PD-1, PD-L1, and PD-L2 Expression as Predictive Markers in Rare Feline Mammary Tumors
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Immunohistochemical Staining and Analysis
2.2. Statistical Analysis
3. Results
3.1. Animals
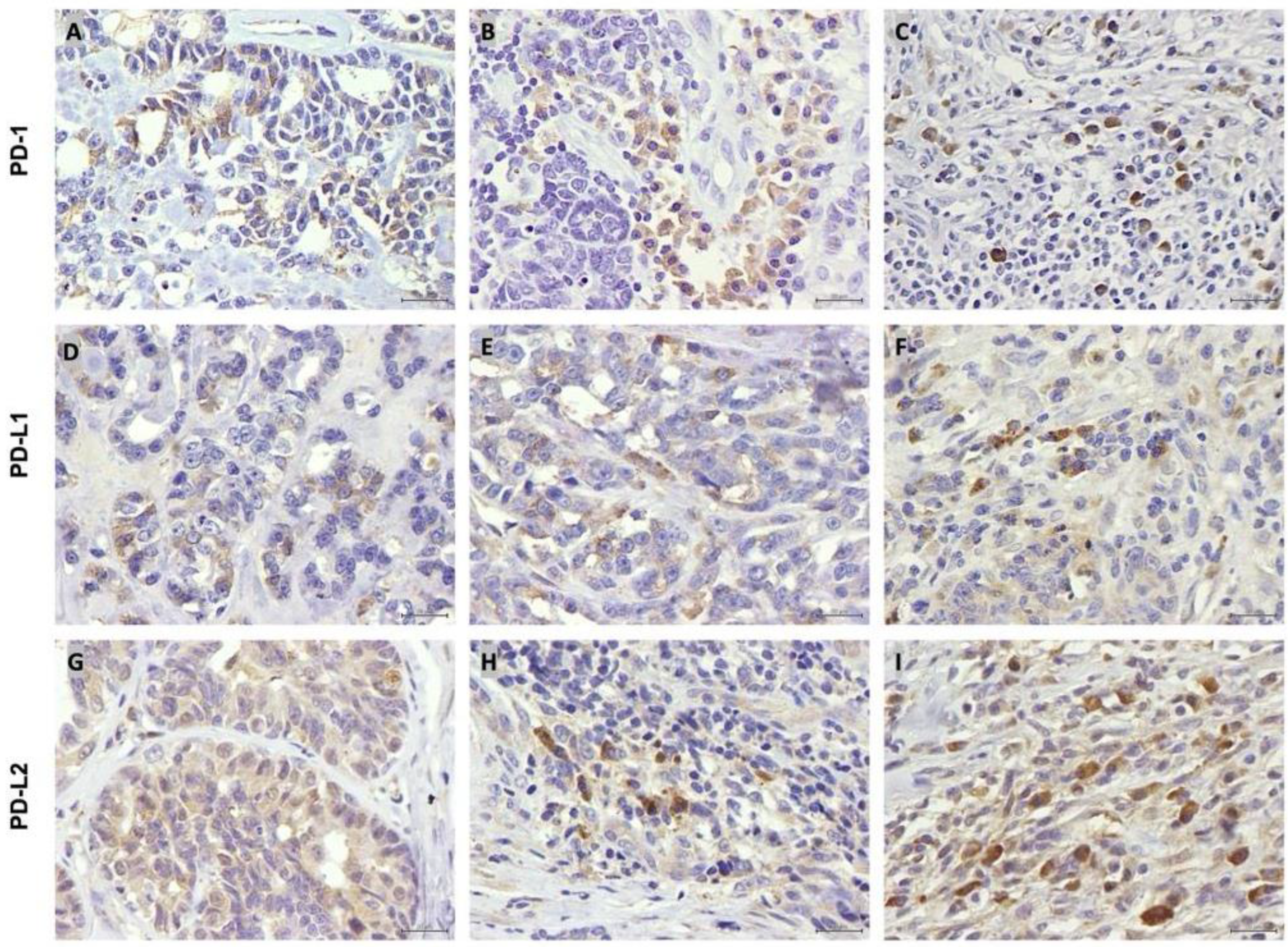
3.2. PD-1, PD-L1 and PD-L2 Are Expressed by Tumor Cells and TILs
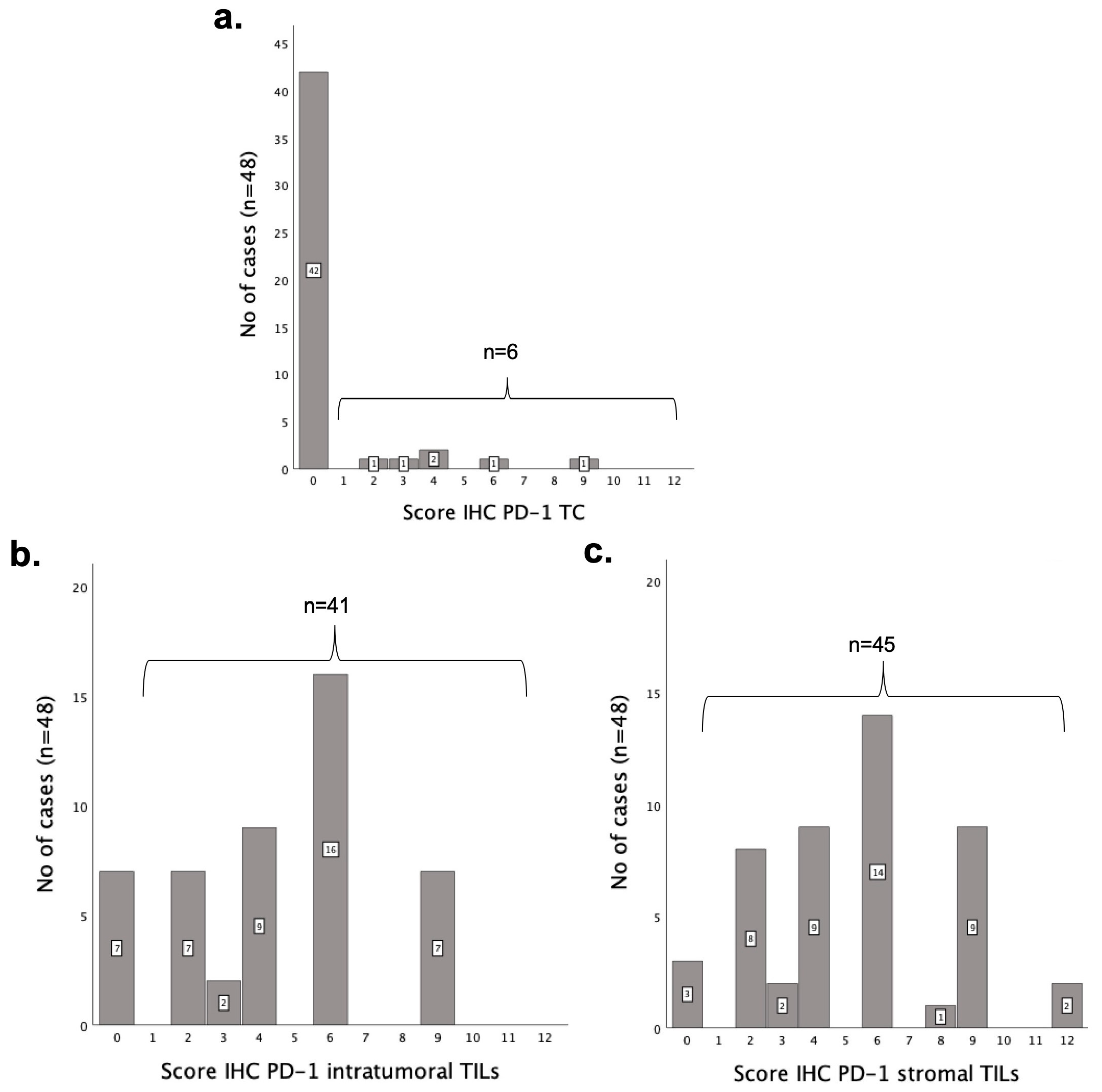
3.3. PD-1 Is More Highly Expressed in TILs than in Tumor Cells
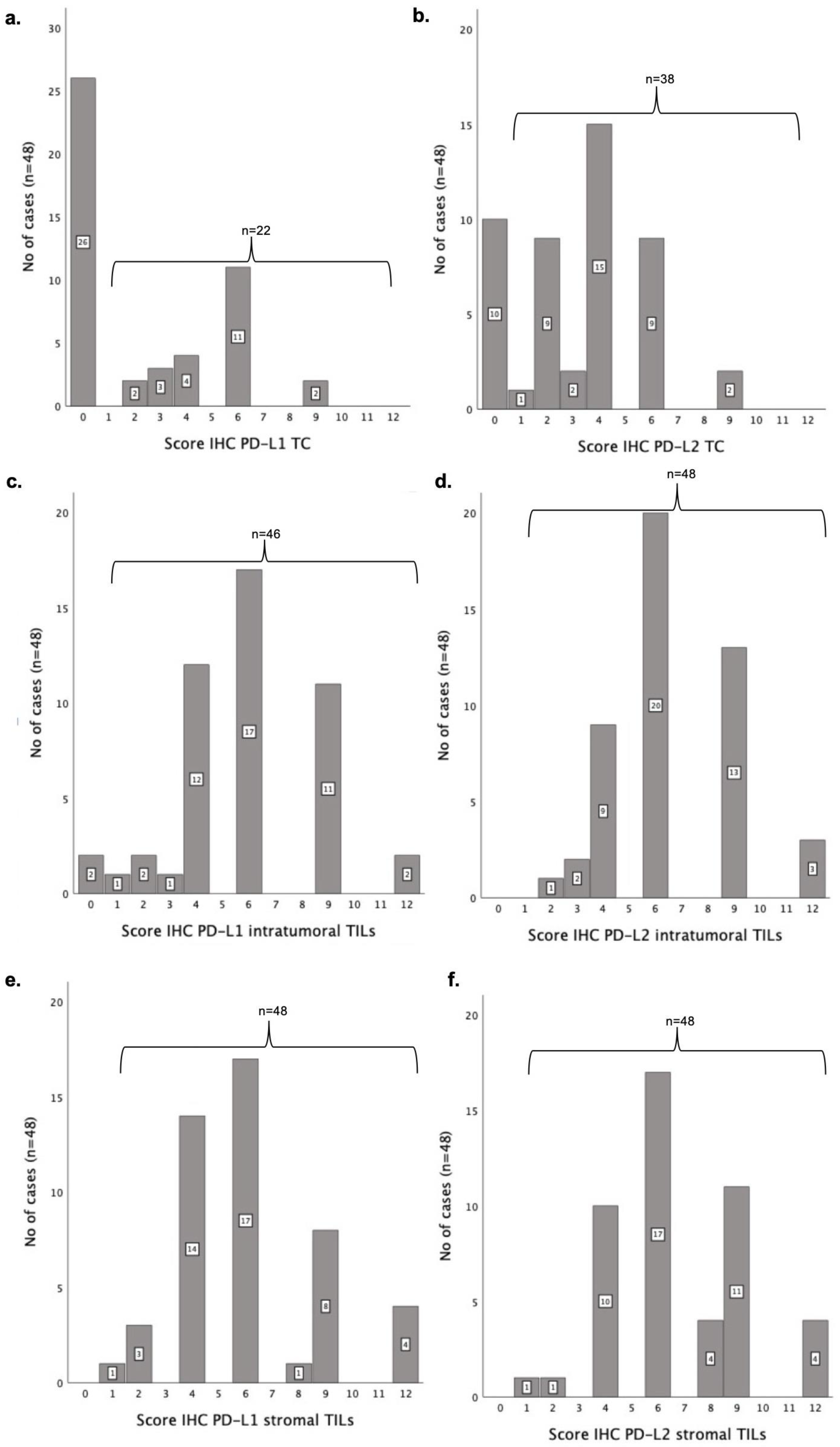
3.4. PD-L1 Is Expressed at Lower Levels than PD-L2 Both in Tumoral Cells and TILs
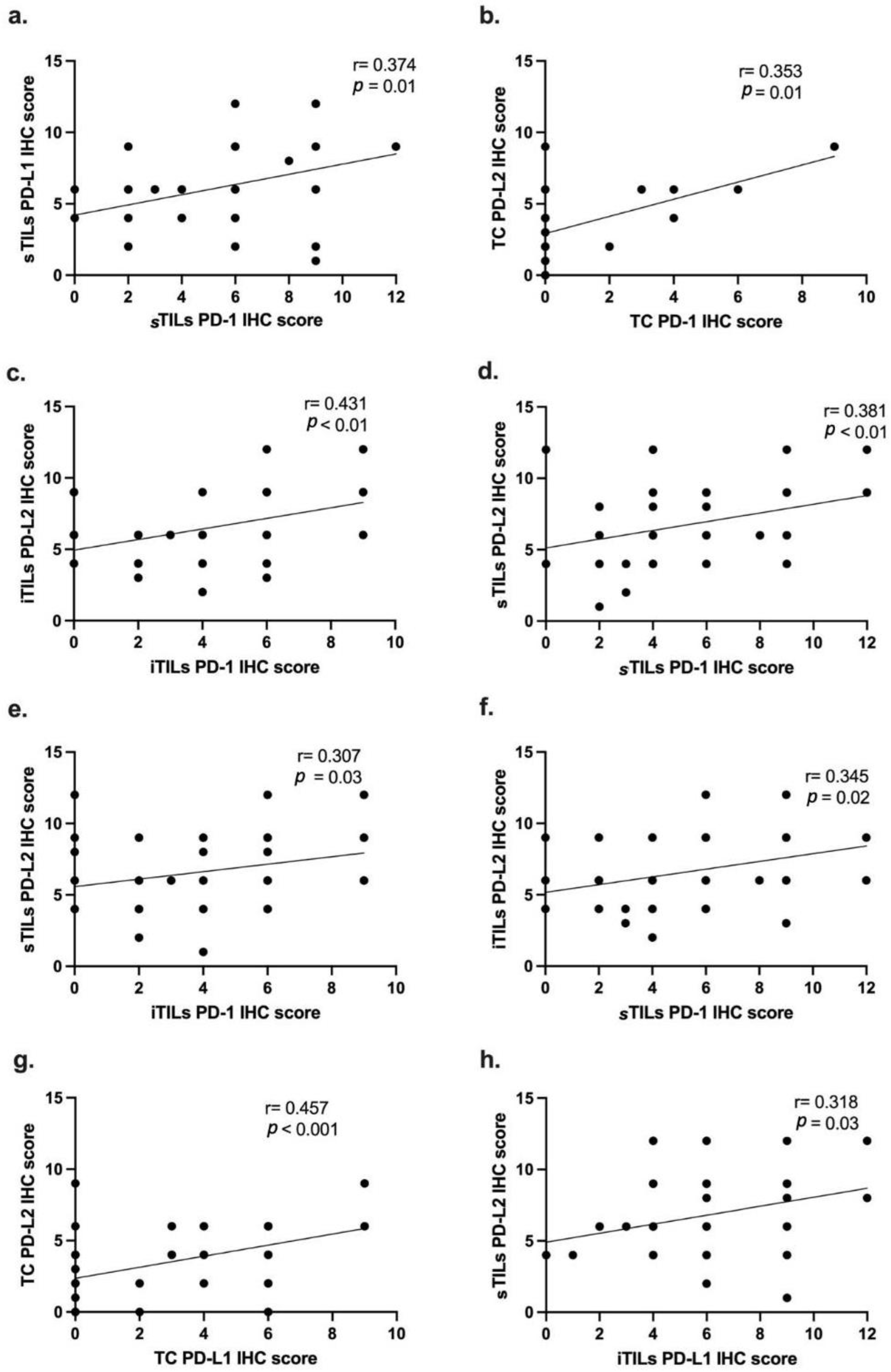
3.5. PD-1, PD-L1 and PD-2 IHC Scores Are Correlated with Each Other and with Cell Type
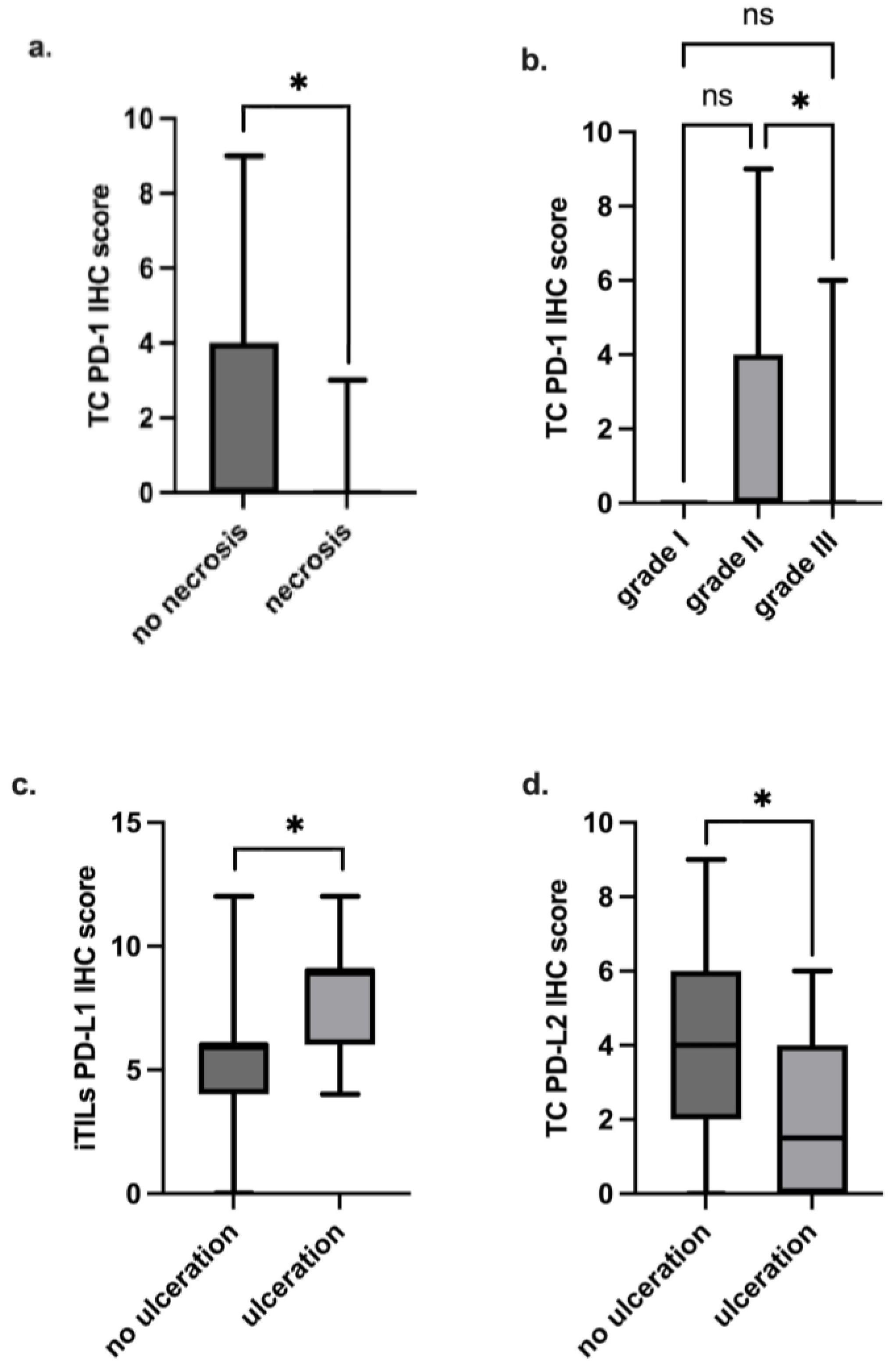
3.6. Higher PD-1 IHC Scores in TCs Are Associated with Less Aggressive Clinicopathological Features
3.7. PD-L1 and PD-L2 IHC Scores Are Significantly Associated with Some Clinicopathological Features
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gameiro, A.; Urbano, A.; Ferreira, F. Emerging Biomarkers and Targeted Therapies in Feline Mammary Carcinoma. Vet. Sci. 2021, 8, 164. [Google Scholar] [CrossRef] [PubMed]
- Zappulli, V.; De Zan, G.; Cardazzo, B.; Bargelloni, L.; Castagnaro, M. Feline mammary tumours in comparative oncology. J. Dairy Res. 2005, 72, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Gama, A.; Seixas, F.; Anjos, P.M.; Alves, A.; Marrinhas, C.; Vilhena, H.; Santos, A.; Ferreira, F.; Santos, J.; Pereira, P.D.; et al. Rare and uncommon malignant mammary tumours in dogs and cats: Preliminary results from a portuguese multicentric study. J. Comp. Pathol. 2024, 210, 110–111. [Google Scholar] [CrossRef]
- Goldschmidt, M.; Peña, L.; Zappulli, V. Tumors of the Mammary Gland. In Tumors in Domestic Animals, 5th ed.; Meuten, J.D., Ed.; Wiley Blackwell: Chichester, UK, 2017; pp. 723–765. [Google Scholar]
- Soares, M.; Ribeiro, R.; Carvalho, S.; Peleteiro, M.; Correia, J.; Ferreira, F. Ki-67 as a Prognostic Factor in Feline Mammary Carcinoma: What Is the Optimal Cutoff Value? Vet. Pathol. 2016, 53, 37–43. [Google Scholar] [CrossRef]
- Giménez, F.; Hecht, S.; Craig, L.E.; Legendre, A.M. Early detection, aggressive therapy: Optimizing the management of feline mammary masses. J. Feline Med. Surg. 2010, 12, 214–224. [Google Scholar] [CrossRef]
- Nascimento, C.; Urbano, A.C.; Gameiro, A.; Ferreira, J.; Correia, J.; Ferreira, F. Serum PD-1/PD-L1 Levels, Tumor Expression and PD-L1 Somatic Mutations in HER2-Positive and Triple Negative Normal-Like Feline Mammary Carcinoma Subtypes. Cancers 2020, 12, 1386. [Google Scholar] [CrossRef]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the Treatment of Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef]
- Abad, M.N.; Calabuig-Fariñas, S.; Lobo de Mena, M.; Torres-Martínez, S.; González, C.G.; García, J.Á.G.; Iranzo González-Cruz, V.; Camps Herrero, C. Programmed Death-Ligand 1 (PD-L1) as Immunotherapy Biomarker in Breast Cancer. Cancers 2022, 14, 307. [Google Scholar] [CrossRef]
- Ghosh, C.; Luong, G.; Sun, Y. A snapshot of the PD-1/PD-L1 pathway. J. Cancer 2021, 12, 2735–2746. [Google Scholar] [CrossRef]
