Ultrasound Imaging Modalities in the Evaluation of the Dog’s Stifle Joint
Simple Summary
Abstract
1. Introduction
2. Methodology of the Examination of the Stifle Joint
- Angle of insonation: The angle between the direction of blood flow and the ultrasound waves should be less than 60°.
- Doppler gate: The cursor is positioned in the center of the vessel, occupying half to two-thirds of its diameter [52].
- Doppler angle: Should be set between 45° and 60° [53].
- Pulse repetition frequency: Should be adjusted based on the blood flow velocity.
- PW Gain: Should be set to ensure optimal contrast between the Doppler waveform and the background [54].
- The time interval from contrast injection to its arrival at the region of interest and subsequent detection by ultrasound.
- The maximum intensity of the contrast agent, known as Peak Enhancement (PE), and the time taken to reach PE.
- The regional blood volume area.
- The maximum value of the enhancement curve, referred to as the Wash-In Rate.
- The time from contrast agent injection until PE.
- The duration during which the intensity exceeds the mean value.
3. Imaging of Normal and Pathological Musculoskeletal Structure of the Stifle
3.1. Imaging of Normal Musculoskeletal Structure
3.1.1. Muscle
3.1.2. Tendon and Ligament
3.1.3. Bone
3.1.4. Stifle Joint
- Cranial Cruciate Ligament (CCL)
- Patellar ligament
- Other tendons and ligaments
- Meniscus
3.1.5. Peripheral Nerve
3.1.6. Arteries
3.2. Imaging of Pathological Musculoskeletal Structure
3.2.1. Muscle
3.2.2. Tendon and Ligament
3.2.3. Bone
3.2.4. Stifle Joint
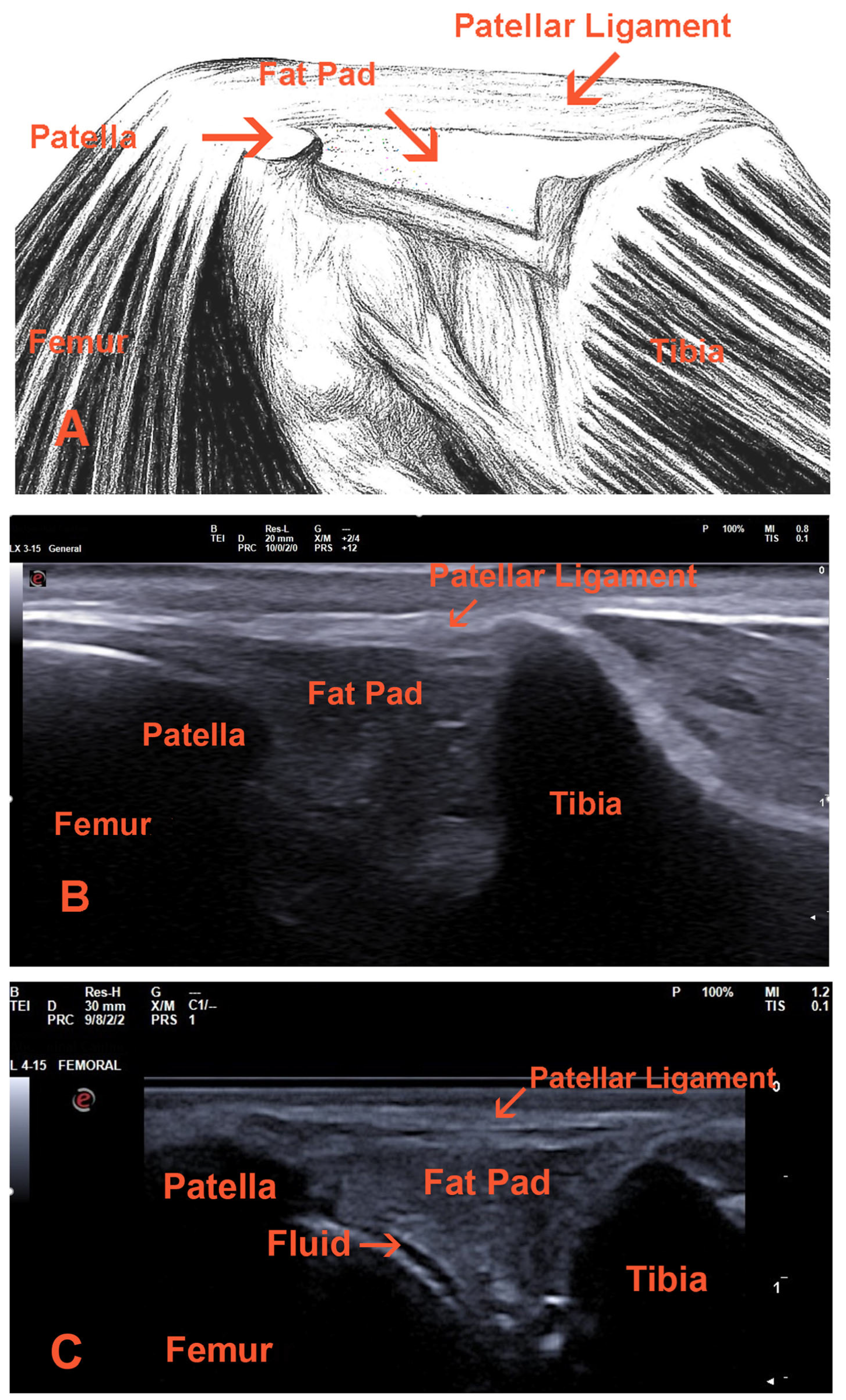

- Cranial Cruciate Ligament (CCL)
- Patellar Ligament
- Other tendons and ligaments
- Meniscus
3.2.5. Peripheral Nerve
3.2.6. Arteries
4. Other Imaging Modalities
5. Discussion
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Richardson, M.L.; Selby, B.; Montana, M.A.; Mack, L.A. Ultrasonography of the knee. Radiol. Clin. N. Am. 1988, 26, 63–75. [Google Scholar] [CrossRef]
- Laine, H.R.; Harjula, A.; Peltokallio, P. Ultrasound in the evaluation of the knee and patellar regions. Ultrasound Med. 1987, 6, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Friedman, L.; Finlay, K.; Jurriaans, E. Ultrasound of the Knee. Skelet. Radiol. 2001, 30, 361–377. [Google Scholar] [CrossRef] [PubMed]
- Cauvin, E.R.; Munroe, G.A.; Boyd, J.S.; Paterson, C. Ultrasonographic examination of the femorotibial articulation in horses: Imaging of the caudal aspects. Equine Vet. J. 1996, 28, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Penninck, G.D.; Nyland, G.T.; O’Brien, R.T.; Wheat, D.J.; Berry, R.C. Ultrasonography of the equine stifle. Vet. Radiol. 1990, 31, 293–298. [Google Scholar] [CrossRef]
- Reed, L.A.; Payne, T.J.; Constantinescu, M.G. Ultrasonographic anatomy of the normal canine stifle. Vet. Radiol. Ultrasound 1995, 36, 315–321. [Google Scholar] [CrossRef]
- Tyron, A.K.; Clark, R.C. Ultrasonographic examination of the distal limb of cattle. Vet. Clin. N. Am. Food Anim. Pract. 1999, 15, 275–300. [Google Scholar] [CrossRef]
- Kofler, J.; Geissbühler, U.; Steiner, A. Diagnostic imaging in bovine orthopedics. Vet. Clin. N. Am. Food Anim. Pract. 2014, 30, 11–53. [Google Scholar] [CrossRef]
- de Rooster, H.; van Ryssen, B.; van Bree, H. Diagnosis of cranial cruciate ligament injury in dogs by tibial compression radiography. Vet. Rec. 1998, 142, 366–368. [Google Scholar] [CrossRef]
- Farrell, M. The stifle. In BSAVA Manual of Canine and Feline Musculoskeletal Disorders: A Practical Guide to Lameness and Joint Disease, 2nd ed.; Bennett, D., Finn-Bodner, A.T.N., Eds.; British Small Animal Veterinary Association: Gloucester, UK, 2021; pp. 404–446. [Google Scholar]
- de Rooster, H.; van Bree, H. Radiographic measurement of craniocaudal instability in stifle joints of clinically normal dogs and dogs with injury of a cranial cruciate ligament. Am. J. Vet. Res. 1999, 60, 1567–1570. [Google Scholar] [CrossRef]
- Marino, J.D.; Loughin, A.C. Diagnostic imaging of the canine stifle: A review. Vet. Surg. 2010, 39, 284–295. [Google Scholar] [CrossRef]
- Kealy, J.K.; McAllister, H. Diagnostic Radiology and Ultrasonography of the Dog and Cat, 3rd ed.; Saunders: Philadelphia, PA, USA, 2000; pp. 298–384. [Google Scholar]
- Burk, L.R.; Ackerman, N. Small Animal Radiology and Ultrasonography, 2nd ed.; Elsevier Science Inc.: New York, NY, USA, 1996; pp. 478–600. [Google Scholar]
- Cullen, R.; Canapp, D.; Dycus, D.; Carr, B.; Ibrahim, V.; Canapp, S. Evaluation of iliopsoas strain with findings from diagnostic musculoskeletal ultrasound in agility performance canines–73 cases. Vet. Evid. 2017, 2, 1–17. [Google Scholar] [CrossRef]
- Khoury, V.; Cardinal, E.; Bureau, J.N. Musculoskeletal sonography: A dynamic tool for usual and unusual disorders. AJR Am. J. Roentgenol. 2007, 188, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Kremkau, F. Diagnostic Ultrasound: Principles and Instruments, 6th ed.; Saunders: Philadelphia, PA, USA, 2000; pp. 128–170. [Google Scholar]
