1. Introduction
Alimentary lymphoma (AL) is well documented in cats, but studies on dogs remain scarce. AL is one of the most prevalent forms of extranodal lymphoma in dogs, accounting for 5 to 7% of all canine lymphoma cases [
1]. According to previous studies, most canine intestinal lymphomas are of a T-cell origin [
2,
3,
4], with high-grade AL the most frequently described, and only a few cases of low-grade AL [
2]. The majority of studies describe histological and immunohistochemical aspects [
3,
5,
6,
7,
8], but pay less attention to clinical features, endoscopic findings, and prognosis of dogs with AL that do not present a well-defined mass.
In human medicine, gastroenterologists aim to standardize the description of endoscopic lesions observed in gastrointestinal lymphomas by using endoscopic terminology that has been consistently reported across multiple publications [
9,
10]. Although no endoscopic lesion is pathognomonic of the AL type, some lesions are known to be significantly associated with aggressive non-Hodgkin’s lymphoma [
10]. In veterinary medicine, no such criteria exist yet.
Endoscopic biopsies are a suitable sampling method for diagnosing AL, particularly because approximately 75% of these lymphomas exhibit epitheliotropism [
2]. Endoscopic gastrointestinal biopsies offer several clinical advantages, including minimal invasiveness, shorter anesthesia time, and a lower risk of intestinal dehiscence—factors particularly beneficial in dogs with poor general condition. The ability to obtain multiple samples from different gastrointestinal segments also increases diagnostic sensitivity. However, this technique has inherent limitations: focal lesions or abnormalities located beyond the reach of the endoscope may be missed, and because endoscopic biopsies are restricted to the mucosal layer, deeper submucosal or transmural lesions may not be adequately assessed [
11].
Immunohistochemistry (IHC) and molecular clonality testing are valuable ancillary tools that can assist pathologists in diagnosing AL, particularly in cases where lymphoid morphology is ambiguous, such as with small-cell infiltrates. IHC provides phenotypic information by identifying the expression of specific lymphocyte markers (e.g., CD3 for T-cells, CD79a for B-cells), helping to characterize the lineage and distribution of infiltrating cells within the digestive system [
2]. Clonality testing of lymphocyte populations detects gene rearrangements in T-cell receptor or immunoglobulin genes. A clonal population is suggestive of lymphoma, whereas a polyclonal pattern typically supports a diagnosis of non-neoplastic inflammatory enteropathy [
12]. While these techniques are complementary and can improve diagnostic confidence, they are not interchangeable, as each has distinct strengths and limitations. Their combined use is particularly helpful in challenging cases where the histopathological findings are inconclusive.
The objective of this study was to characterize the clinical, biological, and endoscopic features of dogs with a diffuse form of AL, i.e., without an intestinal mass detectable upon abdominal ultrasound, at a veterinary referral center. We hypothesized that a diffuse form of AL is more prevalent in older dogs, presents with abnormal plasma albumin and serum cobalamin concentrations, has a poor prognosis without a specific treatment, and may present with recurrent endoscopic lesions.
3. Results
3.1. Signalment and Clinical Features (Table 1)
Thirty-nine dogs were recruited based on histological reports of alimentary lymphoma, including thirty dogs with a diffuse form of AL, eight dogs with a jejunal mass, and one dog with an esophageal mass. All nine dogs with a well-defined mass were excluded. Twelve dogs were further excluded due to a lack of complete histological and immunohistochemical analysis (n = 8) or because of incomplete endoscopic reports (n = 4). Eighteen dogs met the inclusion criteria.