- Schütz, F.; Stefanovic, S.; Mayer, L.; Von Au, A.; Domschke, C.; Sohn, C. PD-1/PD-L1 Pathway in Breast Cancer. Oncol. Res. Treat. 2017, 40, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, L.; Jiang, J. Role of programmed cell death ligand-1 expression on prognostic and overall survival of breast cancer. Medicine 2019, 98, e15201. [Google Scholar] [CrossRef] [PubMed]
- Mkrtichyan, M.; Najjar, Y.G.; Raulfs, E.C.; Abdalla, M.Y.; Samara, R.; Rotem-Yehudar, R.; Cook, L.; Khleif, S.N. Anti-PD-1 synergizes with cyclophosphamide to induce potent anti-tumor vaccine effects through novel mechanisms. Eur. J. Immunol. 2011, 41, 2977–2986. [Google Scholar] [CrossRef]
- Ghebeh, H.; Mohammed, S.; Al-Omair, A.; Qattan, A.; Lehe, C.; Al-Qudaihi, G.; Elkum, N.; Alshabanah, M.; Bin Amer, S.; Tulbah, A.; et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: Correlation with important high-risk prognostic factors. Neoplasia 2006, 8, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Akiba, H.; Iwai, H.; Matsuda, H.; Aoki, M.; Tanno, Y.; Shin, T.; Tsuchiya, H.; Pardoll, D.M.; Okumura, K.; et al. Expression of programmed death 1 ligands by murine T cells and APC. J. Immunol. 2002, 169, 5538–5545. [Google Scholar] [CrossRef]
- Baptista, M.Z.; Sarian, L.O.; Derchain, S.F.M.; Pinto, G.A.; Vassallo, J. Prognostic significance of PD-L1 and PD-L2 in breast cancer. Hum. Pathol. 2016, 47, 78–84. [Google Scholar] [CrossRef]
- Pardoll, D.M. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Zou, W.; Wolchok, J.D.; Chen, L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci. Transl. Med. 2016, 8, 328rv4. [Google Scholar] [CrossRef]
- Vranic, S.; Cyprian, F.S.; Gatalica, Z.; Palazzo, J. PD-L1 status in breast cancer: Current view and perspectives. Semin. Cancer Biol. 2021, 72, 146–154. [Google Scholar] [CrossRef]
- Noske, A.; Möbus, V.; Weber, K.; Schmatloch, S.; Weichert, W.; Köhne, C.H.; Solbach, C.; Ingold Heppner, B.; Steiger, K.; Müller, V.; et al. Relevance of tumour-infiltrating lymphocytes, PD-1 and PD-L1 in patients with high-risk, nodal-metastasised breast cancer of the German Adjuvant Intergroup Node-positive study. Eur. J. Cancer 2019, 114, 76–88. [Google Scholar] [CrossRef]
- Yam, C.; Mani, S.A.; Moulder, S.L. Targeting the Molecular Subtypes of Triple Negative Breast Cancer: Understanding the Diversity to Progress the Field. Oncologist 2017, 22, 1086–1093. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020, 396, 1817–1828. [Google Scholar] [CrossRef]
- Huang, W.; Ran, R.; Shao, B.; Li, H. Prognostic and clinicopathological value of PD-L1 expression in primary breast cancer: A meta-analysis. Breast Cancer Res. Treat. 2019, 178, 17–33. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, X.; Bai, X.; Yang, J.; Song, J. Programmed cell death-ligand 2: New insights in cancer. Front. Immunol. 2024, 15, 1359532. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, N.; Konnai, S.; Takagi, S.; Kagawa, Y.; Okagawa, T.; Nishimori, A.; Ikebuchi, R.; Izumi, Y.; Deguchi, T.; Nakajima, C.; et al. A canine chimeric monoclonal antibody targeting PD-L1 and its clinical efficacy in canine oral malignant melanoma or undifferentiated sarcoma. Sci. Rep. 2017, 7, 8951. