The Effect of Cannabidiol in Conjunction with Radiation Therapy on Canine Glioma Cell Line Transplanted in Immunodeficient Mice
Simple Summary
Abstract
1. Introduction
2. Material and Methods
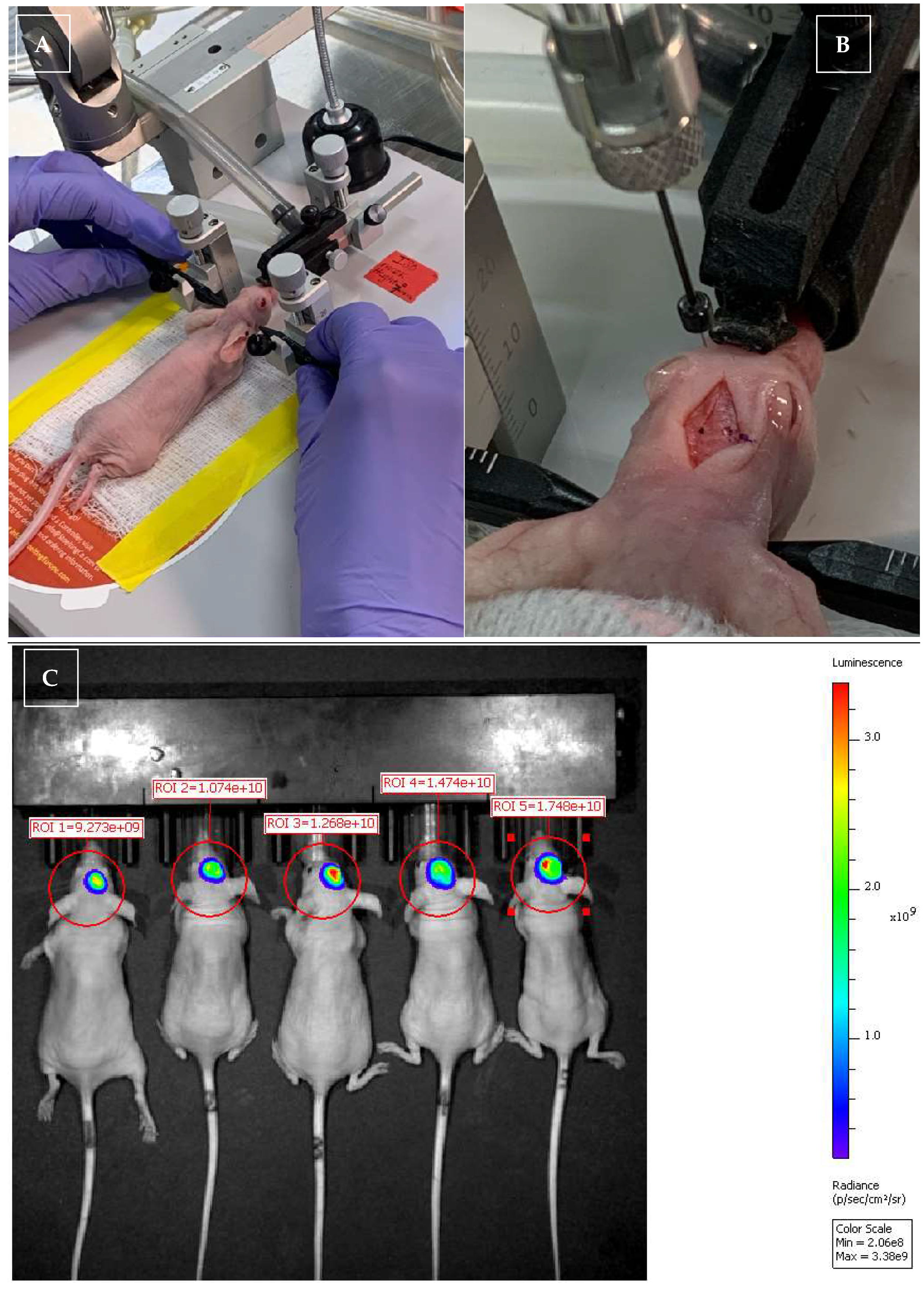
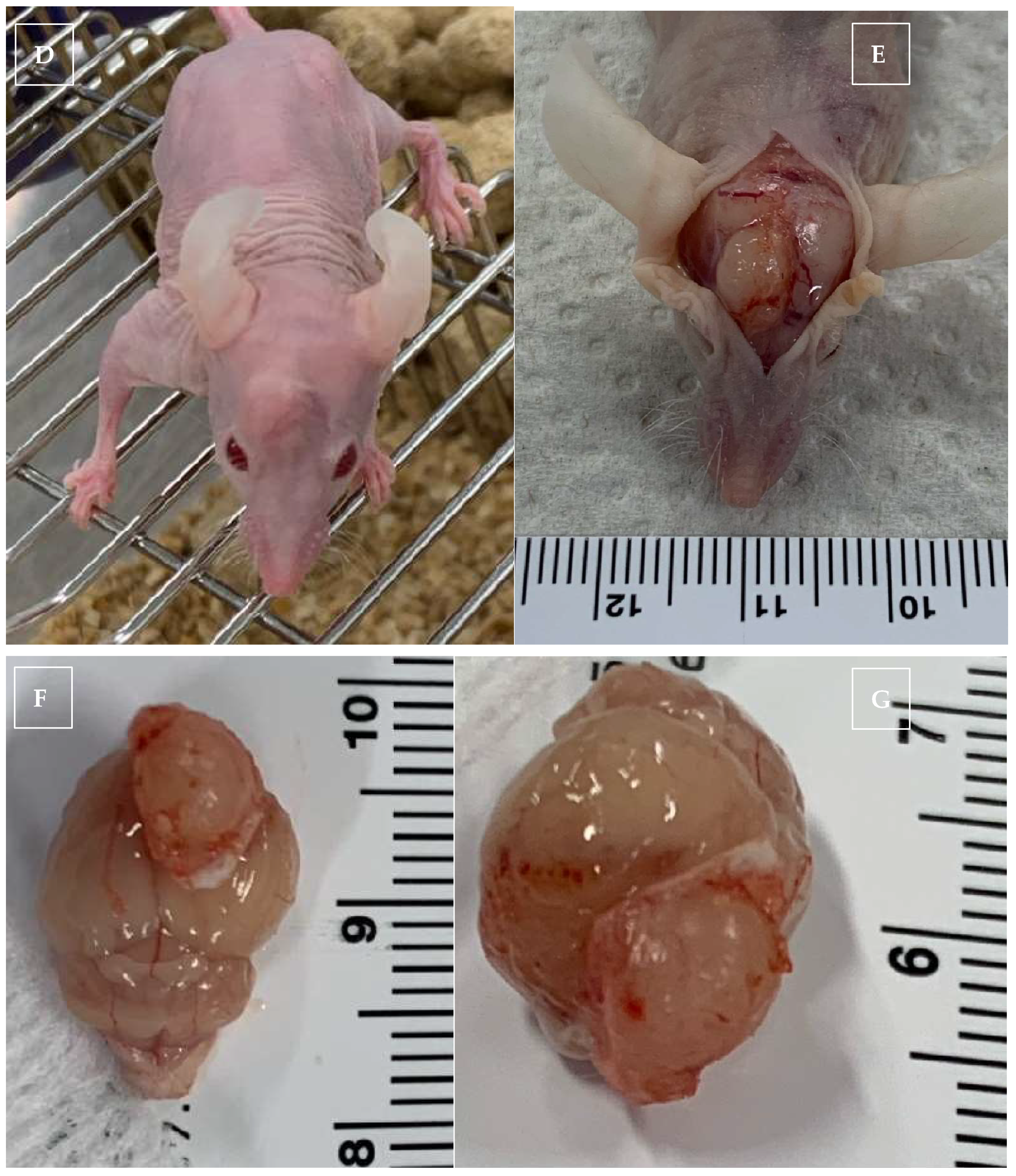
2.1. In Vivo Xenogeneic Orthotopic Glioma Transplantation
2.2. Radiation Therapy (RT)
2.3. CBD IP Injection
2.4. IVIS Imaging for Monitoring Glioma Size in the Brain
2.5. Monitoring for Short-Term and Long-Term Effects
2.6. Harvesting of the Blood and Brain for CBD Concentration and Histopathology
2.7. CBD Extraction Procedure for Plasm
2.8. CBD Extraction Procedure for Brain Samples
2.9. Statistical Analysis
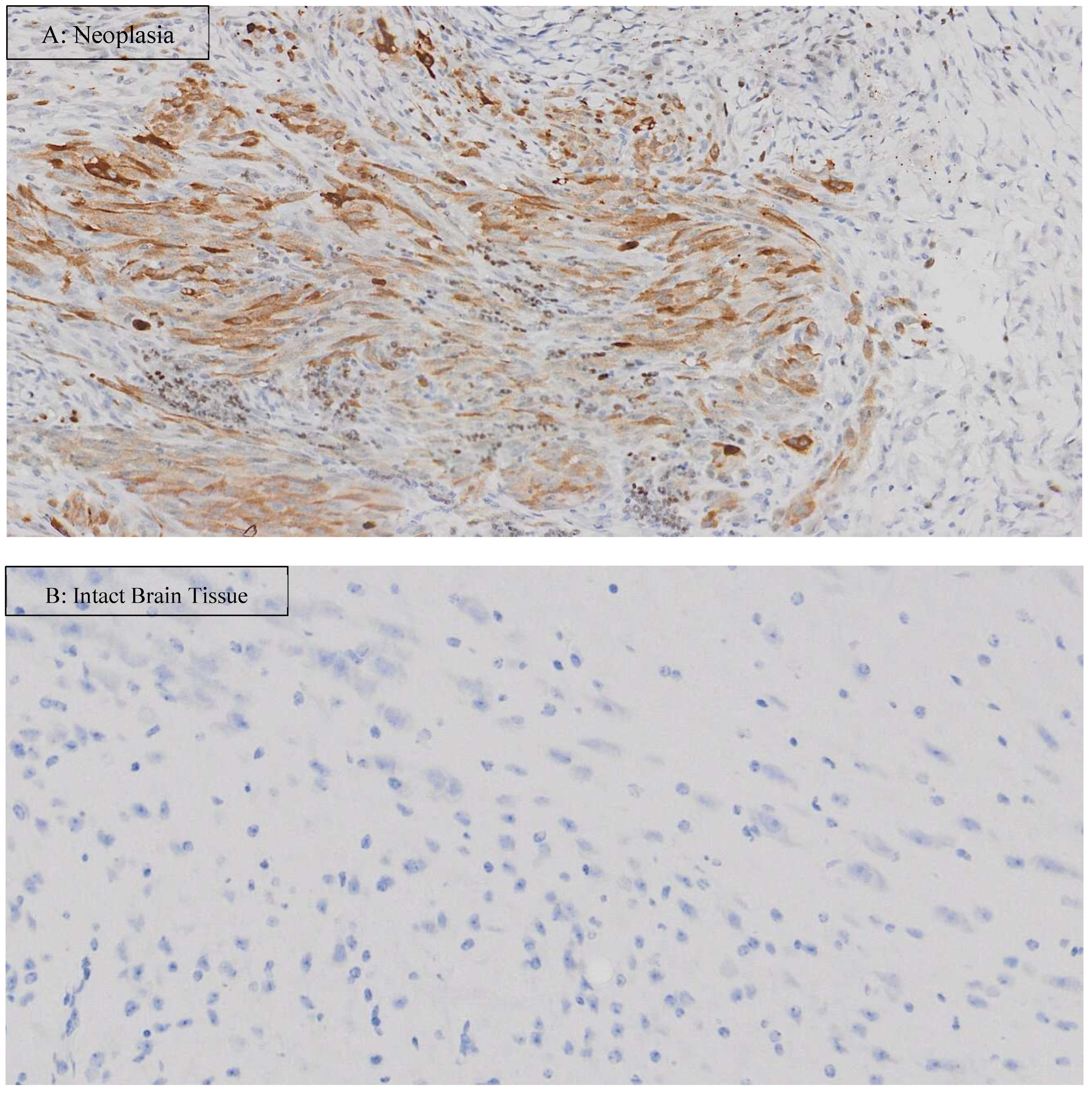
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koehler, J.W.; Miller, A.D.; Miller, C.R.; Porter, B.; Aldape, K.; Beck, J.; Brat, D.; Cornax, I.; Corps, K.; Frank, C.; et al. A Revised Diagnostic Classification of Canine Glioma: Towards Validation of the Canine Glioma Patient as a Naturally Occurring Preclinical Model for Human Glioma. J. Neuropathol. Exp. Neurol. 2018, 77, 1039–1054. [Google Scholar] [CrossRef]
- Krane, G.A.; Shockley, K.R.; Malarkey, D.E.; Miller, A.D.; Miller, C.R.; Tokarz, D.A.; Jensen, H.L.; Janardhan, K.S.; Breen, M.; Mariani, C.L. Inter-Pathologist Agreement on Diagnosis, Classification and Grading of Canine Glioma. Vet. Comp. Oncol. 2022, 20, 881–889. [Google Scholar] [CrossRef]
- Belluco, S.; Avallone, G.; Di Palma, S.; Rasotto, R.; Oevermann, A. Inter- and Intraobserver Agreement of Canine and Feline Nervous System Tumors. Vet. Pathol. 2019, 56, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of Radiotherapy with Concomitant and Adjuvant Temozolomide versus Radiotherapy Alone on Survival in Glioblastoma in a Randomised Phase III Study: 5-Year Analysis of the EORTC-NCIC Trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Rossmeisl, J.H.; Jones, J.C.; Zimmerman, K.L.; Robertson, J.L. Survival Time Following Hospital Discharge in Dogs with Palliatively Treated Primary Brain Tumors. J. Am. Vet. Med. Assoc. 