- Cook, R.C. Ultrasound Imaging of the Musculoskeletal System. Vet. Clin. N. Am. Small Anim. Pract. 2016, 46, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Hangiandreou, J.N. AAPM/RSNA physics tutorial for residents. Topics in US: B- mode US: Basic concepts and new technology. Radiographics 2003, 23, 1019–1033. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Finnoff, T.F. Diagnostic and interventional musculoskeletal ultrasound: Part 1. Fundamentals. Am. Acad. Phys. Med. Rehabil. 2009, 1, 64–75. [Google Scholar] [CrossRef]
- Kramer, M.; Gerwing, M.; Hach, V.; Schimke, E. Sonography of the musculoskeletal system in dogs and cats. Vet. Radiol. Ultrasound 1997, 38, 139–149. [Google Scholar] [CrossRef]
- Oglat, A.A.; Alshipli, M.; Sayah, M.A.; Ahmad, M.S. Artifacts in Diagnostic Ultrasonography. J. Vasc. Ultrasound 2020, 44, 212–219. [Google Scholar] [CrossRef]
- Figurova, M.; Kulinova, V. Ultrasonographic examination of some vessels in dogs and the characteristics of blood flow in these vessels. Folia Vet. 2017, 61, 44–52. [Google Scholar] [CrossRef][Green Version]
- Hoffmann, K.L.; Wood, A.K.; Griffiths, K.A.; Evans, D.L.; Gill, R.W.; Kirby, A.C. Doppler sonographic measurements of arterial blood flow and their repeatability in the equine foot during weight bearing and non-weight bearing. Res. Vet. Sci. 2001, 70, 199–203. [Google Scholar] [CrossRef]
- Wongaumnuaykul, S.; Siedler, C.; Schobesberger, H.; Stanek, C. Doppler sonographic evaluation of the digital blood flow in horses with laminitis or septic pododermatitis. Vet. Radiol. Ultrasound 2006, 47, 199–205. [Google Scholar] [CrossRef]
- Risselada, M.; Kramer, M.; Saunders, H.J.; Verleyen, P.; Van Bree, H. Power Doppler assessment of the neovascularization during uncomplicated fracture healing of long bones in dogs and cats. Vet. Radiol. Ultrasound 2006, 47, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Strouse, J.P.; DiPietro, A.M.; Teo, L.E.; Doi, K.; Chrisp, E.C. Power Doppler evaluation of joint effusions: Investigation in a rabbit model. Pediatr. Radiol. 1999, 29, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, S.; Huang, C.; Zheng, Q.; Ye, Z.; Lye, G. Longitudinal Changes in Knee Joint Synovial Vascularity in a Rabbit Model of Rheumatoid Arthritis: Quantification Using Power Doppler Ultrasound and Contrast-Enhanced Ultrasound. Ultrasound Med. Biol. 2021, 47, 2430–2441. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Wang, Y.W.; Chen, W.S.; Hsiao, M.Y. Update of Contrast-enhanced Ultrasound in Musculoskeletal Medicine: Clinical Perspectives—A Review. J. Med. Ultrasound 2023, 31, 92–100. [Google Scholar] [CrossRef]
- Chang, K.V.; Lew, L.H.; Wang, T.G.; Chen, W.S. Use of contrast-enhanced ultrasonography in musculoskeletal medicine. Am. J. Phys. Med. Rehabil. 2012, 91, 449–457. [Google Scholar] [CrossRef]
- Gitto, S.; Messins, C.; Vitale, N.; Albano, D.; Sconfienza, M.L. Quantitative Musculoskeletal Ultrasound. Semin. Musculoskelet. Radiol. 2020, 24, 367–374. [Google Scholar] [CrossRef]
- Shimizu, M.; Ito, Y. Change in Shear Elastic Modulus of Thigh Muscle by Changing Muscle Length Using Ultrasound Shear Wave Elastography in Beagle Dogs. Vet. Comp. Orthop. Traumatol. 2019, 32, 454–459. [Google Scholar] [CrossRef]
- Piccionello, P.A.; Serrani, D.; Busoni, V.; Salvaggio, A.; Bonazzi, M.; Bergamino, C.; Volta, A. Sonoelastographic Features of the Patellar Ligament in Clinically Normal Dogs. Vet. Comp. Orthop. Traumatol. 2018, 31, 279–284. [Google Scholar] [CrossRef]
- McCagherty, J.; Longo, M.; Pennington, C.; Liuti, T.; Morrison, R.L.; Brown, H.; Clements, N.D. Effect of Stifle Flexion Angle on the Repeatability of Real-Time Elastosonography of the Patellar Ligament in Medium- to Large-Breed Dogs. Vet. Comp. Orthop. Traumatol. 2020, 33, 391–397. [Google Scholar] [CrossRef]
- Del Signore, F.; De Dominicis, S.; Mastromatteo, G.; Simeoni, F.; Scapolo, P.A.; Tamburro, R.; Vignoli, M. Sonoelastography of Normal Canine Common Calcaneal Tendon: Preliminary Results. Vet. Comp. Orthop. Traumatol. 2021, 34, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Diogo, L.M.I.; Andrade, C.R.; Faria, L.G.; Uscategui, R.A.R.; Maronezi, M.C.; Cruz, I.K.; Aires, L.P.N.; Nociti, R.P.; Dias, L.G.G.G.; Feliciano, M.A.R.; et al. Acoustic radiation force impulse (ARFI) elastography of the stifle joint of healthy beagles. Arq. Bras. Med. Vet. Zootec. 2020, 72, 1646–1652. [Google Scholar] [CrossRef]
- Pennasilico, L.; Volta, A.; Sassaroli, S.; Di Bella, C.; Riccio, V.; Pilati, N.; Tambella, A.M.; Dini, F.; Piccionello, P.A. Ultrasound and Elastosonographic Features of the Patellar Ligament in Dogs Affected by Cranial Cruciate Ligament Disease. Vet. Sci. 2024, 11, 126. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, A.; Grajo, R.J.; Dhyani, M.; Antony, W.B.; Samir, E.A. Principles of ultrasound elastography. Abdom. Radiol. 2018, 43, 773–785. [Google Scholar] [CrossRef]