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, N.; Konnai, S.; Nishimura, M.; Kagawa, Y.; Takagi, S.; Hosoya, K.; Ohta, H.; Kim, S.; Okagawa, T.; Izumi, Y.; et al. PD-L1 immunohistochemistry for canine cancers and clinical benefit of anti-PD-L1 antibody in dogs with pulmonary metastatic oral malignant melanoma. NPJ Precis. Oncol. 2021, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, N.; Konnai, S.; Asano, Y.; Otsuka, T.; Aoki, E.; Takeuchi, H.; Kato, Y.; Kaneko, M.K.; Yamada, S.; Kagawa, Y.; et al. Molecular characterization of feline immune checkpoint molecules and establishment of PD-L1 immunohistochemistry for feline tumors. PLoS ONE 2023, 18, e0281143. [Google Scholar] [CrossRef] [PubMed]
- Hendry, S.; Salgado, R.; Gevaert, T.; Russell, P.A.; John, T.; Thapa, B.; Christie, M.M.; van de Vijver, K.; Estrada, M.; Gonzalez-Ericsson, P.I.; et al. Assessing Tumor-infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method From the International Immunooncology Biomarkers Working Group: Part 1: Assessing the Host Immune Response, TILs in Invasive Breast Carcinoma and Ductal Carcinoma In Situ, Metastatic Tumor Deposits and Areas for Further Research. Adv. Anat. Pathol. 2017, 24, 235–251. [Google Scholar] [CrossRef]
- Urbano, A.C.; Nascimento, C.; Soares, M.; Correia, J.; Ferreira, F. Clinical Relevance of the serum CTLA-4 in Cats with Mammary Carcinoma. Sci. Rep. 2020, 10, 3822. [Google Scholar] [CrossRef]
- Mehan, A.; Anthony, M.L.; Paul, P.; Syed, A.; Chowdhury, N.; Rao, S.; Hussain, N.; Ravi, B. Expression of Programmed Cell Death-1 (PD-1) and Its Ligand (PD-L1) in Breast Cancers and Its Association with Clinicopathological Parameters. J. Lab. Physicians 2022, 14, 27–31. [Google Scholar] [CrossRef]
- Choi, J.E.; Lee, J.S.; Jin, M.S.; Nikas, I.P.; Kim, K.; Yang, S.; Park, S.Y.; Koh, J.; Yang, S.; Im, S.-A.; et al. The prognostic value of a combined immune score in tumor and immune cells assessed by immunohistochemistry in triple-negative breast cancer. Breast Cancer Res. 2023, 25, 134. [Google Scholar] [CrossRef]
- Zappulli, V.; Peña, L.; Rasotto, R.; Goldschmidt, M.; Gama, A.; Scruggs, J.; Kiupel, M. Surgical Pathology of Tumors of Domestic Animals; Kiupel, M., Ed.; Davis-Thompson DVM Foundation: Gurnee, IL, USA, 2008; Volume 2. [Google Scholar]
- Cannon, C.M. Cats, Cancer and Comparative Oncology. Vet. Sci. 2015, 2, 111–126. [Google Scholar] [CrossRef]
- Chen, M.; Bie, L.; Ying, J. Cancer cell-intrinsic PD-1: Its role in malignant progression and immunotherapy. Biomed. Pharmacother. 2023, 167, 115514. [Google Scholar] [CrossRef]
- Yao, H.; Wang, H.; Li, C.; Fang, J.Y.; Xu, J. Cancer Cell-Intrinsic PD-1 and Implications in Combinatorial Immunotherapy. Front. Immunol. 2018, 9, 1774. [Google Scholar] [CrossRef] [PubMed]
- McLane, L.M.; Abdel-Hakeem, M.S.; Wherry, E.J. CD8 T Cell Exhaustion During Chronic Viral Infection and Cancer. Annu. Rev. Immunol. 2019, 37, 457–495. [Google Scholar] [CrossRef]