2013, 242, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Barker, A.; Harcourt-Brown, T.; Jeffery, N. Systematic Review of Brain Tumor Treatment in Dogs. J. Vet. Intern. Med. 2015, 29, 1456–1463. [Google Scholar] [CrossRef]
- Uhm, J.H.; Porter, A.B. Treatment of Glioma in the 21st Century: An Exciting Decade of Postsurgical Treatment Advances in the Molecular Era. Mayo Clin. Proc. 2017, 92, 995–1004. [Google Scholar] [CrossRef]
- Elsohly, M.A.; Slade, D. Chemical Constituents of Marijuana: The Complex Mixture of Natural Cannabinoids. Life Sci. 2005, 78, 539–548. [Google Scholar] [CrossRef]
- Liu, W.M.; Scott, K.A.; Shamash, J.; Joel, S.; Powles, T.B. Enhancing the in Vitro Cytotoxic Activity of Delta9-Tetrahydrocannabinol in Leukemic Cells through a Combinatorial Approach. Leuk. Lymphoma 2008, 49, 1800–1809. [Google Scholar] [CrossRef]
- Powles, T.; te Poele, R.; Shamash, J.; Chaplin, T.; Propper, D.; Joel, S.; Oliver, T.; Liu, W.M. Cannabis-Induced Cytotoxicity in Leukemic Cell Lines: The Role of the Cannabinoid Receptors and the MAPK Pathway. Blood 2005, 105, 1214–1221. [Google Scholar] [CrossRef]
- Solinas, M.; Massi, P.; Cantelmo, A.R.; Cattaneo, M.G.; Cammarota, R.; Bartolini, D.; Cinquina, V.; Valenti, M.; Vicentini, L.M.; Noonan, D.M.; et al. Cannabidiol Inhibits Angiogenesis by Multiple Mechanisms. Br. J. Pharmacol. 2012, 167, 1218–1231. [Google Scholar] [CrossRef]
- Liu, W.M.; Fowler, D.W.; Dalgleish, A.G. Cannabis-Derived Substances in Cancer Therapy--an Emerging Anti-Inflammatory Role for the Cannabinoids. Curr. Clin. Pharmacol. 2010, 5, 281–287. [Google Scholar] [CrossRef]