- Prodo-Costa, R.; Rebelo, J.; Monteiro-Barroso, J.; Preto, S.A. Ultrasound elastography: Compression elastography and shear-wave elastography in the assessment of tendon injury. Insights Imaging 2018, 9, 791–814. [Google Scholar] [CrossRef]
- Taljanovic, S.M.; Gimber, H.L.; Becker, W.G.; Latt, D.L.; Klauser, S.A.; Melville, M.D.; Gao, L.; Witte, S.R. Shear-Wave Elastography: Basic Physics and Musculoskeletal Applications. Radiographics 2017, 37, 855–870. [Google Scholar] [CrossRef]
- Pedersen, M.; Fredberg, U.; Langberg, H. Sonoelastography as a diagnostic tool in the assessment of musculoskeletal alterations: A systematic review. Ultraschall Med. 2012, 33, 441–446. [Google Scholar] [CrossRef]
- Ooi, C.C.; Richards, J.P.; Maffulli, N.; Ede, D.; Schneider, E.M.; Connell, D.; Morrissey, D.; Malliaras, P. A soft patellar tendon on ultrasound elastography is associated with pain and functional deficit in volleyball players. J. Sci. Med. Sport 2016, 19, 373–378. [Google Scholar] [CrossRef]
- Lustgarten, M.; Redding, W.R.; Labens, R.; Davis, W.; Daniel, M.T.; Griffith, E.; Seiler, S.G. Elastoographic evaluation of naturally occurring tendon and ligament injuries of the equine distal limb. Vet. Radiol. Ultrasound 2015, 56, 670–679. [Google Scholar] [CrossRef]
- Lento, H.P.; Primack, S. Advances and utility of diagnostic ultrasound in musculoskeletal medicine. Curr. Rev. Musculoskelet. Med. 2008, 1, 24–31. [Google Scholar] [CrossRef]
- Nazarian, N.L.; McShane, M.J.; Ciccotti, G.M.; O’Kane, L.P.; Harwood, I.M. Dynamic US of the anterior band of the ulnar collateral ligament of the elbow in asymptomatic major league baseball pitchers. Radiology 2003, 227, 149–154. [Google Scholar] [CrossRef]
- O’Connor, J.P.; Grainger, J.A. Ultrasound imaging of joint disease. In Practical Musculoskeletal Ultrasound; McNally, E.G., Ed.; Elsevier: Philadelphia, PA, USA, 2005; pp. 245–262. [Google Scholar]
- Kramer, M.; Stengel, H.; Gerwing, M.; Schimke, E.; Sheppard, C. Sonography of the canine stifle. Vet. Radiol. Ultrasound 1999, 40, 282–293. [Google Scholar] [CrossRef]
- Arnault, F.; Cauvin, E.; Viguier, E.; Kraft, E.; Sonet, J.; Carozzo, C. Diagnostic value of ultrasonography to assess stifle lesions in dogs after cranial cruciate ligament rupture: 13 cases. Vet. Comp. Orthop. Traumatol. 2009, 22, 479–485. [Google Scholar]
- Mahn, M.M.; Cook, L.J.; Cook, R.C.; Balke, T.B. Arthroscopic verification of ultrasonographic diagnosis of meniscal pathology in dogs. Vet. Surg. 2005, 34, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.J. Doppler ultrasonography of the lower extremity arteries: Anatomy and scanning guidelines. Ultrasonography 2017, 36, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Kruskal, B.J.; Newman, A.P.; Sammons, G.L.; Kane, A.R. Optimizing Doppler and color flow US: Application to hepatic sonography. Radiographics 2004, 24, 657–675. [Google Scholar] [CrossRef] [PubMed]
- Knox, A.R.; Phillips, J.D.; Breslau, J.P.; Lawrence, R.; Primozich, J.; Strandness, D.E., Jr. Empirical findings relating sample volume size to diagnostic accuracy in pulsed Doppler cerebrovascular studies. J. Clin. Ultrasound 1982, 10, 227–232. [Google Scholar] [CrossRef]
- Zwiebel, W.J.; Pellerito, J.S. Basic concepts of Doppler frequency spectrum analysis and ultrasound blood flow imaging. In Introduction to Vascular Ultrasonography, 5th ed.; Pellerito, J.S., Ed.; Elsevier Saunders: Philadelphia, PA, USA, 2004; pp. 61–89. [Google Scholar]
- Hofer, M. Ein Arbeitsbuch für den Einstieg in die Farbkodierte Duplexsonographie und Echokardiographie. In FKDS-Trainer, 3rd ed.; Hofer, M., Ed.; Didamed: Düsseldorf, Germany, 2009; pp. 10–18. [Google Scholar]
- Tang, M.X.; Mulvana, H.; Gauthier, T.; Lim, A.K.; Cosgrove, D.O.; Eckersley, R.J.; Stride, E. Quantitative contrast-enhanced ultrasound imaging: A review of sources of variability. Interface Focus 2011, 1, 520–539. [Google Scholar] [CrossRef]
- Embriano, K.; Holland, M.; Corriveau, K.M.; Hofmeister, E.; McCarthy, J. Shear-wave elastography of canine patellar tendons in healthy dogs and the influence of stifle joint angle. Vet. Radiol. Ultrasound 2025, 66, e13447. [Google Scholar] [CrossRef]
- Hardy, A.; Rodaix, C.; Vergari, C.; Vialle, R. Normal range of patellar tendon elasticity using the shear wave elastography technique: An in vivo study in normal volunteers. Surg. Technol. Int. 2017, 31, 227–230. [Google Scholar]
- Kim, S.Y.; Cheon, J.H.; Seo, W.J.; Yang, G.Y.; Choi, Y.M.; Kim, K.H. A pictorial review of signature patterns living in musculoskeletal ultrasonography. Korean J. Pain 2016, 29, 217–228. [Google Scholar] [CrossRef]
- Zwingenberger, A.; Benigni, L.; Lamb, C.R. Musculoskeletal system. In Small Animal Diagnostic Ultrasound, 3rd ed.; Mattoon, J.S., Nyland, T.G., Eds.; Elsevier Saunders: St. Louis, MO, USA, 2015; pp. 517–540. [Google Scholar]
- van Holsbeeck, M.; Strouse, P.J. Sonography of the shoulder: Evaluation of the subacromial-subdeltoid bursa. AJR Am. J. Roentgenol. 1993, 160, 561–564. [Google Scholar] [CrossRef]
- Sideri, A.; Tsioli, V. Ultrasonographic examination of the musculoskeletal system in sheep. Small Rumin. Res. 2017, 152, 158–161. [Google Scholar] [CrossRef]