- Du, S.; McCall, N.; Park, K.; Guan, Q.; Fontina, P.; Ertel, A.; Zhan, T.; Dicker, A.P.; Lu, B. Blockade of Tumor-Expressed PD-1 promotes lung cancer growth. Oncoimmunology 2018, 7, e1408747. [Google Scholar] [CrossRef]
- Ieranò, C.; Righelli, D.; D’ALterio, C.; Napolitano, M.; Portella, L.; Rea, G.; Auletta, F.; Santagata, S.; Trotta, A.M.; Guardascione, G.; et al. In PD-1+ human colon cancer cells NIVOLUMAB promotes survival and could protect tumor cells from conventional therapies. J. Immunother. Cancer 2022, 10, e004032. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Xu, Y.; Li, X.; Liu, H.; You, T.; Cai, T.; Yang, F. YB-1 promotes cell proliferation and metastasis by targeting cell-intrinsic PD-1/PD-L1 pathway in breast cancer. Int. J. Biochem. Cell Biol. 2022, 153, 106314. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Diao, L.; Cuentas, E.R.P.; Denning, W.L.; Chen, L.; Fan, Y.H.; Byers, L.A.; Wang, J.; Papadimitrakopoulou, V.A.; Behrens, C.; et al. Epithelial-mesenchymal transition is associated with a distinct tumor microenvironment including elevation of inflammatory signals and multiple immune checkpoints in lung adenocarcinoma. Clin. Cancer Res. 2016, 22, 3630–3642. [Google Scholar] [CrossRef]
- Yao, C.; Su, L.; Shan, J.; Zhu, C.; Liu, L.; Liu, C.; Xu, Y.; Yang, Z.; Bian, X.; Shao, J.; et al. IGF/STAT3/NANOG/Slug signaling axis simultaneously controls epithelial-mesenchymal transition and stemness maintenance in colorectal cancer. Stem Cells 2016, 34, 820–831. [Google Scholar] [CrossRef]
- Ghebeh, H.; Barhoush, E.; Tulbah, A.; Elkum, N.; Al-Tweigeri, T.; Dermime, S. FOXP3+ Tregs and B7-H1+/PD-1+ T lymphocytes co-infiltrate the tumor tissues of high-risk breast cancer patients: Implication for immunotherapy. BMC Cancer 2008, 8, 57. [Google Scholar] [CrossRef]
- Saito, H.; Kuroda, H.; Matsunaga, T.; Osaki, T.; Ikeguchi, M. Increased PD-1 expression on CD4+ and CD8+ T cells is involved in immune evasion in gastric cancer. J. Surg. Oncol. 2013, 107, 517–522. [Google Scholar] [CrossRef]
- Yamamoto, R.; Nishikori, M.; Kitawaki, T.; Sakai, T.; Hishizawa, M.; Tashima, M.; Kondo, T.; Ohmori, K.; Kurata, M.; Hayashi, T.; et al. PD-1-PD-1 ligand interaction contributes to immunosuppressive microenvironment of Hodgkin lymphoma. Blood 2008, 111, 3220–3224. [Google Scholar] [CrossRef]
- Antony, G.R.; Littleflower, A.B.; Parambil, S.T.; Subhadradevi, L. PD-1/PD-L1 blockade inhibits epithelial-mesenchymal transition and improves chemotherapeutic response in breast cancer. Med. Oncol. 2023, 40, 108. [Google Scholar] [CrossRef] [PubMed]
- Angelico, G.; Broggi, G.; Tinnirello, G.; Puzzo, L.; Vecchio, G.M.; Salvatorelli, L.; Memeo, L.; Santoro, A.; Farina, J.; Mulé, A.; et al. Tumor Infiltrating Lymphocytes (TILS) and PD-L1 Expression in Breast Cancer: A Review of Current Evidence and Prognostic Implications from Pathologist’s Perspective. Cancers 2023, 15, 4479. [Google Scholar] [CrossRef]