The mean age was 7.81 years (n = 18; SD, 1.99; and range, 4–11). Seven dogs were intact males (39%), seven were spayed females (39%), three were neutered males (17%), and one was an intact female (5%). The mean body weight was 19.3 kg (n = 18; SD, 10.3; and range, 4.2–35.6). The represented breeds were Pugs (n = 3), French bulldogs (n = 2), and a minority of other pure breeds (one dog per breed, n = 13). All of the dogs were presented for the investigation of chronic gastrointestinal signs. Fifteen dogs had weight loss (83%), fourteen dogs had diarrhea (78%), twelve dogs had vomiting (67%), and five dogs showed signs of gastrointestinal bleeding (28%). The CCECAI scores were assessed in 17 dogs and were insignificant in none of the dogs, mild in 1 dog (6%), moderate in 4 dogs (24%), severe in 7 dogs (41%), and very severe in 5 dogs (29%). The mean CCECAI score was 9.65 (n = 17; SD, 2.42; and range, 5–13).
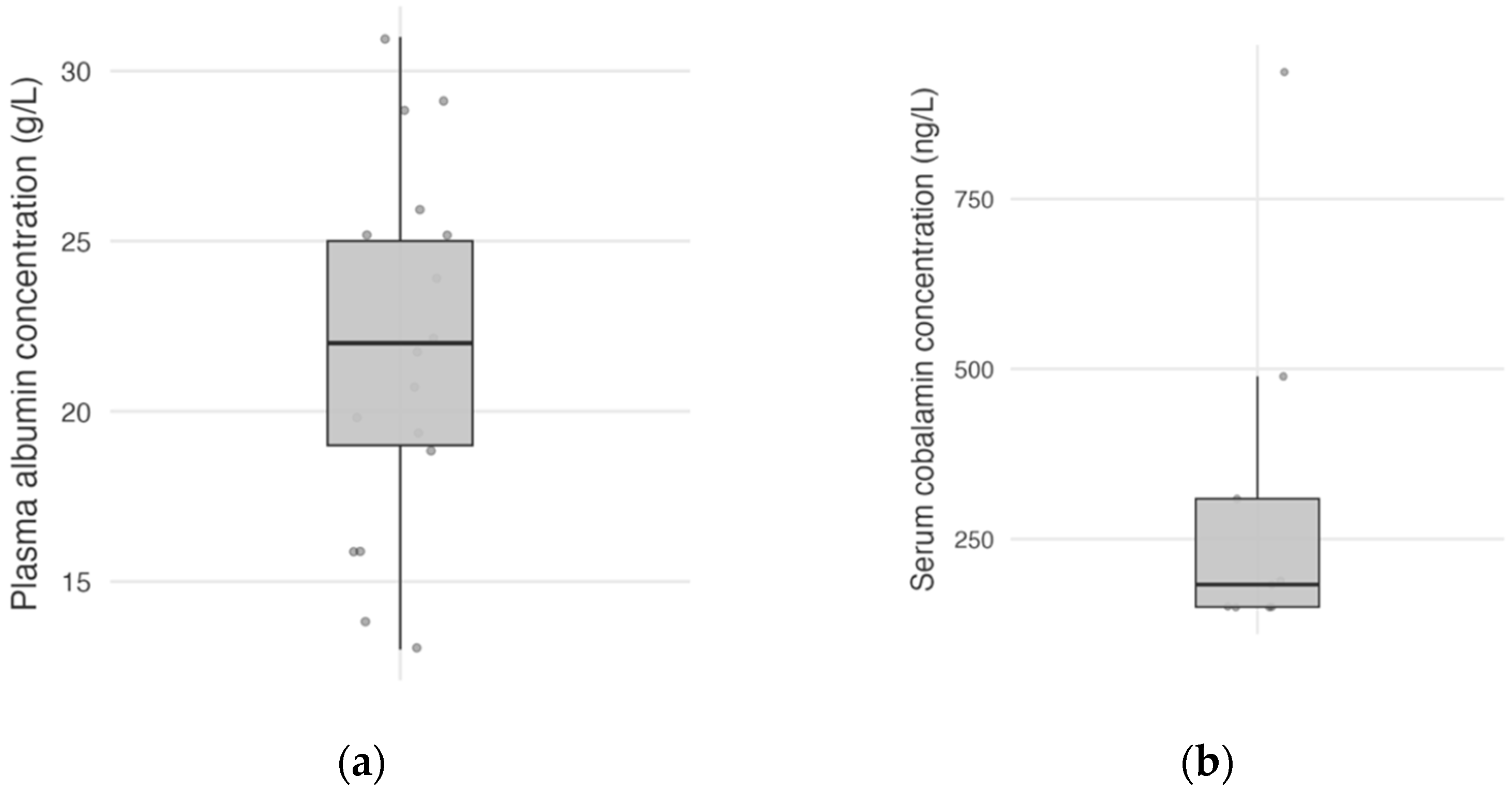
Plasma albumin concentration was available for 17 dogs (94%), with a mean plasma albumin concentration at the time of diagnosis of 21.8 g/L (
n = 17; SD, 5.34; and range, 13–31,
Figure 1a). Plasma albumin concentration was normal in 11 dogs (65%), mildly decreased in 4 dogs (23%), and moderately decreased in 2 dogs (12%), and none of the dogs had severe hypoalbuminemia. Serum cobalamin concentration was available in nine dogs (50%), with a median serum cobalamin concentration of 183 ng/L (
n = 9; IQR, 150–309; and range, <150–937;
Figure 1b).
Figure 1.
Distribution of plasma albumin concentrations in 17 dogs (a), and serum cobalamin concentrations in 9 dogs (b). Each point represents a dog.
Figure 1.
Distribution of plasma albumin concentrations in 17 dogs (a), and serum cobalamin concentrations in 9 dogs (b). Each point represents a dog.
Table 1.
Signalment and clinical data of 18 dogs with a diffuse form of alimentary lymphoma. Abbreviations: NM, neutered male; IM, intact male; SF, spayed female; IF, intact female; CCECAI, Canine Chronic Enteropathy Clinical Activity Index; and NA, not available.
Table 1.
Signalment and clinical data of 18 dogs with a diffuse form of alimentary lymphoma. Abbreviations: NM, neutered male; IM, intact male; SF, spayed female; IF, intact female; CCECAI, Canine Chronic Enteropathy Clinical Activity Index; and NA, not available.
| Case Number | Gender | Age (Years) | Lymphoma Location | CCECAI Score | Survival Time (Days) |
|---|
| 1 | NM | 9 | Stomach and duodenum | 5 | 297 |
| 2 | SF | 11 | Duodenum and ileum | 9 | 8 |
| 3 | SF | 8 | Stomach | 8 | 279 |
| 4 | SF | 8 | Duodenum, ileum, and colon | 11 | 24 |
| 5 | SF | 6 | Duodenum | 10 | 58 |
| 6 | SF | 6 | Duodenum and ileum | 12 | 9 |
| 7 | IM | 8 | Duodenum | 10 | 82 |
| 8 | SF | 10 | Duodenum | 12 | 9 |
| 9 | NM | 4.5 | Duodenum | 13 | 5 |
| 10 | SF | 9 | Duodenum | 6 | 350 |
| 11 | IM | 4 | Duodenum | 9 | 12 |
| 12 | IM | 10 | Duodenum | 10 | 4 |
| 13 | IM | 9 | Duodenum | 13 | Still alive |
| 14 | IM | 7 | Ileum | 7 | 55 |
| 15 | IM | 9 | Ileum and colon | 7 | 29 |
| 16 | IM | 5 | Duodenum | 10 | 32 |
| 17 | IF | 9 | Stomach and duodenum | 12 | Still alive |
| 18 | NM | 8 | Duodenum | NA | 92 |
3.2. Diagnostic Imaging Findings
The main ultrasonographic findings included paralytic ileus with corrugated intestinal loops (72%), and heterogeneous and hyperechoic intestinal walls (56%). Intestinal wall thickening was identified in the stomachs of five dogs (28%), in the duodenums of eight dogs (44%), in the jejunums of five dogs (28%), in the ileums of five dogs (28%), and in the colons of four dogs (22%). In three cases (17%), a segmental loss of normal wall layering was observed without a clearly defined mass. Regional mesenteric lymphadenopathy was present in eight dogs (44%; range: 5.2–22.5 mm).
3.3. Endoscopic Findings
Ten dogs (10/18, 56%) had a combination of gastroduodenoscopy and ileocolonoscopy, seven dogs (39%) had gastroduodenoscopy alone, and one dog (5%) had ileocolonoscopy alone. Concordance between the three internist clinicians upon reviewing the endoscopic images was considered moderate on the basis of Kendall W correlation and was similar between the three specialists (Kendall’s tau-b ranging from 0.534 to 0.582). The highest agreement was observed between clinicians 2 and 3, with a tau-b of 0.582 (
p < 0.001). The results of the correlations are shown in
Table 2.
All of the endoscopic findings are summarized in
Table 3.
