Evaluating a Targeted Antimicrobial Stewardship Program and Its Temporal Association with Resistance Trends in a Veterinary Referral Hospital †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
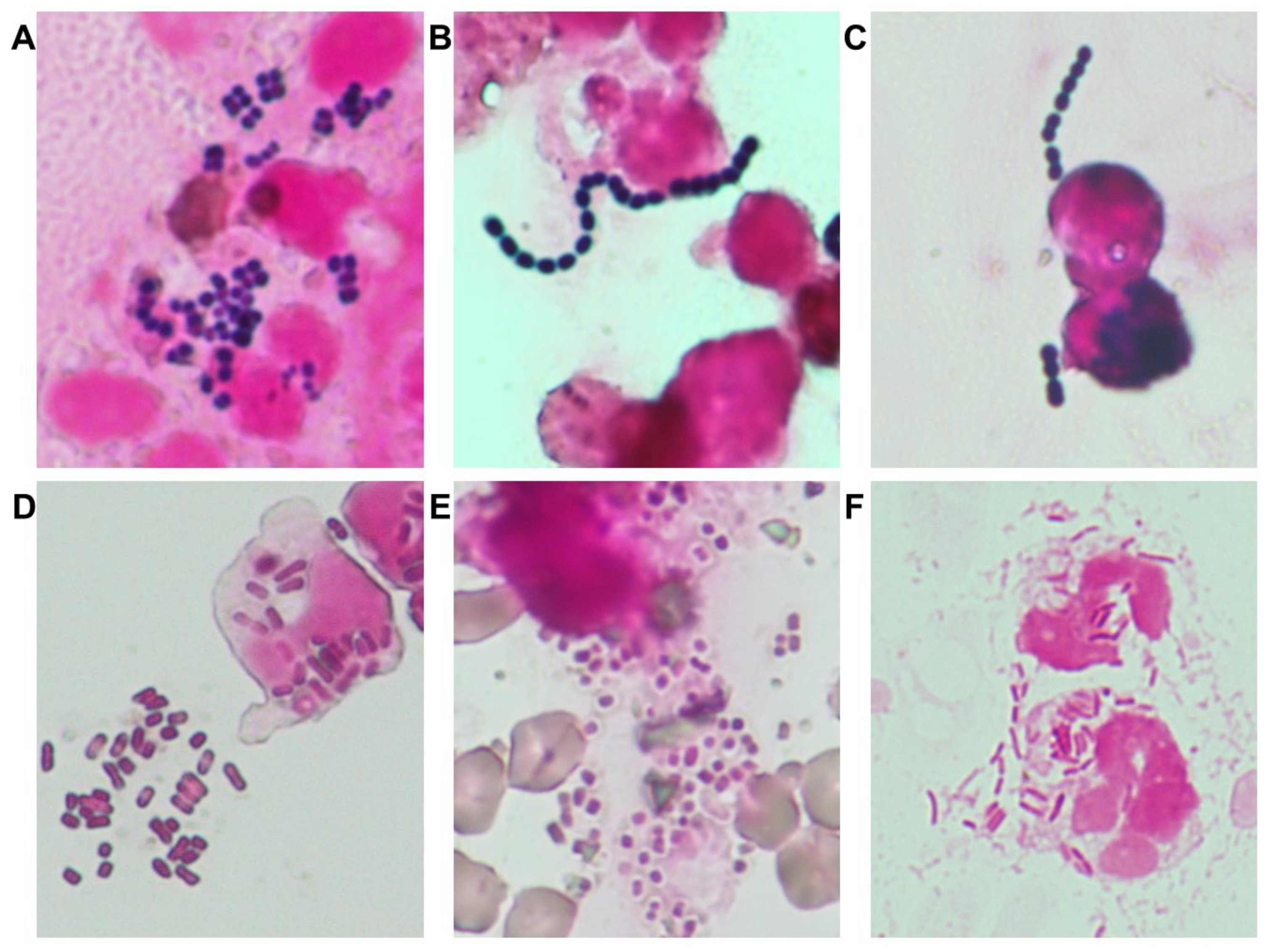
2.1. Analysis of Antimicrobial Use and Resistance for Identification of Critical Control Points
- − β-lactams: ampicillin (ABPC), amoxicillin (AMPC), amoxicillin-clavulanic acid (AMPC-CVA), cefazolin (CEZ), cefalexin (CEX), faropenem (FRPM), imipenem/cilastatin (IPM/CS)
- − Fluoroquinolones: enrofloxacin (ERFX), orbifloxacin (OBFX)
- − Aminoglycosides: amikacin (AMK)
- − Macrolides: clindamycin (CLDM)
- − Tetracyclines: doxycycline (DOXY), minocycline (MINO)
- − Others: trimethoprim-sulfamethoxazole (ST), chloramphenicol (CP), and fosfomycin (FOM)
2.2. Intervention Strategy
- Proactive use of Gram staining:
- 2.