- Thiele, E.A.; Bebin, E.M.; Bhathal, H.; Jansen, F.E.; Kotulska, K.; Lawson, J.A.; O’Callaghan, F.J.; Wong, M.; Sahebkar, F.; Checketts, D.; et al. Add-on Cannabidiol Treatment for Drug-Resistant Seizures in Tuberous Sclerosis Complex: A Placebo-Controlled Randomized Clinical Trial. JAMA Neurol. 2021, 78, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.D.; Mazurkiewicz-Bełdzińska, M.; Chin, R.F.; Gil-Nagel, A.; Gunning, B.; Halford, J.J.; Mitchell, W.; Scott Perry, M.; Thiele, E.A.; Weinstock, A.; et al. Long-Term Safety and Efficacy of Add-on Cannabidiol in Patients with Lennox-Gastaut Syndrome: Results of a Long-Term Open-Label Extension Trial. Epilepsia 2021, 62, 2228–2239. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Verducci, C.; Thiele, E.A.; Laux, L.C.; Patel, A.D.; Filloux, F.; Szaflarski, J.P.; Wilfong, A.; Clark, G.D.; Park, Y.D.; et al. Open-Label Use of Highly Purified CBD (Epidiolex®) in Patients with CDKL5 Deficiency Disorder and Aicardi, Dup15q, and Doose Syndromes. Epilepsy Behav. EB 2018, 86, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Hong, M.; Han, J.-H.; Ryu, B.R.; Lim, Y.S.; Lim, J.D.; Kim, C.H.; Lee, S.-U.; Kwon, T.-H. In Vitro and In Vivo Anti-Inflammatory Effects of Cannabidiol Isolated from Novel Hemp (Cannabis Sativa L.) Cultivar Pink Pepper. Molecules 2023, 28, 6439. [Google Scholar] [CrossRef]
- Massi, P.; Solinas, M.; Cinquina, V.; Parolaro, D. Cannabidiol as Potential Anticancer Drug. Br. J. Clin. Pharmacol. 2013, 75, 303–312. [Google Scholar] [CrossRef]
- Jacobsson, S.O.P.; Rongård, E.; Stridh, M.; Tiger, G.; Fowler, C.J. Serum-Dependent Effects of Tamoxifen and Cannabinoids upon C6 Glioma Cell Viability. Biochem. Pharmacol. 2000, 60, 1807–1813. [Google Scholar] [CrossRef]
- Calvaruso, G.; Pellerito, O.; Notaro, A.; Giuliano, M. Cannabinoid-Associated Cell Death Mechanisms in Tumor Models (Review). Int. J. Oncol. 2012, 41, 407–413. [Google Scholar] [CrossRef][Green Version]
- Gross, C.; Ramirez, D.A.; McGrath, S.; Gustafson, D.L. Cannabidiol Induces Apoptosis and Perturbs Mitochondrial Function in Human and Canine Glioma Cells. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef]