- Moore, R.E. 2010 Musculoskeletal Ultrasound for the Extremities: A Practical Guide to Sonography of the Extremities; Createspace: Valley, CA, USA, 2010; pp. 9–11. [Google Scholar]
- Martinoli, C.; Bianchi, S.; Derchi, E.L. Tendon and nerve sonography. Radiol. Clin. N. Am. 1999, 37, 691–711. [Google Scholar] [CrossRef] [PubMed]
- Adler, S.R.; Buly, R.; Ambrose, R.; Sculco, T. Diagnostic and therapeutic use of sonography-guided iliopsoas peritendinous injections. AJR Am. J. Roentgenol. 2005, 185, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.; McNally, E.; Scott, P. Ultrasound of peripheral nerves. Imaging 2002, 14, 217–222. [Google Scholar] [CrossRef]
- Ebrahim, F.; De Maeseneer, M.; Jager, T.; Marcelis, S.; Jamadar, D.A.; Jacobson, J.A. US diagnosis of UCL tears of the thumb and Stener lesions: Technique, pattern-based approach, and differential diagnosis. Radiographics 2006, 26, 1007–1020. [Google Scholar] [CrossRef]
- Smith, J.; Finnoff, T.J. Diagnostic and interventional musculoskeletal ultrasound: Part 2. Phys. Med. Rehabil. J. 2009, 1, 162–177. [Google Scholar]
- Østergaard, M.; Wiell, C. Ultrasonography in rheumatoid arthritis: A very promising method still needing more validation. Curr. Opin. Rheumatol. 2004, 16, 223–230. [Google Scholar] [CrossRef]
- Tarhan, S.; Unlu, Z. Magnetic resonance imaging and ultrasonographic evaluation of the patients with knee osteoarthritis: A comparative study. Clin. Rheumatol. 2003, 22, 181–188. [Google Scholar] [CrossRef]
- Gnudi, G.; Bertoni, G. Echographic examination of the stifle joint affected by cranial cruciate ligament rupture in the dog. Vet. Radiol. Ultrasound 2001, 42, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Porta, F.; Damjanov, N.; Galluccio, F.; Iagnocco, A.; Matucci-Cerinic, M. Ultrasound elastography is a reproducible and feasible tool for the evaluation of the patellar tendon in healthy subjects. Int. J. Rheum. 2014, 17, 762–766. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Duan, L.; Liu, Q.; Zhang, W. Application of shear wave elastography and B-mode ultrasound in patellar tendinopathy after extracorporeal shockwave therapy. J. Med. Ultrason. 2020, 47, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Fitch, B.R.; Wilson, R.E.; Hathcock, T.J.; Montgomery, D.R. Radiographic, computed tomographic and magnetic resonance imaging evaluation of a chronic long digital extensor tendon avulsion in a dog. Vet. Radiol. Ultrasound 1997, 38, 177–181. [Google Scholar] [CrossRef]
- Evans, H.E. Miller’s Anatomy of the Dog, 4th ed.; Elsevier: St. Louis, MO, USA, 2013; pp. 650–651. [Google Scholar]
- Toijala, T.M.; Canapp, D.A.; Canapp, S.O. Ultrasonography Findings in the Proximal Sciatic Nerve and Deep Gluteal Muscles in 29 Dogs with Suspected Sciatic Neuritis. Front. Vet. Sci. 2021, 8, 704904. [Google Scholar] [CrossRef]
- Walker, O.F. Imaging nerve and muscle with ultrasound. Suppl. Clin. Neurophysiol. 2004, 57, 243–254. [Google Scholar]
- Szatmari, V.; Sotonyi, P.; Vörös, K. Normal duplex Doppler waveforms of major abdominal blood vessels in dogs: A review. Vet. Radiol. Ultrasound 2001, 42, 93–107. [Google Scholar] [CrossRef]
- Lee, K.; Choi, M.; Yoon, J.; Jung, J. Spectral waveform analysis of major arteries in conscious dogs by Doppler ultrasonography. Vet. Radiol. Ultrasound 2004, 45, 166–171. [Google Scholar] [CrossRef]
- Doyle, J.A.; Miller, V.M.; French, G.J. Ultrasound of soft-tissue masses: Pitfalls in interpretation. Australas. Radiol. 2000, 44, 275–280. [Google Scholar] [CrossRef]
- Giovagnorio, F.; Andreoli, C.; De Cicco, M. Valutazione con ecografia e Tomografia Computerizzata degli ematomi “spontanei” della parete addominale [The echographic and computed tomographic assessment of “spontaneous” hematomas of the abdominal wall]. Radiol. Med. 1997, 94, 481–485. [Google Scholar]
- Campbell, E.S.; Alder, R.; Sofka, M.C. Ultrasound of muscle abnormalities. Ultrasound Q. 2005, 21, 87–94. [Google Scholar] [PubMed]
- Pingel, J.; Harrison, A.; Simonsen, L.; Suetta, C.; Bülow, J.; Langberg, H. The microvascular volume of the Achilles tendon is increased in patients with tendinopathy at rest and after a 1-hour treadmill run. Am. J. Sports Med. 2013, 41, 2400–2408. [Google Scholar] [CrossRef] [PubMed]
- Genovese, E.; Ronga, M.; Recaldini, C.; Fontana, F.; Callegari, L.; Maffulli, N.; Fugazzola, C. Analysis of achilles tendon vascularity with second-generation contrast-enhanced ultrasound. J. Clin. Ultrasound 2011, 39, 141–145. [Google Scholar] [CrossRef]
- Möller, I.; Bong, D.; Naredo, E.; Filippucci, E.; Carrasco, I.; Moragues, C.; Iagnocco, A. Ultrasound in the study and monitoring of osteoarthritis. Osteoarthr. Cartil. 2008, 16, 4–7. [Google Scholar] [CrossRef]
- Cooperberg, L.P.; Tsang, I.; Truelove, L.; Knickerbocker, J.W. Gray scale ultrasound in the evaluation of rheumatoid arthritis of the knee. Radiology 1978, 126, 759–763. [Google Scholar] [CrossRef]
