Reiner, G. (2025). Inflammation and Necrosis Syndrome in Young Piglets—A Longitudinal Study. Veterinary Sciences, 12(8), 752. https://doi.org/10.3390/vetsci12080752