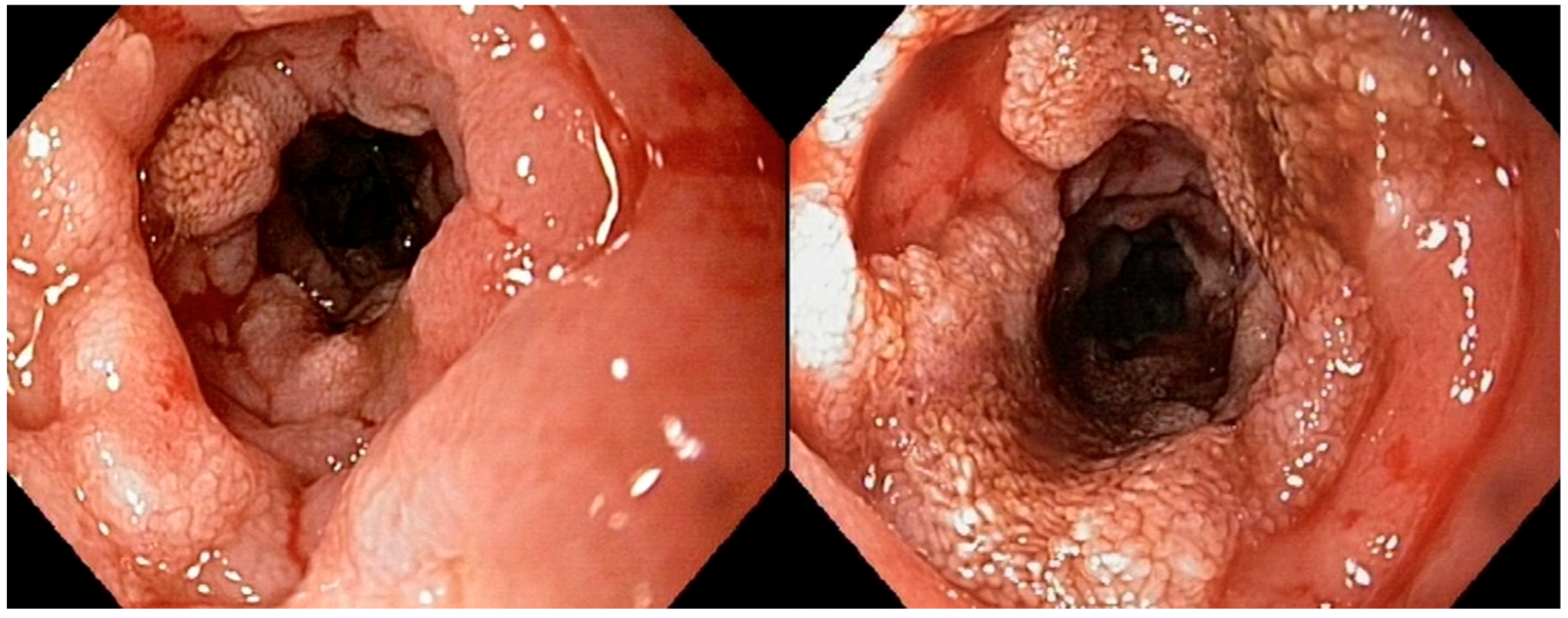
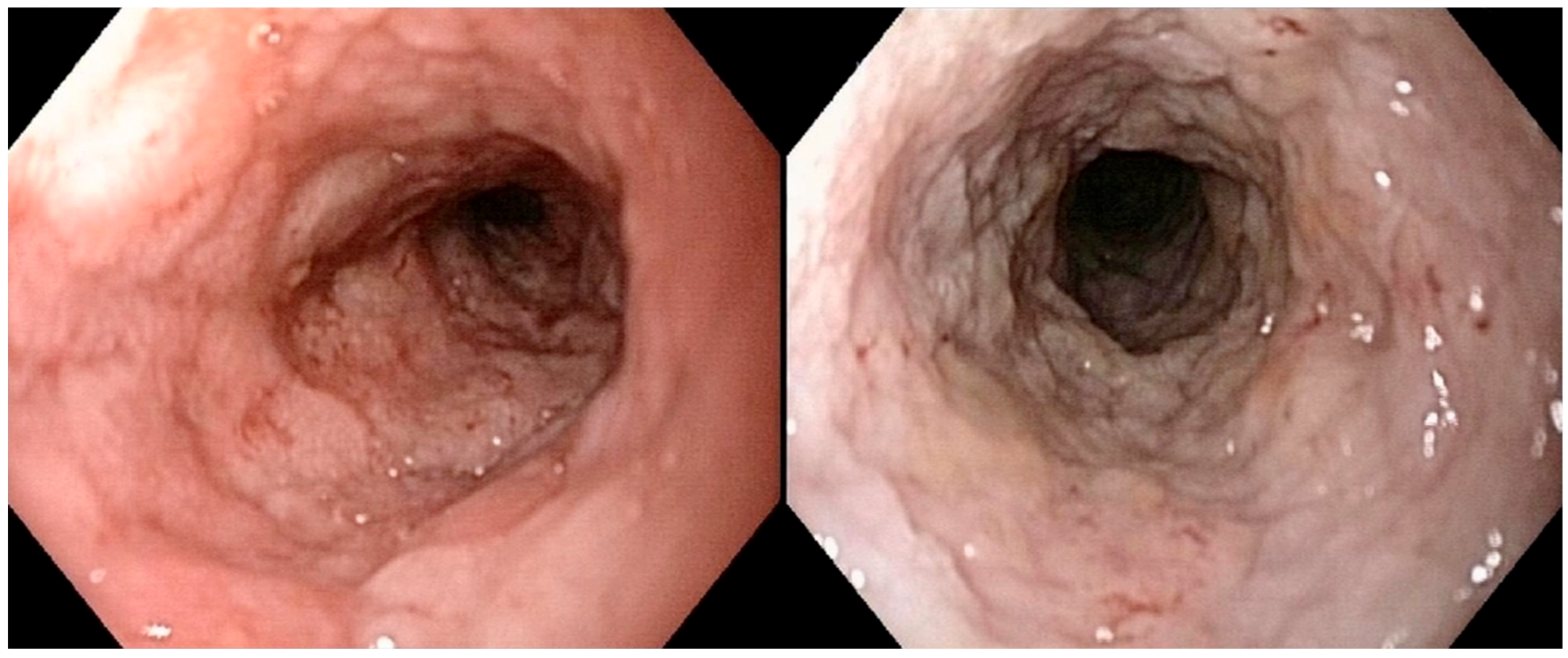
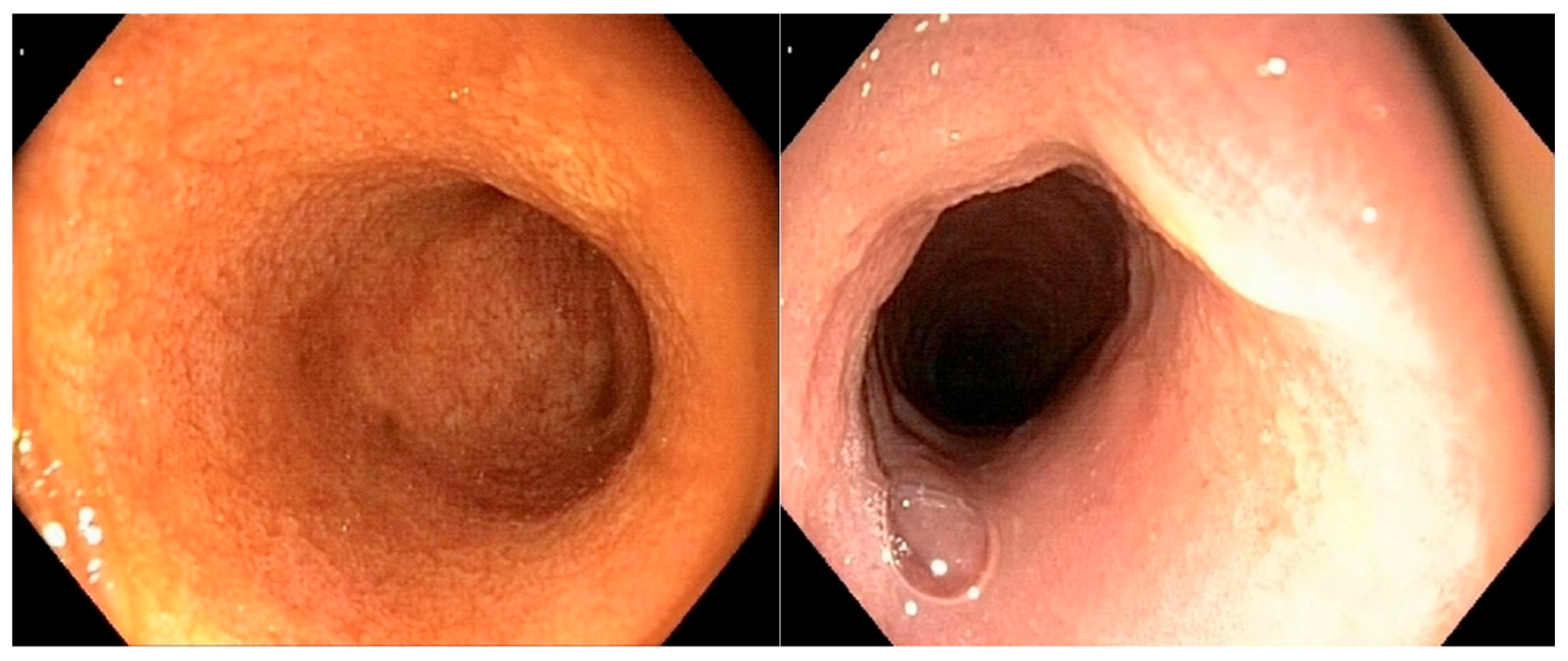
Figure 2 illustrates a severe alteration in the granularity of the duodenal mucosa during duodenoscopy. Duodenal lacteal dilatation was observed in one dog (6%). A “cobblestone” appearance of the duodenal mucosa was identified in nine dogs (53%) (
Figure 3). No dog developed colonic stricture or intussusception.
3.4. Histological Findings
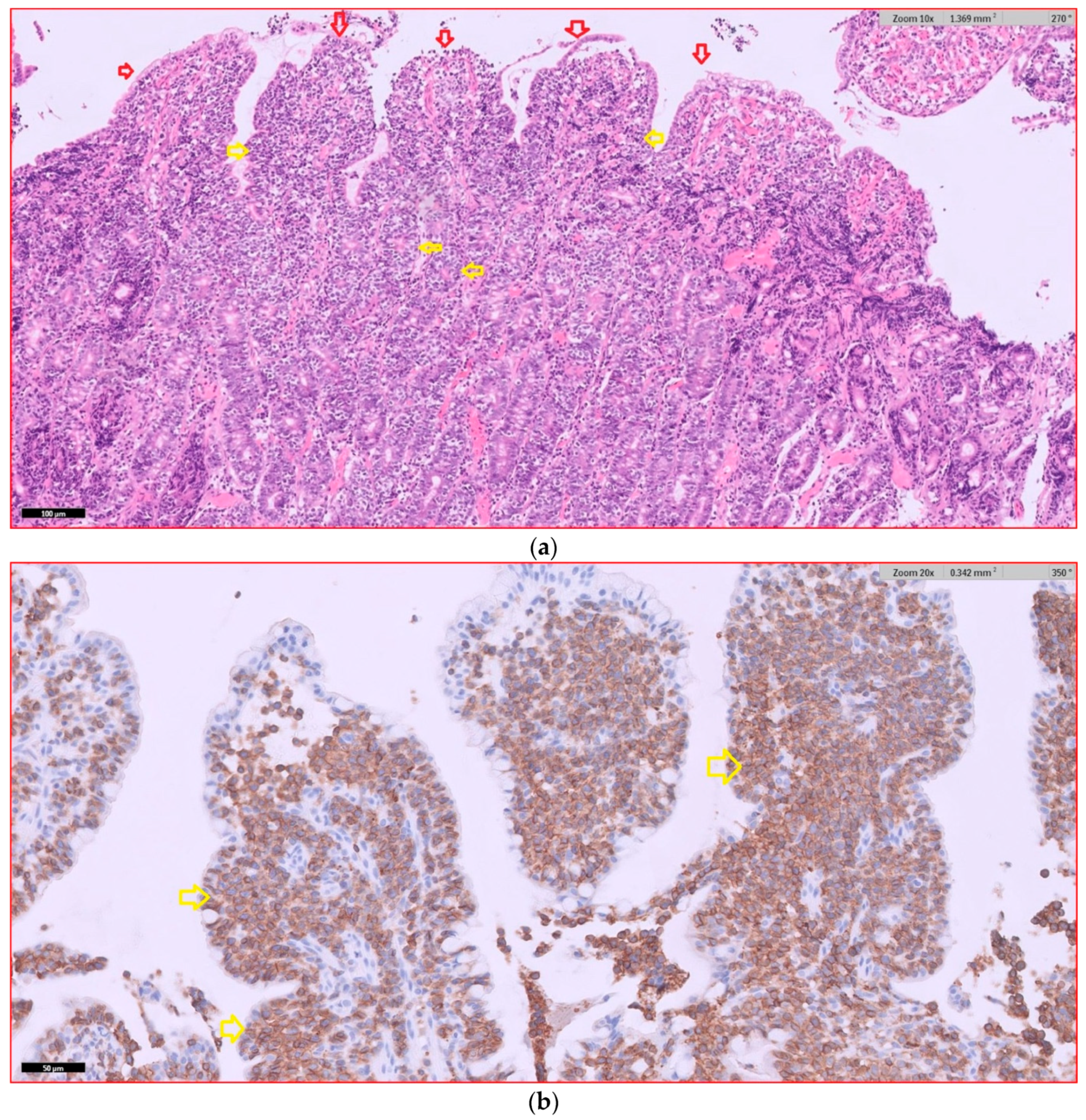
In dogs with diffuse AL that underwent endoscopic examination, 17 dogs had intermediate- to large-cell lymphoma (94%), and 1 dog (6%) had small- to intermediate-cell lymphoma. The duodenum was the part of the gastrointestinal tract most frequently affected by lymphoma. Infiltration by lymphoma was observed in the duodenums of 15 dogs, in the ileums of 5 dogs, in the stomachs of 3 dogs, and in the colons of 2 dogs. Six dogs had lymphomatous infiltration in multiple locations simultaneously. Dogs with duodenal lymphoma exhibited nests of neoplastic cells (80%, 12/15), villous atrophy (60%, 9/15), marked epitheliotropism (60%, 9/15), crypt distension (40%, 6/15), and lacteal dilatation (7%, 1/15). Dogs with ileal lymphoma showed nests of neoplastic cells (100%, 5/5), villous atrophy (80%, 4/5), marked epitheliotropism (80%, 4/5), and crypt distension (40%, 2/5), while no cases of lacteal dilatation were observed. Representative histopathological images of intermediate- to large-T-cell duodenal lymphoma are shown in
Figure 4.