- Fessell, P.D.; Jacobson, A.J.; Craig, J.; Habra, G.; Prasad, A.; Radliff, A.; van Holsbeeck, M.T. Using sonography to reveal and aspirate joint effusions. AJR Am. J. Roentgenol. 2000, 174, 1353–1362. [Google Scholar] [CrossRef]
- Court-Payen, M. Sonography of the knee: Intra-articular pathology. J. Clin. Ultrasound 2004, 32, 481–490. [Google Scholar] [CrossRef]
- Qvistgaard, E.; Kristoffersen, H.; Terslev, L.; Danneskiold-Samsøe, B.; Torp-Pedersen, S.; Bliddal, H. Guidance by ultrasound of intra-articular injections in the knee and hip joints. Osteoarthr. Cartil. 2001, 9, 512–517. [Google Scholar] [CrossRef]
- Hammer, M.; Mielke, H.; Wagener, P.; Schwarzrock, R.; Giebel, G. Sonography and NMR imaging in rheumatoid gonarthritis. Scand. J. Rheumatol. 1986, 15, 157–164. [Google Scholar] [CrossRef]
- van Holsbeeck, M.; van Holsbeeck, K.; Gevers, G.; Marchal, G.; van Steen, A.; Favril, A.; Gielen, J.; Dequeker, J.; Baert, A. Staging and follow-up of rheumatoid arthritis of the knee. Comparison of sonography, thermography, and clinical assessment. J. Ultrasound Med. 1988, 7, 561–566. [Google Scholar] [CrossRef][Green Version]
- Razek, A.A.; Fouda, S.N.; Elmetwaley, N.; Elbogdady, E. Sonography of the knee joint. J. Ultrasound 2009, 12, 53–60. [Google Scholar] [CrossRef]
- Marchal, J.G.; Van Holsbeeck, T.M.; Raes, M.; Favril, A.A.; Verbeken, E.E.; Casteels-Vandaele, M.; Baert, L.A.; Lauweryns, M.J. Transient synovitis of the hip in children: Role of US. Radiology 1987, 162, 825–828. [Google Scholar] [CrossRef]
- Pond, J.M.; Nuki, G. Experimentally-induced osteoarthritis in the dog. Ann. Rheum. Dis. 1973, 32, 387–388. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.E. Metabolic response during early stages of surgically-induced osteoarthritis in mature beagles. J. Rheumatol. 1980, 7, 788–800. [Google Scholar]
- Thrall, D.E. Textbook of Veterinary Diagnostic Radiology, 4th ed.; W. B. Saunders: Philadelphia, PA, USA, 2002; pp. 350–380. [Google Scholar]
- Carrig, C.B. Diagnostic imaging of osteoarthritis. Vet. Clin. N. Am. Small Anim. Pract. 1997, 27, 777–814. [Google Scholar] [CrossRef] [PubMed]
- Hulse, D.A.; Shires, P.K. Observation of the posteromedial compartment of the stifle joint. J. Am. Anim. Hosp. Assoc. 1981, 17, 575–578. [Google Scholar]
- Bennett, D.; May, C. Meniscal damage associated with cruciate disease in the dog. J. Small Anim. Pract. 1991, 32, 111–117. [Google Scholar] [CrossRef]
- Flo, L.G. Meniscal injuries. Vet. Clin. N. Am. Small Anim. Pract. 1993, 23, 831–843. [Google Scholar] [CrossRef]
- Mattern, K.L.; Berry, C.R.; Peck, J.N.; De Haan, J.J. Radiographic and ultrasonographic evaluation of the patellar ligament following tibial plateau leveling osteotomy. Vet. Radiol. Ultrasound 2006, 47, 185–191. [Google Scholar] [CrossRef]
- Kuhn, K.; Ohlerth, S.; Makara, M.; Hässig, M.; Guerrero, T.G. Radiographic and ultrasonographic evaluation of the patellar ligament following tibial tuberosity advancement. Vet. Radiol. Ultrasound 2011, 52, 466–471. [Google Scholar] [CrossRef]
- Seong, Y.; Eom, K.; Lee, H.; Lee, J.; Park, J.; Lee, K.; Jang, K.; Oh, T.; Yoon, J. Ultrasonographic evaluation of cranial cruciate ligament rupture via dynamic intra-articular saline injection. Vet. Radiol. Ultrasound 2005, 46, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Signore, F.D.; De Dominicis, S.; Smoglica, C.; Rosto, M.; De Bonis, A.; Paolini, A.; Vignoli, M. Strain Elastography Evaluation of Patellar Tendons in Dogs after TPLO/TTA for Cranial Cruciate Ligament Rupture, Qualitative and Semiquantitative Evaluation Compared with Healthy Subjects. Animals 2024, 14, 2946. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, S.; Martinoli, M.C.; Boutry, N. Ultrasound imaging of the hand and wrist. In Practical Musculoskeletal Ultrasound; McNally, E.G., Ed.; Elsevier: Philadelphia, PA, USA, 2005; pp. 95–117. [Google Scholar]
- Upchurch, D.A.; Ogden, D.M.; Baker, D.G. Bilateral femoral arterial dirofilariasis caused by Dirofilaria immitis in a dog. Vet. Rec. Case Rep. 2015, 3, e000184. [Google Scholar] [CrossRef]
- Newman, S.J.; Adler, S.R.; Bude, O.R.; Rubin, M.J. Detection of soft-tissue hyperemia: Value of power Doppler sonography. AJR Am. J. Roentgenol. 1994, 163, 385–389. [Google Scholar] [CrossRef]
- Hussain, S.M.; Dawson, C.; Wang, Y.; Tonkin, A.M.; Chou, L.; Wluka, A.E.; Cicuttini, F.M. Vascular Pathology and Osteoarthritis: A Systematic Review. J. Rheumatol. 2020, 47, 748–760. [Google Scholar] [CrossRef]
- Imhof, H.; Breitenseher, M.; Kainberger, F.; Trattnig, S. Degenerative joint disease: Cartilage or vascular disease? Skelet. Radiol. 1997, 26, 398–403. [Google Scholar] [CrossRef]
- Wu, J.; Li, Y.; Zhang, X.; Liu, J.; Qian, Z.; Ren, P.; Xu, R.; Ren, L.; Ren, L. Assessment of blood flow around the knee joint in patients with knee osteoarthritis by color Doppler ultrasound. Eur. J. Radiol. 2023, 166, 111005. [Google Scholar] [CrossRef]
- Findlay, M.D. Vascular pathology and osteoarthritis. Rheumatology 2007, 46, 1763–1768. [Google Scholar] [CrossRef]