- Herranz, C.; Fernández, F.; Martín-Ibáñez, R.; Blasco, E.; Crespo, E.; De la Fuente, C.; Añor, S.; Rabanal, R.M.; Canals, J.M.; Pumarola, M. Spontaneously Arising Canine Glioma as a Potential Model for Human Glioma. J. Comp. Pathol. 2016, 154, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Duke, S.E.; Wang, H.J.; Breen, T.E.; Higgins, R.J.; Linder, K.E.; Ellis, P.; Langford, C.F.; Dickinson, P.J.; Olby, N.J.; et al. ‘Putting Our Heads Together’: Insights into Genomic Conservation between Human and Canine Intracranial Tumors. J. Neurooncol. 2009, 94, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.A.; Dalgleish, A.G.; Liu, W.M. The Combination of Cannabidiol and Δ9-Tetrahydrocannabinol Enhances the Anticancer Effects of Radiation in an Orthotopic Murine Glioma Model. Mol. Cancer Ther. 2014, 13, 2955–2967. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, M.E.; Arnold, S.; Bin Zahid, A.; McPheeters, M.; Gerard O’Sullivan, M.; Tabaran, A.-F.; Hunt, M.A.; Pluhar, G.E. Naturally Occurring Canine Glioma as a Model for Novel Therapeutics. Cancer Investig. 2018, 36, 415–423. [Google Scholar] [CrossRef]
- Hicks, W.H.; Bird, C.E.; Pernik, M.N.; Haider, A.S.; Dobariya, A.; Abdullah, K.G.; Aoun, S.G.; Bentley, R.T.; Cohen-Gadol, A.A.; Bachoo, R.M.; et al. Large Animal Models of Glioma: Current Status and Future Prospects. Anticancer. Res. 2021, 41, 5343–5353. [Google Scholar] [CrossRef]
- Snyder, J.M.; Shofer, F.S.; Van Winkle, T.J.; Massicotte, C. Canine Intracranial Primary Neoplasia: 173 Cases (1986–2003). J. Vet. Intern. Med. 2006, 20, 669–675. [Google Scholar] [CrossRef]
- Fuchs, D.; Rohrer Bley, C.; Morandi, L.; Tonon, C.; Weyland, M.S.; Nytko, K.J. Triple Combination of Lomustine, Temozolomide and Irradiation Reduces Canine Glioma Cell Survival in Vitro. Vet. Med. Sci. 2023, 9, 1573–1583. [Google Scholar] [CrossRef]