- Kim, A.; Lee, S.J.; Kim, Y.K.; Park, W.Y.; Park, D.Y.; Kim, J.Y.; Lee, C.H.; Gong, G.; Huh, G.Y.; Choi, K.U. Programmed death-ligand 1 (PD-L1) expression in tumour cell and tumour infiltrating lymphocytes of HER2-positive breast cancer and its prognostic value. Sci. Rep. 2017, 7, 11671. [Google Scholar] [CrossRef] [PubMed]
- Paver, E.C.; Cooper, W.A.; Colebatch, A.J.; Ferguson, P.M.; Hill, S.K.; Lum, T.; Shin, J.-S.; O’TOole, S.; Anderson, L.; Scolyer, R.A.; et al. Programmed death ligand-1 (PD-L1) as a predictive marker for immunotherapy in solid tumours: A guide to immunohistochemistry implementation and interpretation. Pathology 2021, 53, 141–156. [Google Scholar] [CrossRef]
- Gatalica, Z.; Snyder, C.; Maney, T.; Ghazalpour, A.; Holterman, D.A.; Xiao, N.; Overberg, P.; Rose, I.; Basu, G.D.; Vranic, S.; et al. Programmed Cell Death 1 (PD-1) and Its Ligand (PD-L1) in Common Cancers and Their Correlation with Molecular Cancer Type. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2965–2970. [Google Scholar] [CrossRef] [PubMed]
- Muenst, S.; Schaerli, A.R.; Gao, F.; Däster, S.; Trella, E.; Droeser, R.A.; Muraro, M.G.; Zajac, P.; Zanetti, R.; Gillanders, W.E.; et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res. Treat. 2014, 146, 15–24. [Google Scholar] [CrossRef]
- Li, Z.; Dong, P.; Ren, M.; Song, Y.; Qian, X.; Yang, Y.; Li, S.; Zhang, X.; Liu, F. PD-L1 Expression Is Associated with Tumor FOXP3+ Regulatory T-Cell Infiltration of Breast Cancer and Poor Prognosis of Patient. J. Cancer 2016, 7, 784–793. [Google Scholar] [CrossRef]
- Park, I.H.; Kong, S.-Y.; Ro, J.Y.; Kwon, Y.; Kang, J.H.; Mo, H.J.; Jung, S.-Y.; Lee, S.; Lee, K.S.; Kang, H.-S.; et al. Prognostic Implications of Tumor-Infiltrating Lymphocytes in Association With Programmed Death Ligand 1 Expression in Early-Stage Breast Cancer. Clin. Breast Cancer 2016, 16, 51–58. [Google Scholar] [CrossRef]
- Chen, S.; Wang, R.X.; Liu, Y.; Yang, W.T.; Shao, Z.M. PD-L1 expression of the residual tumor serves as a prognostic marker in local advanced breast cancer after neoadjuvant chemotherapy. Int. J. Cancer 2017, 140, 1384–1395. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, D.M.; Lee, A. Prognostic Role and Clinical Association of Tumor-Infiltrating Lymphocyte, Programmed Death Ligand-1 Expression with Neutrophil-Lymphocyte Ratio in Locally Advanced Triple-Negative Breast Cancer. Cancer Res. Treat 2019, 51, 649–663. [Google Scholar] [CrossRef]
- Jeong, H.; Koh, J.; Kim, S.; Song, S.G.; Lee, S.H.; Jeon, Y.; Lee, C.-H.; Keam, B.; Lee, S.-H.; Chung, D.H. Epithelial−mesenchymal transition induced by tumor cell-intrinsic PD-L1 signaling predicts a poor response to immune checkpoint inhibitors in PD-L1-high lung cancer. Br. J. Cancer 2024, 131, 23–36. [Google Scholar] [CrossRef]
- Zhang, Y.-C.; Zhang, Y.-T.; Wang, Y.; Zhao, Y.; He, L.-J. What role does PDL1 play in EMT changes in tumors and fibrosis? Front. Immunol. 2023, 14, 1226038. [Google Scholar] [CrossRef]