- Boyaci, A.; Tutoglu, A.; Boyaci, N.; Koca, I.; Aridici, R.; Daglioglu, E.; Yildiz, S. Assessment of lower extremity arterial blood flow in females with knee osteoarthritis. Clin. Rheumatol. 2015, 34, 329–335. [Google Scholar] [CrossRef]
- Dudley, M.R.; Kowaleski, P.M.; Drost, T.W.; Dyce, J. Radiographic and computed tomographic determination of femoral varus and torsion in the dog. Vet. Radiol. Ultrasound 2006, 47, 546–552. [Google Scholar] [CrossRef]
- Mostafa, A.A.; Griffon, J.D.; Thomas, W.M.; Constable, D.P. Proximodistal alignment of the canine patella: Radiographic evaluation and association with medial and lateral patellar luxation. Vet. Surg. 2008, 37, 201–211. [Google Scholar] [CrossRef]
- Langley-Hobbs, S.J.; Brown, G.; Matis, U. Traumatic fracture of the patella in 11 cats. Vet. Comp. Orthop. Traumatol. 2008, 21, 427–433. [Google Scholar]
- Guillaumot, P.; Scotti, S.; Carozzo, C.; Bouvy, B.; Genevois, J.P. Two cases of surgically treated feline patellar fractures. Vet. Comp. Orthop. Traumatol. 2008, 21, 156–158. [Google Scholar]
- Shipov, A.; Shahar, R.; Joseph, R.; Milgram, J. Successful management of bilateral patellar tendon rupture in a dog. Vet. Comp. Orthop. Traumatol. 2008, 21, 181–184. [Google Scholar]
- Mink, J.H.; Levy, T.; Crues, J.V. Tears of the anterior cruciate ligament and menisci of the knee: MR imaging evaluation. Radiology 1988, 167, 769–774. [Google Scholar] [CrossRef]
- Vande Berg, B.C.; Lecouvet, F.E.; Poilvache, P.; Dubuc, J.E.; Maldague, B.; Malghem, J. Anterior cruciate ligament tears and associated meniscal lesions: Assessment at dual-detector spiral CT arthrography. Radiology 2002, 223, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Moon, K., Jr.; Genant, H.K.; Helms, C.A.; Chafetz, N.I.; Crooks, L.E.; Kaufman, L. Musculoskeletal applications of nuclear magnetic resonance. Radiology 1983, 147, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Stoller, D. Magnetic Resonance Imaging in Orthopaedics and Rheumatology, 3rd ed.; Lippincott: Philadelphia, PA, USA, 1989; pp. 1–284. [Google Scholar]
- Burk, D.L., Jr.; Dalinka, M.K.; Schiebler, M.L.; Cohen, E.K.; Kressel, H.Y. Strategies for musculoskeletal magnetic resonance imaging. Radiol. Clin. N. Am. 1988, 26, 653–672. [Google Scholar] [CrossRef] [PubMed]
- Tivers, M.S.; Mahoney, P.; Corr, S.A. Canine stifle positive contrast computed tomography arthrography for assessment of caudal horn meniscal injury: A cadaver study. Vet. Surg. 2008, 37, 269–277. [Google Scholar] [CrossRef]
- Towle, H.A.; Griffon, D.J.; Thomas, M.W.; Siegel, A.M.; Dunning, D.; Johnson, A. Pre- and postoperative radiographic and computed tomographic evaluation of dogs with medial patellar luxation. Vet. Surg. 2005, 34, 265–272. [Google Scholar] [CrossRef]
- Banfield, C.M.; Morrison, W.B. Magnetic resonance arthrography of the canine stifle joint: Technique and applications in eleven military dogs. Vet. Radiol. Ultrasound 2000, 41, 200–213. [Google Scholar] [CrossRef]
- Samii, V.F.; Dyce, J. Computed tomographic arthrography of the normal canine stifle. Vet. Radiol. Ultrasound 2004, 45, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Cheon, H.; Cho, H.; Kim, J.; Kang, J.H.; Yang, M.P.; Lee, Y.; Lee, H.; Chang, D. Evaluation of partial cranial cruciate ligament rupture with positive contrast computed tomographic arthrography in dogs. J. Vet. Sci. 2008, 9, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.C.; Felson, D.T.; Helmick, C.G.; Arnold, L.M.; Choi, H.; Deyo, R.A.; Gabriel, S.; Hirsch, R.; Hochberg, M.C.; Hunder, G.G.; et al. National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008, 58, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef]
- Carr, J.C.; Hanly, S.; Griffin, J.; Gibney, R. Sonography of the patellar tendon and adjacent structures in pediatric and adult patients. AJR Am. J. Roentgenol. 2001, 176, 1535–1539. [Google Scholar] [CrossRef]
- Baird, D.K.; Hathcock, J.T.; Rumph, P.F.; Kincaid, S.A.; Visco, D.M. Low-field magnetic resonance imaging of the canine stifle joint: Normal anatomy. Vet. Radiol. Ultrasound 1998, 39, 87–97. [Google Scholar] [CrossRef]
- Baird, D.K.; Hathcock, J.T.; Kincaid, S.A.; Rumph, P.F.; Kammermann, J.; Widmer, W.R.; Visco, D.; Sweet, D. Low-field magnetic resonance imaging of early subchondral cyst-like lesions in induced cranial cruciate ligament deficient dogs. Vet. Radiol. Ultrasound 1998, 39, 167–173. [Google Scholar] [CrossRef]
- Engelke, A.; Meyer-Lindenberg, A.; Nolte, I. Die Ultraschalluntersuchung des Kniegelenkes des Hundes [Ultrasonography of the stifle joint in dogs]. Berl. Munch. Tierarztl. Wochenschr. 1997, 110, 24–29. [Google Scholar]
- Engelke, A.; Meyer-Lindenberg, A.; Nolte, I. Die Ultraschalluntersuchung des inneren Kniegelenkes bei Hunden mit Kreuzbandriss [Ultrasonography of the inner stifle joint in dogs with rupture of the cruciate ligaments]. Dtsch. Tierarztl. Wochenschr. 1997, 104, 114–117. [Google Scholar]
- Holcombe, S.J.; Bertone, A.L.; Biller, D.S.; Haider, V. Magnetic resonance imaging of the equine stifle. Vet. Radiol. 1995, 36, 119–125. [Google Scholar] [CrossRef]