- Tresch, N.S.; Fuchs, D.; Morandi, L.; Tonon, C.; Rohrer Bley, C.; Nytko, K.J. Temozolomide Is Additive with Cytotoxic Effect of Irradiation in Canine Glioma Cell Lines. Vet. Med. Sci. 2021, 7, 2124–2134. [Google Scholar] [CrossRef]
- Bonomi, A.; Ghezzi, E.; Pascucci, L.; Aralla, M.; Ceserani, V.; Pettinari, L.; Coccè, V.; Guercio, A.; Alessandri, G.; Parati, E.; et al. Effect of Canine Mesenchymal Stromal Cells Loaded with Paclitaxel on Growth of Canine Glioma and Human Glioblastoma Cell Lines. Vet. J. 2017, 223, 41–47. [Google Scholar] [CrossRef]
- Yaghi, N.K.; Wei, J.; Hashimoto, Y.; Kong, L.-Y.; Gabrusiewicz, K.; Nduom, E.K.; Ling, X.; Huang, N.; Zhou, S.; Kerrigan, B.C.P.; et al. Immune Modulatory Nanoparticle Therapeutics for Intracerebral Glioma. Neuro-Oncol. 2017, 19, 372–382. [Google Scholar] [CrossRef]
- Yoshida, K.; Chambers, J.K.; Uchida, K. Immunohistochemical Study of Neural Stem Cell Lineage Markers in Canine Brains, Gliomas, and a Glioma Cell Line. Vet. Pathol. 2023, 60, 35–46. [Google Scholar] [CrossRef]
- Rainov, N.G.; Koch, S.; Sena-Esteves, M.; Berens, M.E. Characterization of a Canine Glioma Cell Line as Related to Established Experimental Brain Tumor Models. J. Neuropathol. Exp. Neurol. 2000, 59, 607–613. [Google Scholar] [CrossRef]
- Schrock, M.S.; Zalenski, A.A.; Tallman, M.M.; Kollin, L.; Bratasz, A.; Weeks, G.; Miller, M.A.; Sweeney, C.N.; Pluhar, G.E.; Olin, M.R.; et al. Establishment and Characterization of Two Novel Patient-Derived Lines from Canine High-Grade Glioma. Vet. Comp. Oncol. 2023, 21, 492–502. [Google Scholar] [CrossRef]
- Berens, M.E.; Giese, A.; Shapiro, J.R.; Coons, S.W. Allogeneic Astrocytoma in Immune Competent Dogs. Neoplasia 1999, 1, 107–112. [Google Scholar] [CrossRef]
- Hermanson, D.J.; Marnett, L.J. Cannabinoids, Endocannabinoids and Cancer. Cancer Metastasis Rev. 2011, 30, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Laezza, C.; Pagano, C.; Navarra, G.; Pastorino, O.; Proto, M.C.; Fiore, D.; Piscopo, C.; Gazzerro, P.; Bifulco, M. The Endocannabinoid System: A Target for Cancer Treatment. Int. J. Mol. Sci. 2020, 21, 747. [Google Scholar] [CrossRef] [PubMed]