- Fan, Z.; Wu, C.; Chen, M.; Jiang, Y.; Wu, Y.; Mao, R.; Fan, Y. The generation of PD-L1 and PD-L2 in cancer cells: From nuclear chromatin reorganization to extracellular presentation. Acta Pharm. Sin. B 2022, 12, 1041–1053. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, C.; Gameiro, A.; Correia, J.; Ferreira, J.; Ferreira, F. The Landscape of Tumor-Infiltrating Immune Cells in Feline Mammary Carcinoma: Pathological and Clinical Implications. Cells 2022, 11, 2578. [Google Scholar] [CrossRef]
- Emens, L.A.; Cruz, C.; Eder, J.P.; Braiteh, F.; Chung, C.; Tolaney, S.M.; Kuter, I.; Nanda, R.; Cassier, P.A.; Delord, J.P.; et al. Long-term Clinical Outcomes and Biomarker Analyses of Atezolizumab Therapy for Patients With Metastatic Triple-Negative Breast Cancer: A Phase 1 Study. JAMA Oncol. 2019, 5, 74–82. [Google Scholar] [CrossRef]
- Adams, S.; Loi, S.; Toppmeyer, D.; Cescon, D.W.; De Laurentiis, M.; Nanda, R.; Winer, E.P.; Mukai, H.; Tamura, K.; Armstrong, A.; et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: Cohort B of the phase II KEYNOTE-086 study. Ann. Oncol. 2019, 30, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.I.; Jeong, D.; Ji, S.; Ahn, T.S.; Bae, S.H.; Chin, S.; Chung, J.C.; Kim, H.C.; Lee, M.S.; Baek, M.-J. Overexpression of PD-L1 and PD-L2 Is Associated with Poor Prognosis in Patients with Hepatocellular Carcinoma. Cancer Res. Treat. 2017, 49, 246–254. [Google Scholar] [CrossRef]
- Takamori, S.; Takada, K.; Azuma, K.; Jogo, T.; Shimokawa, M.; Toyokawa, G.; Hirai, F.; Tagawa, T.; Kawahara, A.; Akiba, J.; et al. Prognostic Impact of Programmed Death-Ligand 2 Expression in Primary Lung Adenocarcinoma Patients. Ann. Surg. Oncol. 2019, 26, 1916–1924. [Google Scholar] [CrossRef]
- Nakayama, Y.; Mimura, K.; Kua, L.-F.; Okayama, H.; Min, A.K.T.; Saito, K.; Hanayama, H.; Watanabe, Y.; Saito, M.; Momma, T.; et al. Immune suppression caused by PD-L2 expression on tumor cells in gastric cancer. Gastric Cancer 2020, 23, 961–973. [Google Scholar] [CrossRef]
- Qiao, Y.; Liu, C.; Zhang, X.; Zhou, Q.; Li, Y.; Xu, Y.; Gao, Z.; Xu, Y.; Kong, L.; Yang, A.; et al. PD-L2 based immune signature confers poor prognosis in HNSCC. Oncoimmunology 2021, 10, 1947569. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Du, J.; Gao, Z.; Sun, H.; Mei, M.; Wang, Y.; Ren, Y.; Zhou, X. Evolving landscape of PD-L2: Bring new light to checkpoint immunotherapy. Br. J. Cancer 2023, 128, 1196–1207. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wu, Y.; Zhang, S.; Ma, P.; Jin, X.; Wang, Z.; Yao, M.; Zhang, E.; Tao, B.; Qin, Y.; et al. A Tumor-Specific Super-Enhancer Drives Immune Evasion by Guiding Synchronous Expression of PD-L1 and PD-L2. Cell Rep. 2020, 32, 108000. [Google Scholar] [CrossRef] [PubMed]
- Yearley, J.H.; Gibson, C.; Yu, N.; Moon, C.; Murphy, E.; Juco, J.; Lunceford, J.; Cheng, J.; Chow, L.Q.; Seiwert, T.Y.; et al. PD-L2 Expression in Human Tumors: Relevance to Anti-PD-1 Therapy in Cancer. Clin. Cancer Res. 2017, 23, 3158–3167. [Google Scholar] [CrossRef]
- Dioken, D.N.; Ozgul, I.; Yilmazbilek, I.; Yakicier, M.C.; Karaca, E.; Erson-Bensan, A.E. An alternatively spliced PD-L1 isoform PD-L1∆3, and PD-L2 expression in breast cancers: Implications for eligibility scoring and immunotherapy response. Cancer Immunol. Immunother. 2023, 72, 4065–4075. [Google Scholar] [CrossRef]