- Widmer, W.R.; Buckwater, K.A.; Braunstein, E.M.; Hill, M.A.; O’Connor, B.L.; Visco, D.M. Radiographic and magnetic resonance imaging of the stifle joint in experimental osteoarthritis of dogs. Vet. Radiol. 1994, 35, 371–383. [Google Scholar] [CrossRef]
- D’Anjou, M.A.; Moreau, M.; Troncy, E.; Martel-Pelletier, J.; Abram, F.; Raynauld, J.P.; Pelletier, J.P. Osteophytosis, subchondral bone sclerosis, joint effusion and soft tissue thickening in canine experimental stifle osteoarthritis: Comparison between 1.5 T magnetic resonance imaging and computed radiography. Vet. Surg. 2008, 37, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Martig, S.; Boisclair, J.; Konar, M.; Spreng, D.; Lang, J. MRI characteristics and histology of bone marrow lesions in dogs with experimentally induced osteoarthritis. Vet. Radiol. Ultrasound 2007, 48, 105–112. [Google Scholar] [CrossRef]
- Sabiston, C.P.; Adams, M.E.; Li, D.K. Magnetic resonance imaging of osteoarthritis: Correlation with gross pathology using an experimental model. J. Orthop. Res. 1987, 5, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Libicher, M.; Ivancic, M.; Hoffmann, M.; Wenz, W. Early changes in experimental osteoarthritis using the Pond-Nuki dog model: Technical procedure and initial results of in vivo MR imaging. Eur. Radiol. 2005, 15, 390–394. [Google Scholar] [CrossRef]
- Innes, J.F.; Costello, M.; Barr, F.J.; Rudorf, H.; Barr, A.R. Radiographic progression of osteoarthritis of the canine stifle joint: A prospective study. Vet. Radiol. Ultrasound 2004, 45, 143–148. [Google Scholar] [CrossRef]
- Peterfy, C.; Kothari, M. Imaging osteoarthritis: Magnetic resonance imaging versus x-ray. Curr. Rheumatol. Rep. 2006, 8, 16–21. [Google Scholar] [CrossRef]
- Raynauld, J.P.; Martel-Pelletier, J.; Berthiaume, M.J.; Beaudoin, G.; Choquette, D.; Haraoui, B.; Tannenbaum, H.; Meyer, J.M.; Beary, J.F.; Cline, G.A.; et al. Long term evaluation of disease progression through the quantitative magnetic resonance imaging of symptomatic knee osteoarthritis patients: Correlation with clinical symptoms and radiographic changes. Arthritis Res. Ther. 2006, 8, R21. [Google Scholar] [CrossRef]
- Raynauld, J.P.; Martel-Pelletier, J.; Berthiaume, M.J.; Labonté, F.; Beaudoin, G.; de Guise, J.A.; Bloch, D.A.; Choquette, D.; Haraoui, B.; Altman, R.D.; et al. Quantitative magnetic resonance imaging evaluation of knee osteoarthritis progression over two years and correlation with clinical symptoms and radiologic changes. Arthritis Rheum. 2004, 50, 476–487. [Google Scholar] [CrossRef]
- Nolte-Ernsting, C.C.; Adam, G.; Bühne, M.; Prescher, A.; Günther, R.W. MRI of degenerative bone marrow lesions in experimental osteoarthritis of canine knee joints. Skelet. Radiol. 1996, 25, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, J.A. Ultrasound in sports medicine. Radiol. Clin. N. Am. 2002, 40, 363–386. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, R.J.; O’Connor, P.J.; Grainger, A.J. Tendon and ligament imaging. Br. J. Radiol. 2012, 85, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Park, G.Y.; Kim, J.M.; Lee, S.M.; Lee, M.Y. The value of ultrasonography in the detection of meniscal tears diagnosed by magnetic resonance imaging. Am. J. Phys. Med. Rehabil. 2008, 87, 14–20. [Google Scholar] [CrossRef]
- Bruce, W.; Lee, T.S.; Sundarajan, V.; Walker, P.; Magnussen, J.; Van der Wall, H. Performance characteristics of ultrasound of the knee in a general radiological setting. Knee 2004, 11, 303–306. [Google Scholar] [CrossRef]
- Petersen, L.J.; Rasmussen, O.S. UL-scanning som diagnostisk metode ved mistanke om menisklaesion i knaeet. Prospektiv blindet undersøgelse af 52 patienter [Ultrasonography as a diagnostic method in suspected meniscal lesion of the knee. A prospective single blind study of 52 patients]. Ugeskr. Lager 1999, 161, 5679–5682. [Google Scholar]
- Najafi, J.; Bagheri, S.; Lahiji, F.A. The value of sonography with micro convex probes in diagnosing meniscal tears compared with arthroscopy. J. Ultrasound Med. 2006, 25, 593–597. [Google Scholar] [CrossRef]
- Kelly, S.; Bombardieri, M.; Humby, F.; Ng, N.; Marrelli, A.; Riahi, S.; DiCicco, M.; Mahto, A.; Zou, L.; Pyne, D.; et al. Angiogenic gene expression and vascular density are reflected in ultrasonographic features of synovitis in early Rheumatoid Arthritis: An observational study. Arthritis Res. Ther. 2015, 17, 58. [Google Scholar] [CrossRef]
- Kaeley, G.S.; Nishio, M.J.; Goyal, J.R.; MacCarter, D.K.; Wells, A.F.; Chen, S.; Kupper, H.; Kalabic, J. Changes in Ultrasonographic Vascularity Upon Initiation of Adalimumab Combination Therapy in Rheumatoid Arthritis Patients With an Inadequate Response to Methotrexate. Arthritis Rheumatol. 2016, 68, 2584–2592. [Google Scholar] [CrossRef]
- Rawool, N.M.; Goldberg, B.B.; Forsberg, F.; Winder, A.A.; Hume, E. Power Doppler assessment of vascular changes during fracture treatment with low-intensity ultrasound. J. Ultrasound Med. 2003, 22, 145–153. [Google Scholar] [CrossRef]