- Rocha, F.C.M.; Dos Santos Júnior, J.G.; Stefano, S.C.; da Silveira, D.X. Systematic Review of the Literature on Clinical and Experimental Trials on the Antitumor Effects of Cannabinoids in Gliomas. J. Neurooncol. 2014, 116, 11–24. [Google Scholar] [CrossRef]
- Deiana, S.; Watanabe, A.; Yamasaki, Y.; Amada, N.; Arthur, M.; Fleming, S.; Woodcock, H.; Dorward, P.; Pigliacampo, B.; Close, S.; et al. Plasma and Brain Pharmacokinetic Profile of Cannabidiol (CBD), Cannabidivarine (CBDV), Δ9-Tetrahydrocannabivarin (THCV) and Cannabigerol (CBG) in Rats and Mice Following Oral and Intraperitoneal Administration and CBD Action on Obsessive-Compulsive Behaviour. Psychopharmacology 2012, 219, 859–873. [Google Scholar] [CrossRef]
- Chen, W.-J.; He, D.-S.; Tang, R.-X.; Ren, F.-H.; Chen, G. Ki-67 Is a Valuable Prognostic Factor in Gliomas: Evidence from a Systematic Review and Meta-Analysis. Asian Pac. J. Cancer Prev. APJCP 2015, 16, 411–420. [Google Scholar] [CrossRef]
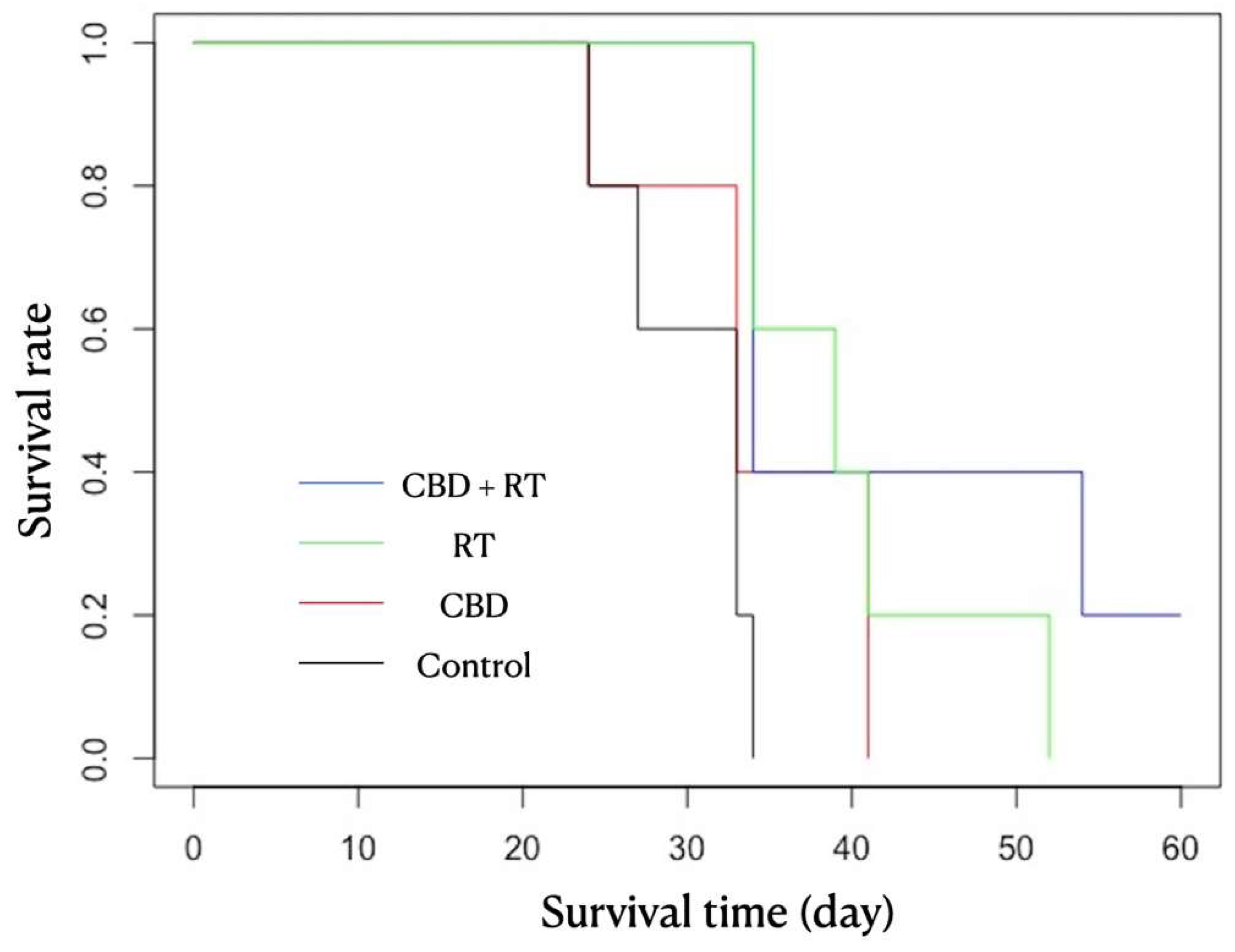
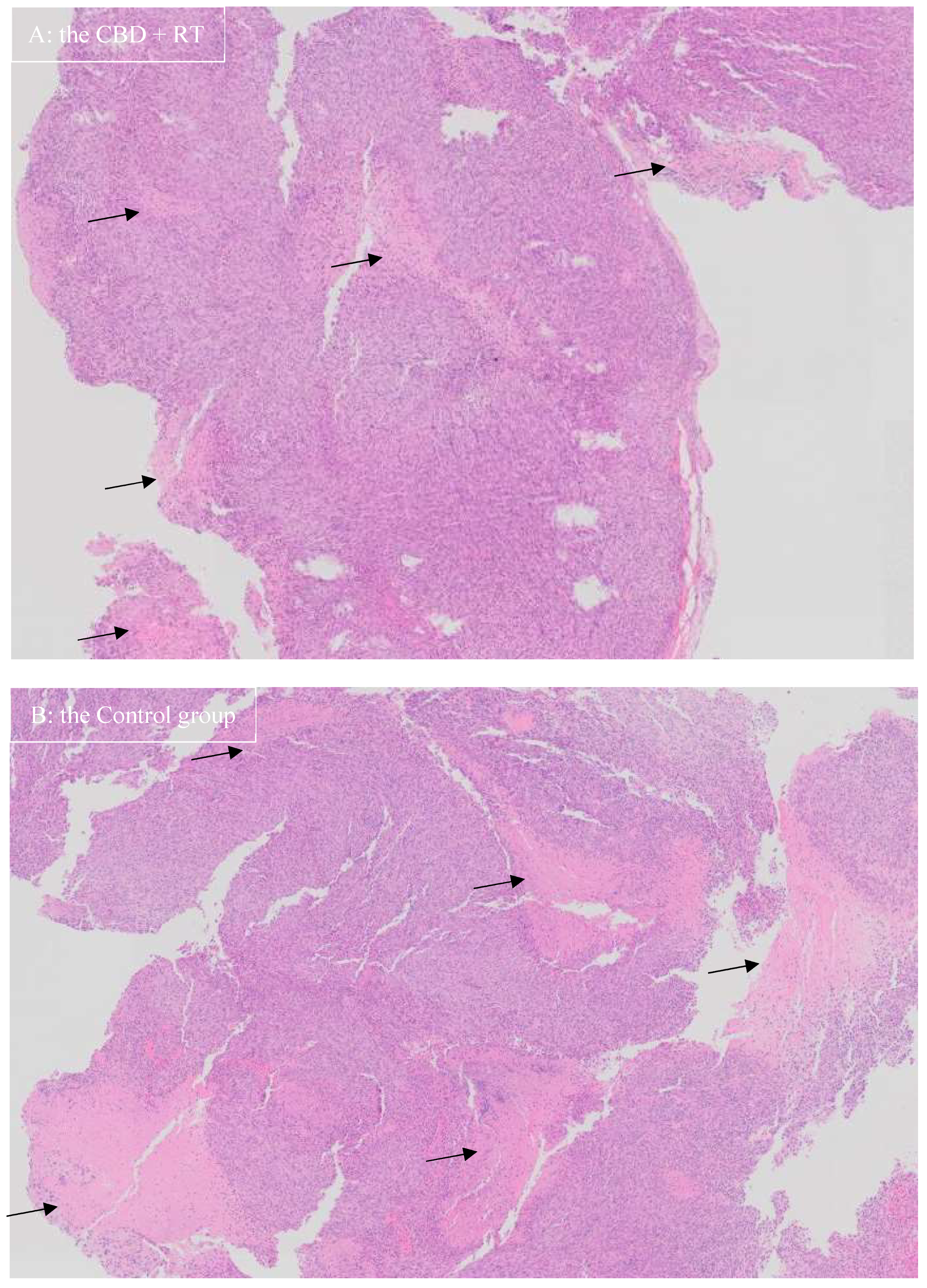
| Group | Mouse | CBD/Vehicle IP Injection Daily | RT | Neurological Signs | Survival Time (day) | Mean (Median) Survival Time |
|---|---|---|---|---|---|---|
| Control | 1 | Vehicle | n/a | - | 33 | 30.2 (33) |
| 2 | - | 33 | ||||
| 3 | - | 34 | ||||
| 4 | - | 24 | ||||
| 5 | - | 27 | ||||
| CBD | 1 | CBD 30 mg/kg q24 h | n/a | - | 24 | 34.4 (33) |
| 2 | + | 33 | ||||
| 3 | - | 41 | ||||
| 4 | + | 41 | ||||
| 5 | - | 33 | ||||
| RT | 1 | Vehicle | 5.5 Gy once | - | 34 | 40 (39) |
| 2 | - | 52 | ||||
| 3 | - | 41 | ||||
| 4 | - | 39 | ||||
| 5 | - | 34 | ||||
| CBD + RT | 1 | CBD 30 mg/kg q24 h | 5.5 Gy once | - | 34 | 43.2 (34) |
| 2 | - | 34 | ||||
| 3 | - | 54 | ||||
| 4 | - | 60 | ||||