- Empirical therapy optimization:
- 3.
- Restriction of high-risk antimicrobials:
- 4.
- Refinement of AST result reporting:
2.3. Statistical Analysis
3. Results
3.1. Bacterial Epidemiology, Antimicrobial Resistance, and Identification of Critical Control Points
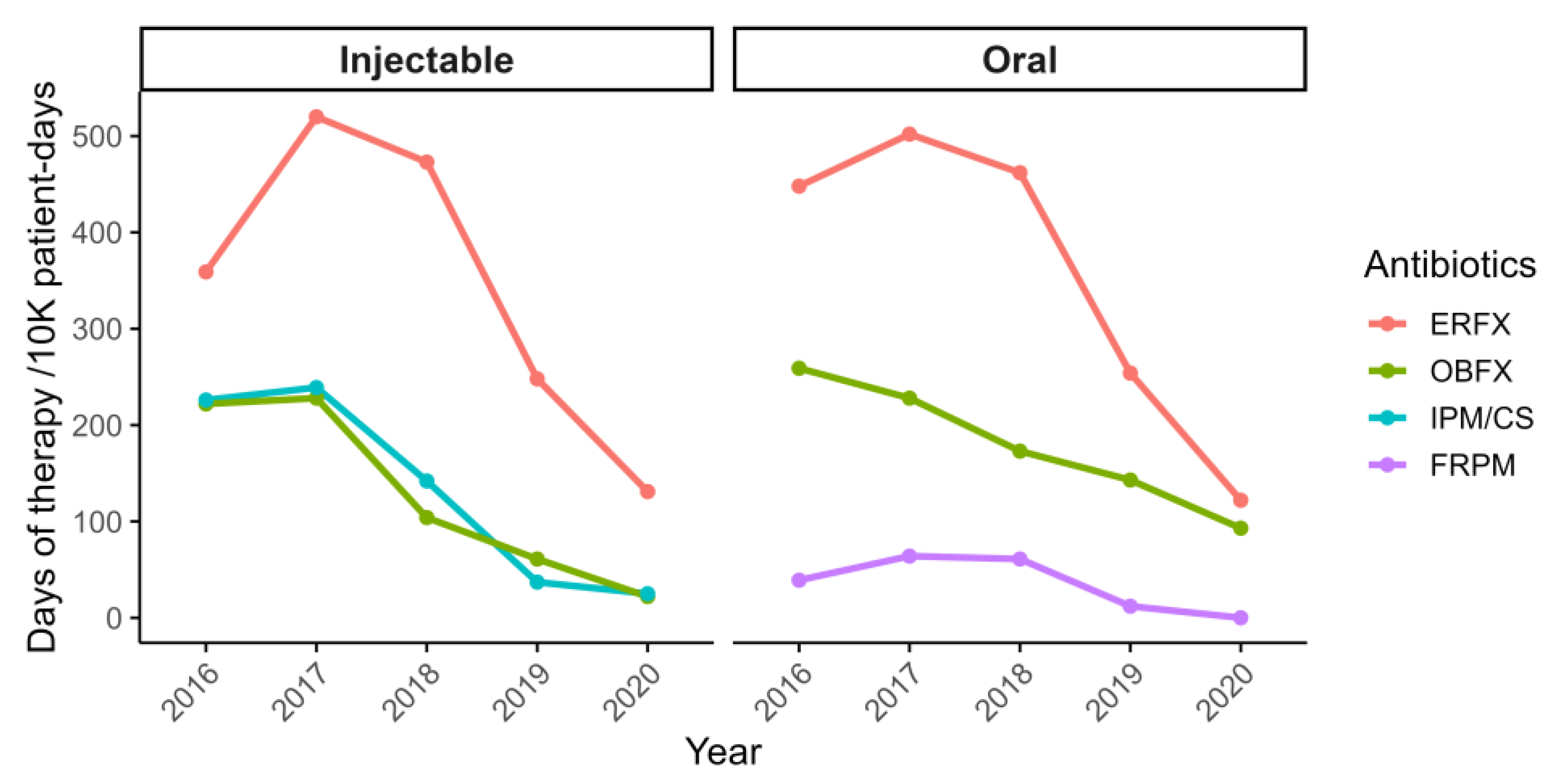
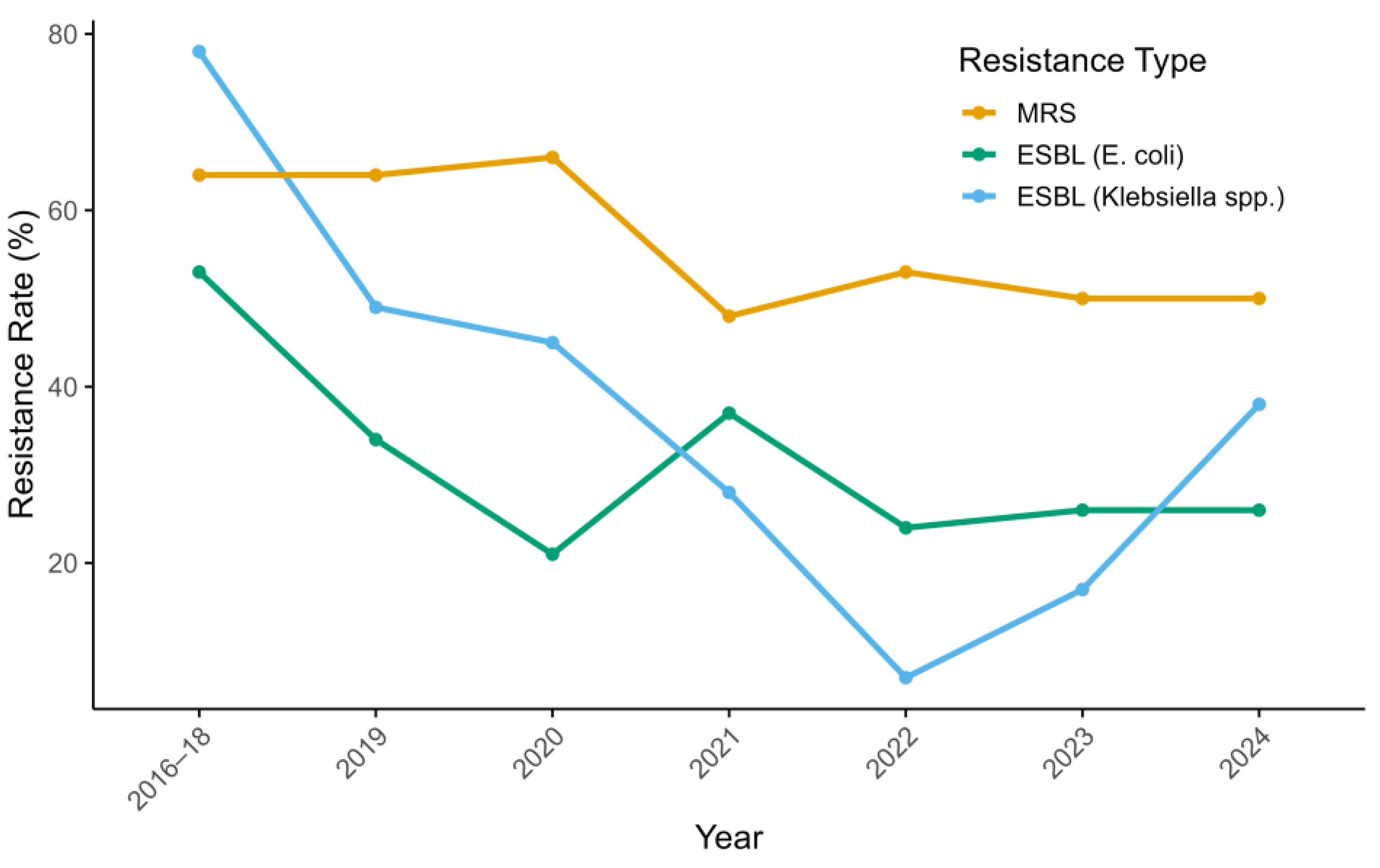
3.2. Impact of Intervention on Antimicrobial Use and Resistance Trends
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABPC | Ampicillin |
| AMK | Amikacin |
| AMPC | amoxicillin |
| AMR | Antimicrobial Resistance |
| ASP | Antimicrobial Stewardship Program |
| AST | Antimicrobial Susceptibility Testing |
| CEX | Cefalexin |
| CEZ | Cefazolin |
| CLDM | Clindamycin |
| CLSI | Clinical and Laboratory Standards Institute |
| CP | Chloramphenicol |
| CVA | Clavulanic acid |
| DOXY | Doxycycline |
| ERFX | Enrofloxacin |
| ESBL | Extended-Spectrum β-Lactamase |
| FOM | Fosfomycin |
| FRPM | Faropenem |
| IMP/CS | Imipenem/cilastatin |
| MINO | Minocycline |
| MRS | Methicillin-Resistant Staphylococci |
| OBFX | Orbifloxacin |
| OR | Odds Ratio |
| PK/PD | Pharmacokinetic/Pharmacodynamic |
| ST | Sulfamethoxazole-trimethoprim |
References
- Allerton, F.; Prior, C.; Bagcigil, A.F.; Broens, E.; Callens, B.; Damborg, P.; Dewulf, J.; Filippitzi, M.E.; Carmo, L.P.; Gomez-Raja, J.; et al. Overview and Evaluation of Existing Guidelines for Rational Antimicrobial Use in Small-Animal Veterinary Practice in Europe. Antibiotics 2021, 10, 409. [Google Scholar] [CrossRef]
- Frey, E.; Costin, M.; Granick, J.; Kornya, M.; Weese, J.S. 2022 AAFP/AAHA Antimicrobial Stewardship Guidelines. J. Am. Anim. Hosp. Assoc. 2022, 58, 1–5. [Google Scholar] [CrossRef]
- Weese, J.S.; Giguere, S.; Guardabassi, L.; Morley, P.S.; Papich, M.; Ricciuto, D.R.; Sykes, J.E. ACVIM consensus statement on therapeutic antimicrobial use in animals and antimicrobial resistance. J. Vet. Intern. Med. 2015, 29, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Hardefeldt, L.Y.; Gilkerson, J.R.; Billman-Jacobe, H.; Stevenson, M.A.; Thursky, K.; Bailey, K.E.; Browning, G.F. Barriers