- Bottinelli, O.; Calliada, F.; Campani, R. Bone callus: Possible assessment with color Doppler ultrasonography. Normal bone healing process. Radiol. Med. 1996, 91, 537–541. [Google Scholar]
- Pappa, E.I.; Barbagianni, M.S.; Georgiou, S.G.; Athanasiou, L.V.; Psalla, D.; Vekios, D.; Katsarou, E.I.; Vasileiou, N.G.C.; Gouletsou, P.G.; Galatos, A.D.; et al. The Use of Stromal Vascular Fraction in Long Bone Defect Healing in Sheep. Animals 2023, 13, 2871. [Google Scholar] [CrossRef]
- Jeon, S.; Jang, J.; Lee, G.; Park, S.; Lee, S.K.; Kim, H.; Choi, J. Assessment of neovascularization during bone healing using contrast-enhanced ultrasonography in a canine tibial osteotomy model: A preliminary study. J. Vet. Sci. 2020, 21, e10. [Google Scholar] [CrossRef]
- Song, I.H.; Althoff, C.E.; Hermann, K.G.; Scheel, A.K.; Knetsch, T.; Schoenharting, M.; Werner, C.; Burmester, G.R.; Backhaus, M. Knee osteoarthritis. Efficacy of a new method of contrast-enhanced musculoskeletal ultrasonography in detection of synovitis in patients with knee osteoarthritis in comparison with magnetic resonance imaging. Ann. Rheum. Dis. 2008, 67, 19–25. [Google Scholar] [CrossRef]
- Drakonaki, E.E.; Allen, G.M.; Wilson, D.J. Ultrasound elastography for musculoskeletal applications. Br. J. Radiol. 2012, 85, 1435–1445. [Google Scholar] [CrossRef] [PubMed]
- Sigrist, R.M.S.; Liau, J.; Kaffas, A.E.; Chammas MCWillmann, J.K. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics 2017, 7, 1303–1329. [Google Scholar] [CrossRef] [PubMed]
- Holdsworth, A.; Bradley, K.; Birch, S.; Browne, W.J.; Barberet, V. Elastography of the normal canine liver, spleen and kidneys. Vet. Radiol. Ultrasound 2014, 55, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Pownder, S.L.; Hayashi, K.; Lin, B.Q.; Meyers, K.N.; Caserto, B.G.; Breighner, R.E.; Potter, H.G.; Koff, M.F. Differences in the magnetic resonance imaging parameter T2* may be identified during the course of canine patellar tendon healing: A pilot study. Quant. Imaging Med. Surg. 2021, 11, 1234–1246. [Google Scholar] [CrossRef]
- Yurdaışık, I. Comparison of two-dimensional shear wave elastography and point shear wave elastography techniques with magnetic resonance findings in detection of patellar tendinopathy. Jt. Dis. Relat. Surg. 2019, 30, 275–281. [Google Scholar] [CrossRef]
- Gennisson, J.L.; Deffieux, T.; Fink, M.; Tanter, M. Ultrasound elastography: Principles and techniques. Diagn. Interv. Imaging 2013, 94, 487–495. [Google Scholar] [CrossRef]
- Ralphs, S.C.; Whitney, W.O. Arthroscopic evaluation of menisci in dogs with cranial cruciate ligament injuries: 100 cases (1999–2000). J. Am. Vet. Med. Assoc. 2002, 221, 1601–1604. [Google Scholar] [CrossRef]
- Williams, J.; Tomlinson; Constantinescu, G.M. Diagnosing and treating meniscal injuries in the dog. Vet. Med. 1994, 89, 42–47. [Google Scholar]
- Widmer, W.R.; Buckwalter, K.A.; Braunstein, E.M.; Visco, D.M.; O’Connor, B.L. Principles of magnetic resonance imaging and application to the stifle joint in dogs. J. Am. Vet. Med. Assoc. 1991, 198, 1914–1922. [Google Scholar] [CrossRef]
- Kivumbi, C.W.; Bennett, D. Arthroscopy of the canine stifle joint. Vet. Rec. 1981, 109, 241–249. [Google Scholar] [CrossRef]
- Warden, S.J.; Kiss, Z.S.; Malara, F.A.; Ooi, A.B.; Cook, J.L.; Crossley, K.M. Comparative accuracy of magnetic resonance imaging and ultrasonography in confirming clinically diagnosed patellar tendinopathy. Am. J. Sports Med. 2007, 35, 427–436. [Google Scholar] [CrossRef]
- Cook, J.L.; Tomlinson, J.L.; Kreeger, J.M.; Cook, C.R. Induction of meniscal regeneration in dogs using a novel biomaterial. Am. J. Sports Med. 1999, 27, 658–665. [Google Scholar] [CrossRef]
- Cook, J.L.; Tomlinson, J.L.; Arnoczky, S.P.; Fox, D.B.; Cook, R.C.; Kreeger, J.M. Kinetic study of the replacement of porcine small intestinal submucosa grafts and the regeneration of meniscal-like tissue in large avascular meniscal defects in dogs. Tissue Eng. 2001, 7, 321–334. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karatrantos, A.T.; Sideri, A.I.; Gouletsou, P.G.; Bektsi, C.G.; Barbagianni, M.S. Ultrasound Imaging Modalities in the Evaluation of the Dog’s Stifle Joint. Vet. Sci. 2025, 12, 734. https://doi.org/10.3390/vetsci12080734
Karatrantos AT, Sideri AI, Gouletsou PG, Bektsi CG, Barbagianni MS. Ultrasound Imaging Modalities in the Evaluation of the Dog’s Stifle Joint. Veterinary Sciences. 2025; 12(8):734. https://doi.org/10.3390/vetsci12080734
Chicago/Turabian StyleKaratrantos, Anargyros T., Aikaterini I. Sideri, Pagona G. Gouletsou, Christina G. Bektsi, and Mariana S. Barbagianni. 2025. "Ultrasound Imaging Modalities in the Evaluation of the Dog’s Stifle Joint" Veterinary Sciences 12, no. 8: 734. https://doi.org/10.3390/vetsci12080734
APA StyleKaratrantos, A. T., Sideri, A. I., Gouletsou, P. G., Bektsi, C. G., & Barbagianni, M. S. (2025). Ultrasound Imaging Modalities in the Evaluation of the Dog’s Stifle Joint. Veterinary Sciences, 12(8), 734. https://doi.org/10.3390/vetsci12080734