3.5. Immunohistochemistry Findings (Table 4)
Sixteen dogs (16/18; 89%) exhibited a T-cell phenotype, while only two dogs (2/18; 11%) showed a B-cell phenotype. The Ki67 score was obtained for all dogs. The mean Ki67 score was 11.7% (
n = 18; SD, 5.23; and range, 3.6–26). The dog with small-cell AL had a Ki67 score of 14.5%.
Table 4.
Alimentary lymphoma in 18 dogs: location, cell size, immunophenotype, and Ki67 score. Abbreviations: GD, gastroduodenoscopy; IC, ileocolonoscopy; +, positive; and −, negative.
Table 4.
Alimentary lymphoma in 18 dogs: location, cell size, immunophenotype, and Ki67 score. Abbreviations: GD, gastroduodenoscopy; IC, ileocolonoscopy; +, positive; and −, negative.
| Case Number | Endoscopic
Evaluation | Lymphoma Location | Cell Sizes | CD3 | CD79a | Ki67 (%) |
|---|
| 1 | GD | Stomach and duodenum | Intermediate to large | + | − | 11.6 |
| 2 | GD and IC | Duodenum and ileum | Intermediate to large | + | − | 10.6 |
| 3 | GD | Stomach | Intermediate to large | − | + | 8 |
| 4 | GD and IC | Duodenum, ileum, and colon | Intermediate to large | + | − | 7.2 |
| 5 | GD | Duodenum | Intermediate to large | + | − | 11 |
| 6 | GD and IC | Duodenum and ileum | Intermediate to large | + | − | 17.8 |
| 7 | GD | Duodenum | Intermediate to large | + | − | 12.6 |
| 8 | GD and IC | Duodenum | Intermediate to large | + | − | 18.2 |
| 9 | GD | Duodenum | Intermediate to large | + | − | 13 |
| 10 | GD and IC | Duodenum | Intermediate to large | + | − | 5.6 |
| 11 | GD and IC | Duodenum | Intermediate to large | + | − | 3.6 |
| 12 | GD and IC | Duodenum | Intermediate to large | + | − | 14.6 |
| 13 | GD and IC | Duodenum | Intermediate to large | + | − | 26 |
| 14 | GD and IC | Ileum | Intermediate to large | + | − | 7.8 |
| 15 | IC | Ileum and colon | Intermediate to large | − | + | 12.2 |
| 16 | GD and IC | Duodenum | Intermediate to large | + | − | 6.9 |
| 17 | GD | Stomach and duodenum | Small to intermediate | + | − | 14.5 |
| 18 | GD | Duodenum | Intermediate to large | + | − | 12.2 |
3.6. Relationship Between Biological Data and Endoscopic and Histological Findings
In the only dog with gastric and duodenal small-cell lymphoma, the endoscopic lesions of the stomach were all classified as ‘moderate’ for mucosal hyperemia, edema, discoloration, and the presence of mucosal ulcerations. The duodenal mucosa of this dog showed severely altered granularity, with no ulcerations or a “cobblestone” appearance.
In dogs with intermediate- to large-cell duodenal lymphoma (
n = 14), none had a normal duodenal mucosa. The alteration in duodenal granularity was reported to be severe in six dogs (43%), moderate in five dogs (36%), and mild in three dogs (21%). Duodenal ulcerations were absent, mild, and moderate in two (14%), eight (57%), and four (29%) dogs, respectively; no dog had severe duodenal ulceration. Duodenal lacteal dilatation was present in only one dog with duodenal lymphoma (moderate- to large-cell). Three dogs (cases 1, 11, and 18) with duodenal intermediate- to large-cell lymphoma showed only mild changes (of any type) in the duodenal mucosa (
Figure 5).