- Ren, T.; Zheng, B.; Huang, Y.; Wang, S.; Bao, X.; Liu, K.; Guo, W. Osteosarcoma cell intrinsic PD-L2 signals promote invasion and metastasis via the RhoA-ROCK-LIMK2 and autophagy pathways. Cell Death Dis. 2019, 10, 261. [Google Scholar] [CrossRef]

| Clinicopathological Feature | No of Animals (%) (n = 47) * | Histopathological Feature | No of Samples (%) (n = 48) ** |
|---|---|---|---|
| Age (years) | HP classification | ||
| <8 years old | 3 (6%) | Mucinous carcinoma | 36 (75%) |
| 8–12 years old | 21 (45%) | Adenosquamous carcinoma | 8 (17%) |
| >12 years old | 21 (45%) | Carcinosarcoma | 4 (8%) |
| Unknown | 2 (4%) | Histologicalgrade | |
| Breed | I | 1 (2%) | |
| European shorthair | 33 (70%) | II | 10 (21%) |
| Siamese | 2 (4%) | III | 37 (77%) |
| Domestic | 2 (4%) | Tumor necrosis | |
| Persian | 3 (7%) | Yes | 33 (69%) |
| Norwegian Forest | 1 (2%) | No | 15 (31%) |
| Birman | 1 (2%) | Lesion distribution | |
| Not determined | 5 (11%) | Unifocal | 26 (54%) |
| Multifocal | 22 (46%) | ||
| Clinicopathological feature | No of samples (%) (n = 48) ** | Vascular permeation | |
| Tumor size (cm) | Yes | 8 (17%) | |
| <2 cm | 9 (19%) | No | 40 (83%) |
| ≥2 cm | 39 (81%) | Lymph node status | |
| Tumor ulceration | Positive | 12 (25%) | |
| Yes | 10 (21%) | Negative | 20 (42%) |
| No | 38 (79%) | Unknown | 16 (33%) |
| Clinicopathological Features | PD-1 IHC Score | |||||
|---|---|---|---|---|---|---|
| TCs Md (IQR) | p Value | iTILs Md (IQR) | p Value | sTILs Md (IQR) | p Value | |
| Age (years) ** | ||||||
| <8 years old | 0 (nd) | 0.23 | 3 (nd) | 0.34 | 3 (nd) | 0.74 |
| 8–12 years old | 0 (0) | 6 (3) | 6 (6) | |||
| >12 years old | 0 (0) | 4 (4) | 6 (3) | |||
| Breed ** | ||||||
| European shorthair | 0 (0) | 0.17 | 4 (4) | 0.96 | 4 (4) | 0.57 |
| Siamese | 0 (0) | 6 (nd) | 6 (nd) | |||
| Domestic | 0 (0) | 4 (nd) | 6 (nd) | |||
| Persian | 0 (nd) | 3 (nd) | 9 (nd) | |||
| Norwegian Forest | 4 (0) | 4 (0) | 4 (0) | |||
| Birman | 0 (0) | 6 (0) | 9 (0) | |||
| Not determined | 0 (2) | 6 (2) | 6 (4) | |||
| Tumor size (cm) | ||||||
| <2 cm | 0 (0) | 0.98 | 4 (5) | 0.72 | 6 (5) | 0.82 |
| ≥2 cm | 0 (0) | 4 (4) | 6 (5) | |||
| Tumor ulceration | ||||||
| Yes | 0 (0) | 0.83 | 6 (4) | 0.35 | 6 (2) | 0.77 |
| No | 0 (0) | 4 (4) | 5 (7) | |||
| HP classification ** | ||||||
| Mucinous carcinoma | 0 (9) | 0.72 | 4 (4) | 0.65 | 6 (4) | 0.99 |
| Adenosquamous carcinoma | 0 (0) | 5 (4) | 6 (6) | |||
| Carcinosarcoma | 0 (0) | 7 (7) | 5 (6) | |||
| Tumor histological grade ** | ||||||
| I | 0 (0) | 0.012 | 4 (0) | 0.70 | 4 (0) | 0.85 |
| II | 0 (4) | 4 (5) | 5 (5) | |||
| III | 0 (0) | 6 (4) | 6 (5) | |||
| Tumor necrosis | ||||||
| Yes | 0 (0) | 0.033 | 4 (4) | 0.21 | 4 (4) | 0.06 |
| No | 0 (4) | 6 (2) | 6 (5) | |||
| Lesion distribution | ||||||
| Unifocal | 0 (0) | 0.14 | 4 (4) | 0.61 | 6 (6) | 0.45 |
| Multifocal | 0 (0) | 5 (3) | 4 (4) | |||
| Vascular permeation | ||||||
| Yes | 0 (0) | 0.95 | 4 (4) | 0.56 | 6 (4) | 0.49 |
| No | 0 (0) | 5 (4) | 6 (5) | |||
| Lymph node status | ||||||