to and enablers of implementing antimicrobial stewardship programs in veterinary practices. J. Vet. Intern. Med. 2018, 32, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- King, C.; Smith, M.; Currie, K.; Dickson, A.; Smith, F.; Davis, M.; Flowers, P. Exploring the behavioural drivers of veterinary surgeon antibiotic prescribing: A qualitative study of companion animal veterinary surgeons in the UK. BMC Vet. Res. 2018, 14, 332. [Google Scholar] [CrossRef] [PubMed]
- Kurita, G.; Tsuyuki, Y.; Murata, Y.; Takahashi, T.; Veterinary Infection Control Association (VICA) AMR Working Group. Reduced rates of antimicrobial resistance in Staphylococcus intermedius group and Escherichia coli isolated from diseased companion animals in an animal hospital after restriction of antimicrobial use. J. Infect. Chemother. 2019, 25, 531–536. [Google Scholar] [CrossRef]
- Frenzer, S.K.; Feuer, L.; Bartel, A.; Bethe, A.; Lubke-Becker, A.; Klein, B.; Baumer, W.; Merle, R. Third-generation cephalosporin resistant Escherichia coli in dogs and cats in Germany in 2019–2021. PLoS ONE 2024, 19, e0309554. [Google Scholar] [CrossRef]
- Iyori, K.; Shishikura, T.; Shimoike, K.; Minoshima, K.; Imanishi, I.; Toyoda, Y. Influence of hospital size on antimicrobial resistance and advantages of restricting antimicrobial use based on cumulative antibiograms in dogs with Staphylococcus pseudintermedius infections in Japan. Vet. Dermatol. 2021, 32, 668-e178. [Google Scholar] [CrossRef]
- Jacobsen, A.; Ogden, J.; Ekiri, A.B. Antimicrobial resistance interventions in the animal sector: Scoping review. Front. Antibiot. 2023, 2, 1233698. [Google Scholar] [CrossRef]
- Lloyd, D.H.; Page, S.W. Antimicrobial Stewardship in Veterinary Medicine. Microbiol. Spectr. 2018, 6, 3. [Google Scholar] [CrossRef]