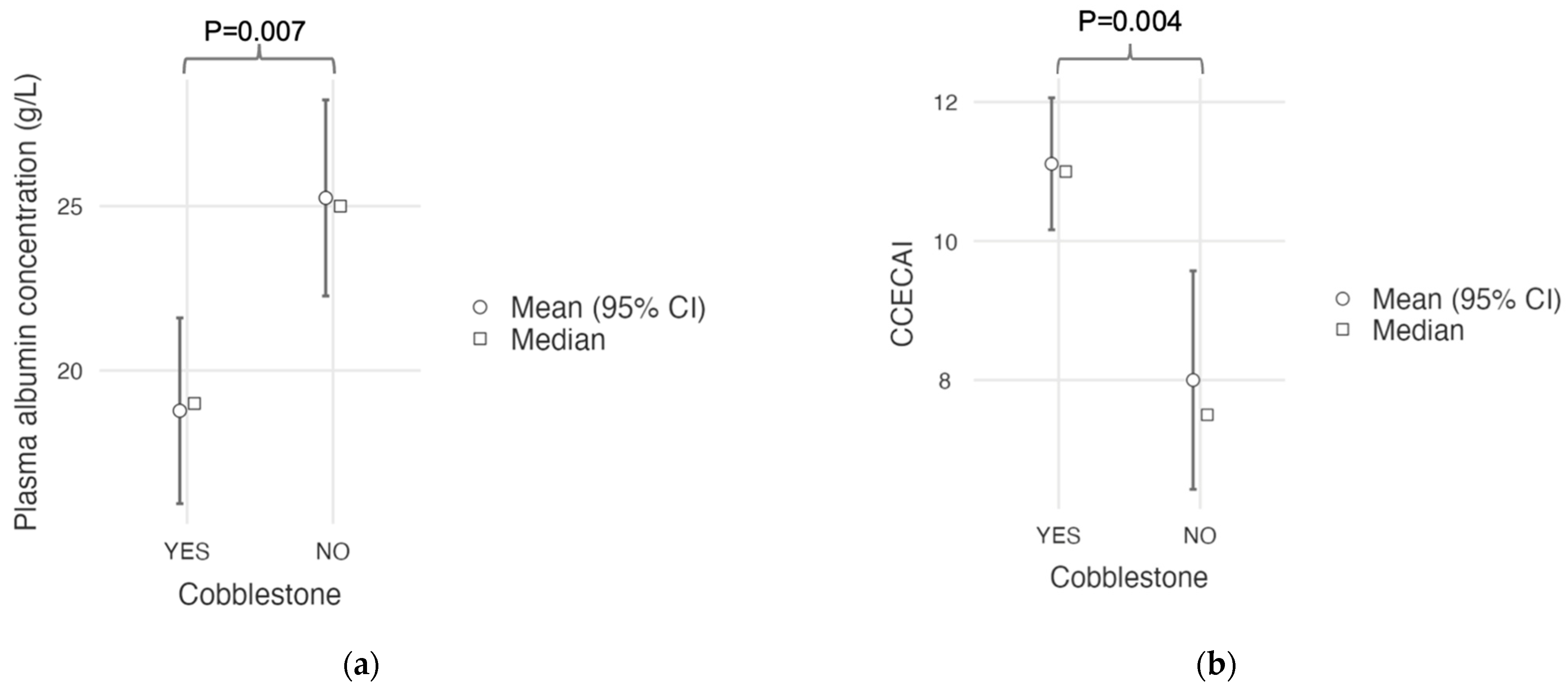
A “cobblestone” appearance of the duodenal mucosa was identified in nine dogs with duodenal lymphoma (9/15; 60%) and in neither of the two dogs without duodenal involvement who underwent gastroduodenoscopy. All nine of these dogs (100%) with a “cobblestone” appearance of the duodenal mucosa had confirmed duodenal involvement from intermediate- to large-cell lymphoma. A comparative analysis of clinical and histological quantitative parameters between dogs with and without this endoscopic feature is shown in
Table 5. Dogs with a “cobblestone” appearance of the duodenal mucosa showed a significantly lower plasma albumin concentration (mean, 18.8 g/L; SD, 4.32; and range, 13–26) compared to dogs without a “cobblestone” appearance (mean, 25.3 g/L; SD, 4.3; and range, 19–31;
p = 0.007;
Figure 6a). They also had a significantly higher CCECAI score (mean, 11.1; SD, 1.45; and range, 9–13) than dogs without a “cobblestone” appearance (mean, 8.0; SD, 2.27; and range, 5–12.
p = 0.004;
Figure 6b). Age, body weight, serum cobalamin concentration, and Ki67 score did not differ between groups (
Table 5).
3.7. Treatments and Outcomes
After endoscopic examination, all dogs received prednisolone at variable doses (0.7–1.3 mg/kg/day), and six dogs (33%) received antibiotics, primarily metronidazole (n = 3) followed by marbofloxacine (n = 2) and amoxicillin-clavulanate (n = 1). After histopathological confirmation of the diagnosis, maximal-tolerated-dose chemotherapy protocols (CHOP protocols) were proposed for dogs still alive at the time; however, none of the owners elected to pursue this treatment option. Three dogs received a combination of prednisolone (1 mg/kg/day) and chlorambucil (4 mg/m2 q24–48 h), resulting in survival times of 9 days, 58 days, and 349 days, respectively. These treatments were not part of the standardized chemotherapy protocol and were administered at the clinician’s discretion.
At the time of writing, 16 dogs (89%) have died, all because of AL. Two dogs (11%) were still alive at 170 (case 13) and 563 days (case 17) since diagnosis; these two dogs were censored for the survival analysis. The median survival time was 30.5 days (n = 16; IQR, 9–84.5; and range, 4–350). Among the dogs that finally died, eight, four, and zero dogs were still alive at 1, 3, and 12 months after diagnosis, respectively.
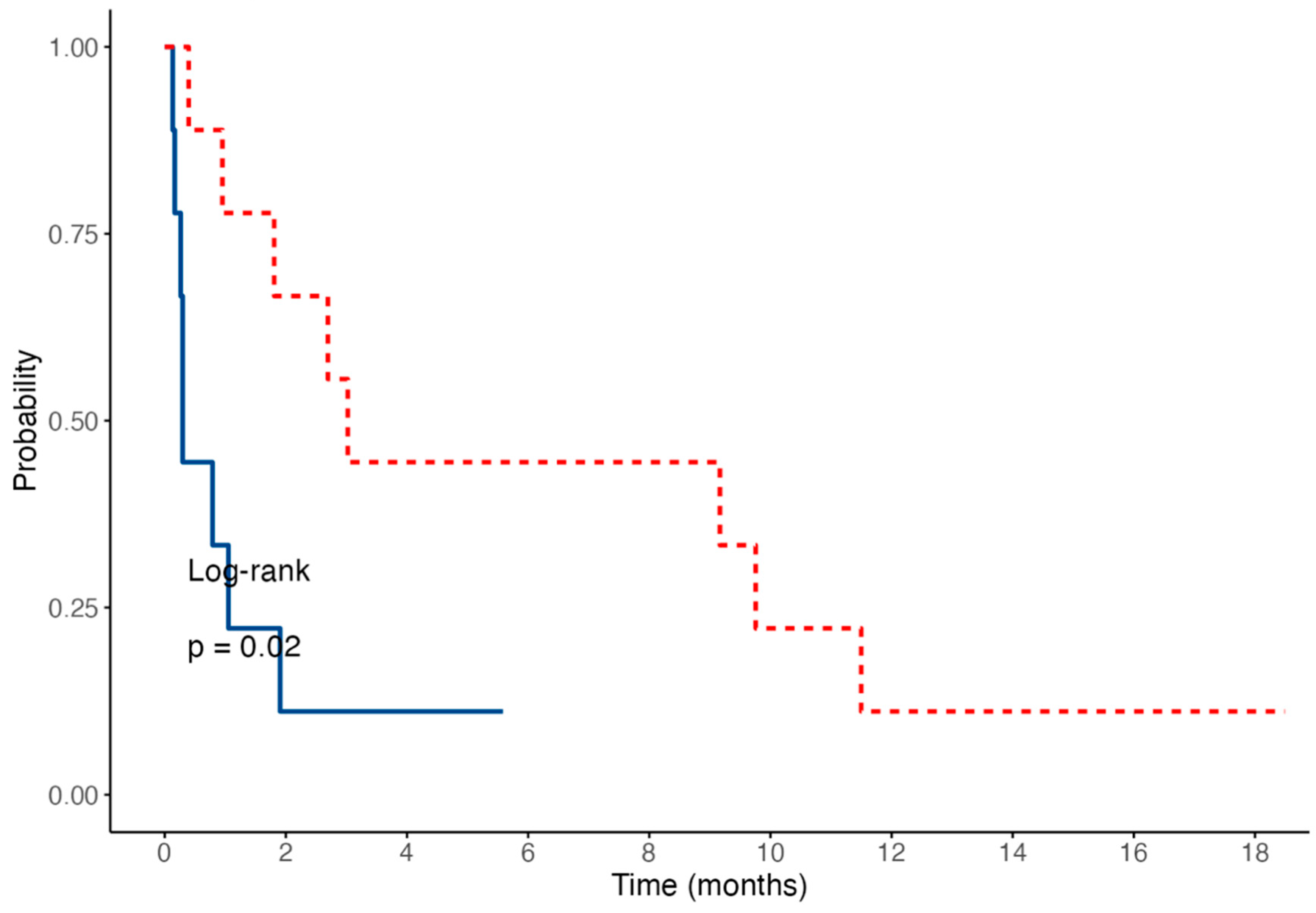
The median survival time was significantly shorter in dogs with a “cobblestone” appearance of the duodenal mucosa (median: 9 days; range, 4–58) compared to those without one (median: 92 days; range, 12–350; log-rank test:
p = 0.02;
Figure 7).