| 5 | - | 34 |
| Group | Mouse | CBD Level in Plasma (ng/mL) | Mean (Median) (ng/mL) | CBD Level in Neoplasia (ng/mL) | Mean (Median) (ng/mL) | CBD Level in Intact Brain (ng/mL) | Mean (Median) (ng/mL) |
|---|---|---|---|---|---|---|---|
| Control | 1 | 0 | 0 (0) | 0 | 0 (0) | 0 | 0 (0) |
| 2 | 0 | 0 | 0 | ||||
| 3 | 0 | 0 | 0 | ||||
| 4 | 0 | 0 | 0 | ||||
| 5 | 0 | 0 | 0 | ||||
| CBD | 1 | 181 | 181.6 (181) | 496 | 355.6 (376) | 579 | 403.2 (456) |
| 2 | 208 | 376 | 471 | ||||
| 3 | 158 | 182 | 277 | ||||
| 4 | 166 | 257 | 233 | ||||
| 5 | 195 | 467 | 456 | ||||
| RT | 1 | 0 | 0 (0) | 0 | 0 (0) | 0 | 0 (0) |
| 2 | 0 | 0 | 0 | ||||
| 3 | 0 | 0 | 0 | ||||
| 4 | 0 | 0 | 0 | ||||
| 5 | 0 | 0 | 0 | ||||
| CBD + RT | 1 | 101 | 145.2 (163) | 181 | 258.68 (320) | 176 | 269.2 (253) |
| 2 | 167 | 320 | 250 | ||||
| 3 | 163 | 93.4 | 379 | ||||
| 4 | 164 | 334 | 253 | ||||
| 5 | 131 | 365 | 288 |
| Group | Mouse | Neoplasia | Intact Brain Tissue | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Positive Nuclei% | Mean (Median) | Positive Cell number/μm2 | Mean (Median) | Positive Nuclei | Mean (Median) | Positive Cell Number/μm2 | Mean (Median) | ||
| Control | 1 | 0.201 | 0.235 (0.177) SD 0.291 | 14.580 | 15.364 (10.866) SD 18.893 | 0.037 | 0.051 (0.051) SD 0.010 | 1.060 | 1.427 (1.420) SD 0.256 |
| 2 | 0.736 | 47.637 | 0.049 | 1.411 | |||||
| 3 | 0.177 | 10.886 | 0.051 | 1.420 | |||||
| 4 | 0.016 | 1.228 | 0.065 | 1.781 | |||||
| 5 | 0.046 | 2.492 | 0.051 | 1.460 | |||||
| CBD | 1 | 3.049 | 1.124 (0.774) SD 1.097 | 158.374 | 62.336 (47.833) SD 56.198 | 0.245 | 0.620 (0.342) SD 0.733 | 5.733 | 16.086 (9.070) SD 18.384 |
| 2 | 0.774 | 60.226 | 0.447 | 12.841 | |||||
| 3 | 0.811 | 47.833 | 1.916 | 48.444 | |||||
| 4 | 0.707 | 27.120 | 0.150 | 4.340 | |||||
| 5 | 0.280 | 18.125 | 0.342 | 9.070 | |||||
| RT | 1 | 3.589 | 1.590 (1.282) SD 1.196 | 223.949 | 95.587 (64.529) SD 77.196 | 0.108 | 0.485 (0.370) SD 0.446 | 3.269 | 11.927 (8.374) SD 10.516 |