- Vernaccini, M.; De Marchi, L.; Briganti, A.; Lippi, I.; Marchetti, V.; Meucci, V.; Intorre, L. Antimicrobial Use in Cats in a University Veterinary Hospital in Central Italy: A Retrospective Study. Antibiotics 2024, 13, 927. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests, 26th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2016. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated From Animals, 7th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2024. [Google Scholar]
- Redding, L.E.; Grunwald, H.; Melofchik, C.; Meily, P.; Henry, A.; Stefanovski, D. Comparison of animal daily doses and days of therapy for antimicrobials in species of veterinary importance. Prev. Vet. Med. 2020, 176, 104942. [Google Scholar] [CrossRef]
- Fukuyama, H.; Yamashiro, S.; Kinjo, K.; Tamaki, H.; Kishaba, T. Validation of sputum Gram stain for treatment of community-acquired pneumonia and healthcare-associated pneumonia: A prospective observational study. BMC Infect. Dis. 2014, 14, 534. [Google Scholar] [CrossRef]
- Way, L.I.; Sullivan, L.A.; Johnson, V.; Morley, P.S. Comparison of routine urinalysis and urine Gram stain for detection of bacteriuria in dogs. J. Veter Emerg. Crit. Care 2013, 23, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, J.; Kinoshita, T.; Yamakawa, K.; Matsushima, A.; Nakamoto, N.; Hamasaki, T.; Fujimi, S. Impact of Gram stain results on initial treatment selection in patients with ventilator-associated pneumonia: A retrospective analysis of two treatment algorithms. Crit. Care 2017, 21, 156. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, M.; Motegi, T.; Uno, H.; Yokono, M.; Harada, K. Pharmacokinetic-pharmacodynamic analysis of cefmetazole against extended-spectrum beta-lactamase-producing Enterobacteriaceae in dogs using Monte Carlo Simulation. Front. Vet. Sci. 2023, 10, 1270137. [Google Scholar] [CrossRef] [PubMed]
- Simner, P.J.; Hindler, J.A.; Bhowmick, T.; Das, S.; Johnson, J.K.; Lubers, B.V.; Redell, M.A.; Stelling, J.; Erdman, S.M. What’s New in Antibiograms? Updating CLSI M39 Guidance with Current Trends. J. Clin. Microbiol. 2022, 60, e0221021. [Google Scholar] [CrossRef]
- Giamarellou, H.; Galani, L.; Karavasilis, T.; Ioannidis, K.; Karaiskos, I. Antimicrobial Stewardship in the Hospital Setting: A Narrative Review. Antibiotics 2023, 12, 1557. [Google Scholar] [CrossRef]