4. Discussion
Diffuse AL can be more challenging to diagnose than mass-associated forms, due to its clinical resemblance to severe chronic enteropathies. Our study aims to describe the clinical, biological, endoscopic, histological, and immunohistochemical findings in dogs with diffuse AL. In the present study, dogs diagnosed with diffuse AL were generally middle-aged or older, the youngest dog being 4 years old. An earlier study reported that males had a predisposition for developing high-grade AL, but no sex predilection was identified in our study [
21]. In a previous study of canine transmural lymphoma [
3], Pug dogs were overrepresented, accounting for 17% of T-cell AL cases. Similarly, in our study, the Pug was the most commonly affected breed (17%).
Plasma albumin concentration is a key prognostic biomarker in protein-losing enteropathies, with one study reporting that normalization within 50 days of treatment initiation was associated with a longer survival time [
22]. Surprisingly, in our study, 65% of dogs had normal plasma albumin concentrations according to the CCECAI scoring system, despite the severity of endoscopic lesions observed in most cases and the presence of duodenal or ileal infiltration in all but one dog. This discrepancy may be explained by the segmental nature of the lymphoproliferative infiltrate, which may spare large portions of the intestinal mucosa and thus have a limited impact on nutrient absorption. Alternatively, albumin levels may have been overestimated in some dogs due to dehydration secondary to gastrointestinal losses. The prognostic value of hypoalbuminemia in diffuse AL should be evaluated in future studies including larger cohorts of dogs with a severe degree of hypoalbuminemia.
In dogs with chronic enteropathies, low serum cobalamin levels (<200 ng/L) were significantly associated with poorer outcomes, despite 6 weeks of cobalamin supplementation [
15]. In our study, serum cobalamin concentrations were available for nine dogs, with a median serum cobalamin concentration of 183 ng/L (
n = 9; IQR, 150–309; and range, <150–937). Seven dogs had a serum cobalamin concentration <400 ng/L, including three dogs with concentrations <150 ng/L. Serum cobalamin concentrations < 150 ng/L could not be precisely measured by the reference laboratory due to the extremely low concentrations. Of the seven dogs with cobalamin levels lower than 400 ng/L, involvement of the ileum was reported in only two dogs. However, three out of seven dogs did not undergo ileocolonoscopy; therefore, ileal involvement may have been underestimated. Considering the high proportion of dogs with hypocobalaminemia in our study, the routine assessment of serum cobalamin in cases of diffuse AL appears warranted, even though its prognostic value was not determined in the present cohort.
In the literature, the most commonly reported ultrasonographic findings of AL in dogs include a thickened gastric or intestinal wall, a loss of layering, the presence of a mass, or ulceration [
4]. The ultrasonographic results in our study revealed that the duodenum was the most frequently affected segment of the gastrointestinal tract. This finding aligns with the histological predominance of lymphomatous infiltration observed primarily in the duodenums of affected dogs. However, the absence of duodenal wall thickening does not exclude the presence of duodenal lymphoma.
Human gastroenterologists use endoscopic features to help differentiate aggressive from non-aggressive AL. Mucosal lesions are classified according to their appearance: the superficial type, protruding type without ulceration, protruding type with ulceration, fungating type, multiple-nodule type, and giant fold type [
10]. Although no endoscopic lesion is pathognomonic for the type of AL, human gastroenterologists identify certain lesions, such as fungating lesions and protruding lesions with ulceration, as being significantly associated with aggressive non-Hodgkin’s lymphoma [
10]. Although a standardized approach to gastrointestinal endoscopic evaluation has been proposed in veterinary medicine by the WSAVA Gastrointestinal Standardization Group, no specific consensus exists for the assessment of digestive neoplasms. Consequently, the WSAVA scoring system is primarily intended to ensure a systemic evaluation of each accessible segment of the gastrointestinal tract, rather than to provide a detailed characterization of mucosal abnormalities. This contrasts with human gastroenterology, where mucosal changes are more rigorously assessed when a suspicious lesion is identified.
One of our main aims was to provide an endoscopic description of lesions observed with diffuse AL. To the authors’ knowledge, this is the second study to describe the endoscopic features of AL in dogs, and the first to specifically document endoscopic findings in cases with a diffuse form of the disease. The only previously published study included seven dogs with AL, four of which exhibited a “cobblestone” appearance of the duodenal mucosa, consistent with histologically confirmed duodenal infiltration [
23]. Our findings expand upon these preliminary observations by focusing on the diffuse form of the disease and systematically correlating endoscopic features with histopathological and immunohistochemical data. In our study, the duodenum was the most frequently affected segment of the gastrointestinal tract, with lymphomatous infiltration of the mucosa observed in 15 out of 17 dogs (83%). Altered mucosal granularity was the most commonly reported abnormality and was present in all dogs with duodenal lymphoma. Severe changes in granularity were reported in 41% (7/17) of dogs, one of which had a small- to intermediate-cell duodenal lymphoma. A “cobblestone” appearance of the duodenal mucosa was noted in 9 out of 17 dogs (53%) and was significantly associated with lower plasma albumin concentrations and higher CCECAI scores. All affected dogs were diagnosed with intermediate- to large-cell duodenal lymphoma. Therefore, a “cobblestone” pattern in the duodenum should be considered a potential indicator of disease severity. This endoscopic feature has already been reported in dogs with duodenal lymphoma, leading authors to propose that AL should be strongly suspected when a solitary mass or a cobblestone appearance is observed on endoscopic examination [
23]. However, because our study did not include dogs with chronic inflammatory enteropathies, we cannot determine whether this feature is specific to lymphoma. A comparative study including both healthy control dogs and dogs with non-neoplastic chronic enteropathies would be required to assess the diagnostic relevance of this pattern. Furthermore, due to the limited number of cases with gastric, ileal, or colonic lymphoma in our study, no firm conclusions can be drawn regarding the endoscopic appearance of these segments.