| 2 | 0.586 | 35.399 | 1.254 | 30.236 | |||||
| 3 | 1.282 | 64.529 | 0.272 | 8.180 | |||||
| 4 | 0.808 | 44.746 | 0.422 | 9.576 | |||||
| 5 | 1.686 | 109.311 | 0.370 | 8.374 | |||||
| CBD + RT | 1 | 0.632 | 0.511 (0.531) SD 0.217 | 47.674 | 27.999 (27.658) SD 17.218 | 0.050 | 0.218 (0.128) SD 0.220 | 1.344 | 5.009 (3.071) SD 4.498 |
| 2 | 0.531 | 27.658 | 0.595 | 12.482 | |||||
| 3 | 0.408 | 18.630 | 0.098 | 2.322 | |||||
| 4 | 0.207 | 4.835 | 0.221 | 5.828 | |||||
| 5 | 0.775 | 41.200 | 0.128 | 3.071 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ukai, M.; Kurihara, J.; Antonakakis, M.; Banks, K.; Dow, S.; Gustafson, D.L.; Boss, M.-K.; Prebble, A.; McGrath, S. The Effect of Cannabidiol in Conjunction with Radiation Therapy on Canine Glioma Cell Line Transplanted in Immunodeficient Mice. Vet. Sci. 2025, 12, 735. https://doi.org/10.3390/vetsci12080735
Ukai M, Kurihara J, Antonakakis M, Banks K, Dow S, Gustafson DL, Boss M-K, Prebble A, McGrath S. The Effect of Cannabidiol in Conjunction with Radiation Therapy on Canine Glioma Cell Line Transplanted in Immunodeficient Mice. Veterinary Sciences. 2025; 12(8):735. https://doi.org/10.3390/vetsci12080735
Chicago/Turabian StyleUkai, Masayasu, Jade Kurihara, Markos Antonakakis, Krista Banks, Steve Dow, Daniel L. Gustafson, Mary-Keara Boss, Amber Prebble, and Stephanie McGrath. 2025. "The Effect of Cannabidiol in Conjunction with Radiation Therapy on Canine Glioma Cell Line Transplanted in Immunodeficient Mice" Veterinary Sciences 12, no. 8: 735. https://doi.org/10.3390/vetsci12080735
APA StyleUkai, M., Kurihara, J., Antonakakis, M., Banks, K., Dow, S., Gustafson, D. L., Boss, M.-K., Prebble, A., & McGrath, S. (2025). The Effect of Cannabidiol in Conjunction with Radiation Therapy on Canine Glioma Cell Line Transplanted in Immunodeficient Mice. Veterinary Sciences, 12(8), 735. https://doi.org/10.3390/vetsci12080735