- Chatzopoulou, M.; Reynolds, L. Role of antimicrobial restrictions in bacterial resistance control: A systematic literature review. J. Hosp. Infect. 2020, 104, 125–136. [Google Scholar] [CrossRef]
- Knudsen, J.D.; Andersen, S.E.; Bispebjerg Intervention, G. A multidisciplinary intervention to reduce infections of ESBL- and AmpC-producing, gram-negative bacteria at a University Hospital. PLoS ONE 2014, 9, e86457. [Google Scholar] [CrossRef]
- Gerlich, M.G.; Piegsa, J.; Schafer, C.; Hubner, N.O.; Wilke, F.; Reuter, S.; Engel, G.; Ewert, R.; Claus, F.; Hubner, C.; et al. Improving hospital hygiene to reduce the impact of multidrug-resistant organisms in health care—A prospective controlled multicenter study. BMC Infect. Dis. 2015, 15, 441. [Google Scholar] [CrossRef]
- Landelle, C.; Marimuthu, K.; Harbarth, S. Infection control measures to decrease the burden of antimicrobial resistance in the critical care setting. Curr. Opin. Crit. Care 2014, 20, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Umpleby, H.; Dushianthan, A.; Catton, T.; Saeed, K. Antimicrobial stewardship programmes focused on de-escalation: A narrative review of efficacy and risks. J. Emerg. Crit. Care Med. 2022, 6, 23. [Google Scholar] [CrossRef]
- Tanzarella, E.S.; Cutuli, S.L.; Lombardi, G.; Cammarota, F.; Caroli, A.; Franchini, E.; Sancho Ferrando, E.; Grieco, D.L.; Antonelli, M.; De Pascale, G. Antimicrobial De-Escalation in Critically Ill Patients. Antibiotics 2024, 13, 375. [Google Scholar] [CrossRef] [PubMed]
- Karanika, S.; Paudel, S.; Grigoras, C.; Kalbasi, A.; Mylonakis, E. Systematic Review and Meta-analysis of Clinical and Economic Outcomes from the Implementation of Hospital-Based Antimicrobial Stewardship Programs. Antimicrob. Agents Chemother. 2016, 60, 4840–4852. [Google Scholar] [CrossRef]
- Davati, N.; Ghorbani, A. Comparison of the antibiotic resistance mechanisms in a gram-positive and a gram-negative bacterium by gene networks analysis. PLoS ONE 2024, 19, e0311434. [Google Scholar] [CrossRef]