Endoscopic gastrointestinal biopsies appear to be an appropriate sampling method with which to diagnose lymphoma, since 75% of ALs are epitheliotropic [
2]. This minimally invasive technique allows samples to be taken quickly while reducing anesthetic time and decreasing the risk of intestinal dehiscence, particularly in cases in poor general condition and with hypoalbuminemia. An additional advantage of endoscopy is the ability to obtain multiple biopsies from various sites along the gastrointestinal tract, thereby increasing the diagnostic yield. In contrast, surgical biopsies obtained via laparotomy typically involve only a limited number of intestinal segments, although they allow for full-thickness samples and the assessment of all layers of the intestinal wall. Nevertheless, endoscopy has inherent limitations: focal lesions or those located beyond the reach of the endoscope may be missed, and lesions confined to the submucosa or deeper layers may not be detected through mucosal biopsies alone. These factors may contribute to the underdiagnosis of lymphomas that do not exhibit epitheliotropism when relying exclusively on endoscopic sampling [
2].
There are notable species-specific differences in the forms of AL. In humans, the most common subtype of AL is B-cell non-Hodgkin’s lymphoma, particularly diffuse large-B-cell lymphoma (DLBCL) and mucosa-associated lymphoid tissue (MALT) lymphoma [
24]. Conversely, T-cell lymphomas are rare in humans, accounting for only 4–6% of AL cases [
24]. According to our study, T-cell AL appears to be the most common form of diffuse AL in dogs, underlining a significant divergence in lymphoma subtypes between species.
Diffuse AL in our study was associated with a poor prognosis, resulting in a median survival time of 30.5 days (
n = 16; IQR, 9–84.5; and range, 4–350). The presence of a duodenal mucosa with a “cobblestone” appearance appeared to be associated with a worse outcome: the median survival time was significantly shorter in dogs with this endoscopic feature (median: 9 days; range, 4–58) compared to those without (median: 92 days; range, 12–350;
p = 0.02). The results of our study highlight the aggressive nature of diffuse AL, particularly in the absence of an appropriate chemotherapeutic protocol. These findings are consistent with previous reports of similar outcomes, particularly in cases of intestinal large-cell T lymphoma, for which a median survival time of 62 days has been reported [
5]. Slightly better outcomes were obtained in some studies, particularly when dogs received a multi-drug chemotherapy protocol [
4,
25]. In our study, none of the dogs received a maximum-tolerated-dose chemotherapy protocol. This may be attributed to the rapid deterioration of the clinical condition of many dogs before their histopathological results became available. Additionally, the cost of a chemotherapy protocol combined with the guarded prognosis may have discouraged owners from pursuing treatment. Further studies are required to evaluate the prognosis of dogs with diffuse AL undergoing polychemotherapy.
The majority of previous studies describing AL focused on immunohistochemical and molecular findings [
3,
6,
19,
20,
26]. Canine intestinal lymphoma is morphologically classified into small-cell, intermediate-cell, or large-cell lymphoma according to its nuclear size [
3]. Based on our histological results, most of our dogs (94%) had intermediate- to large-cell AL. IHC carried out on all dogs confirmed the predominance of the T-cell phenotype (89%) associated with canine AL, in accordance with the literature [
3,
5,
6,
27,
28]. In the B-cell phenotype, one dog had a large-B-cell gastric lymphoma and the other one had a large-B-cell ileo-colic lymphoma. Based on these results, it is not possible to determine whether the phenotype could have an impact on the expected survival of these dogs. The two dogs with B-cell AL survived for 279 days and 29 days, but we did not identify any potential factors that might have influenced these widely divergent results. The Ki67 score is a marker of cellular proliferation used in both canine and human lymphoma to correlate cellular proliferation with prognosis [
19]. Although some studies have specifically focused on the use of the Ki67 score to differentiate canine inflammatory bowel disease (IBD) from intestinal lymphoma, it appears that the Ki67 score cannot reliably distinguish between IBD and lymphoma [
19]. In the aforementioned study, lymphomas exhibited significantly higher Ki67 scores (30–62%) compared to inflammatory enteropathy cases, where most showed indices below 25% [
19]. Surprisingly, the mean Ki67 score in our study was much lower; however, the histopathological findings of our dogs with intermediate- to large-cell lymphoma did not suggest that the final diagnosis was questionable. This further emphasizes that the Ki67 score should not be used as the sole tool for diagnosing and prognosticating AL.
The limitations of this study are mainly due to its retrospective nature, including the absence of biochemical analysis results in all cases, particularly serum cobalamin and plasma albumin concentrations, and inconsistent chemotherapeutic protocols. The relatively small number of included cases is also a limitation, as it may have reduced the statistical power of the study. This limited sample size is mainly attributable to the stringent inclusion criteria, which required histopathological and immunohistochemical confirmation and therefore excluded cases diagnosed by cytological analysis alone. This study does not allow us to report the overall prevalence of dogs with AL in a referral clinic, as many cases of AL were diagnosed by fine-needle aspiration during ultrasound examination of the abdomen. This selection bias excluded all dogs with intestinal masses for which surgical management was recommended in the event of an intestinal obstruction. The absence of a control group including both healthy dogs and dogs with non-neoplastic chronic enteropathies prevents any assessment of whether the reported endoscopic lesions are specific to AL. Moreover, the concordance between the descriptions of gastrointestinal lesions by the three board-certified internists was only moderate, based on the Kendall W correlation. This highlights the relative subjectivity of endoscopic observations despite well-defined criteria, and the need to perform systematic multiple biopsies, even if the lesions appear to be of minor significance to the operator, to optimize the chances of obtaining a reliable diagnosis. As a result, the sensitivity and specificity of these endoscopic findings for the diagnosis of AL could not be determined. Further prospective studies with well-defined control groups are needed to clarify the diagnostic value of such endoscopic features. Additionally, clonality assessment via PARR (polymerase chain reaction for antigen receptor rearrangements) was not routinely performed in this study, especially for cases of small- to intermediate-cell lymphoma. This decision was mainly influenced by logistical constraints, including cost and accessibility. Finally, the relatively small cohort restricts the statistical power of our results.