- Gauba, A.; Rahman, K.M. Evaluation of Antibiotic Resistance Mechanisms in Gram-Negative Bacteria. Antibiotics 2023, 12, 1590. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 464–473. [Google Scholar] [CrossRef]
- Vestergaard, M.; Frees, D.; Ingmer, H. Antibiotic Resistance and the MRSA Problem. Microbiol. Spectr. 2019, 7, 2. [Google Scholar] [CrossRef]
- Motegi, T.; Nagakubo, D.; Maeda, S.; Yonezawa, T.; Nishimura, R.; Momoi, Y. Assessing Antimicrobial Resistance in Companion Animals at a Referral Hospital: The Impact of Antimicrobial Stewardship Strategies. In Proceedings of the 2024 ACVIM Forum, Minneapolis, MN, USA, 8 June 2024. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motegi, T.; Fukuoka, R.; Tsuyuki, Y.; Nagakubo, D.; Maeda, S.; Yonezawa, T.; Nishimura, R.; Momoi, Y. Evaluating a Targeted Antimicrobial Stewardship Program and Its Temporal Association with Resistance Trends in a Veterinary Referral Hospital. Vet. Sci. 2025, 12, 743. https://doi.org/10.3390/vetsci12080743
Motegi T, Fukuoka R, Tsuyuki Y, Nagakubo D, Maeda S, Yonezawa T, Nishimura R, Momoi Y. Evaluating a Targeted Antimicrobial Stewardship Program and Its Temporal Association with Resistance Trends in a Veterinary Referral Hospital. Veterinary Sciences. 2025; 12(8):743. https://doi.org/10.3390/vetsci12080743
Chicago/Turabian StyleMotegi, Tomoki, Rei Fukuoka, Yuzo Tsuyuki, Dai Nagakubo, Shingo Maeda, Tomohiro Yonezawa, Ryohei Nishimura, and Yasuyuki Momoi. 2025. "Evaluating a Targeted Antimicrobial Stewardship Program and Its Temporal Association with Resistance Trends in a Veterinary Referral Hospital" Veterinary Sciences 12, no. 8: 743. https://doi.org/10.3390/vetsci12080743
APA StyleMotegi, T., Fukuoka, R., Tsuyuki, Y., Nagakubo, D., Maeda, S., Yonezawa, T., Nishimura, R., & Momoi, Y. (2025). Evaluating a Targeted Antimicrobial Stewardship Program and Its Temporal Association with Resistance Trends in a Veterinary Referral Hospital. Veterinary Sciences, 12(8), 743. https://doi.org/10.3390/vetsci12080743