| Yes | 0 (0) | 0.92 | 6 (4) | 0.25 | 6 (4) | 0.14 |
| No | 0 (0) | 4 (4) | 4 (4) | |||
| Clinicopathological Features | PD-L1 IHC Score | PD-L2 IHC Score | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TCs Md (IQR) | p Value | iTILs Md (IQR) | p Value | sTILs Md (IQR) | p Value | TCs Md (IQR) | p Value | iTILs Md (IQR) | p Value | sTILs Md (IQR) | p Value | |
| Age (years) ** | ||||||||||||
| <8 years old | 6 (nd) | 0.59 | 6 (nd) | 0.56 | 6 (nd) | 0.51 | 6 (nd) | 0.08 | 6 (nd) | 0.71 | 6 (nd) | 0.27 |
| 8–12 years old | 2 (5) | 6 (5) | 6 (5) | 4 (3) | 6 (4) | 6 (3) | ||||||
| >12 years old | 0 (6) | 6 (2) | 6 (5) | 2 (4) | 6 (3) | 8 (4) | ||||||
| Breed ** | ||||||||||||
| European shorthair | 0 (4) | 0.12 | 6 (5) | 0.75 | 6 (2) | 0.29 | 4 (4) | 0.98 | 6 (5) | 0.62 | 6 (5) | 0.67 |
| Siamese | 6 (nd) | 6 (nd) | 9 (nd) | 6 (nd) | 6 (0) | 8 (nd) | ||||||
| Domestic | 2 (nd) | 4 (nd) | 4 (nd) | 3 (nd) | 5 (nd) | 6 (0) | ||||||
| Persian | 2 (nd) | 4 (nd) | 6 (nd) | 4 (nd) | 6 (nd) | 6 (nd) | ||||||
| Norwegian Forest | 3 (0) | 6 (0) | 6 (0) | 4 (0) | 6 (0) | 6 (0) | ||||||
| Birman | 6 (0) | 6 (0) | 12 (0) | 4 (0) | 3 (0) | 4 (0) | ||||||
| Not determined | 0 (3) | 6 (5) | 6 (7) | 2 (5) | 6 (5) | 8 (3) | ||||||
| Tumor size (cm) | ||||||||||||
| <2 cm | 0 (5) | 0.87 | 4 (3) | 0.07 | 4 (7) | 0.96 | 4 (4) | 0.14 | 9 (4) | 0.36 | 6 (4) | 0.74 |
| ≥2 cm | 0 (6) | 6 (5) | 6 (4) | 4 (3) | 6 (5) | 6 (5) | ||||||
| Tumor ulceration | ||||||||||||
| Yes | 1 (6) | 0.61 | 9 (3) | 0.009 | 6 (4) | 0.18 | 2 (4) | 0.048 | 6 (4) | 0.83 | 6 (2) | 0.83 |
| No | 0 (5) | 6 (2) | 6 (3) | 4 (4) | 6 (5) | 6 (5) | ||||||
| HP classification ** | ||||||||||||
| Mucinous carcinoma | 3 (6) | 0.16 | 6 (2) | 0.30 | 6 (5) | 0.56 | 4 (4) | 0.43 | 6 (5) | 0.63 | 6 (5) | 0.58 |
| Adenosquamous carcinoma | 0 (5) | 6 (5) | 6 (2) | 2 (4) | 6 (3) | 7 (3) | ||||||
| Carcinosarcoma | 0 (0) | 8 (5) | 6 (2) | 4 (2) | 8 (8) | 5 (4) | ||||||
| Tumor histological grade ** | ||||||||||||
| I | 0 (0) | 0.58 | 4 (0) | 0.21 | 4 (0) | 0.45 | 2 (0) | 0.13 | 2 (0) | 0.18 | 4 (0) | 0.44 |
| II | 2 (6) | 4 (2) | 5 (3) | 6 (5) | 6 (5) | 6 (4) | ||||||
| III | 0 (5) | 6 (5) | 6 (5) | 4 (3) | 6 (3) | 6 (4) | ||||||
| Tumor necrosis | ||||||||||||
| Yes | 0 (6) | 0.57 | 6 (5) | 0.18 | 6 (5) | 0.76 | 0 (6) | 0.15 | 6 (4) | 0.16 | 6 (5) | 0.61 |
| No | 0 (4) | 4 (6) | 6 (5) | 0 (4) | 9 (3) | 6 (3) | ||||||
| Lesion distribution | ||||||||||||
| Unifocal | 0 (6) | 0.57 | 6 (5) | 0.82 | 6 (2) | 0.06 | 4 (3) | 0.48 | 6 (4) | 0.62 | 6 (5) | 0.64 |
| Multifocal | 0 (4) | 6 (5) | 4 (5) | 3 (5) | 6 (5) | 6 (4) | ||||||
| Vascular permeation | ||||||||||||
| Yes | 0 (3) | 0.42 | 8 (5) | 0.14 | 6 (5) | 0.33 | 1 (6) | 0.23 | 6 (0) | 0.88 | 9 (3) | 0.18 |
| No | 0 (6) | 6 (2) | 6 (4) | 4 (2) | 6 (5) | 6 (5) | ||||||
| Lymph node status | ||||||||||||
| Yes | 1 (6) | 0.39 | 6 (5) | 0.35 | 6 (5) | 0.69 | 1 (4) | 0.24 | 6 (2) | 0.21 | 7 (3) | 0.37 |
| No | 0 (4) | 6 (2) | 6 (2) | 4 (2) | 6 (4) | 6 (5) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco, M.; Seixas, F.; Pires, M.d.A.; Alves, A.; Santos, A.; Marrinhas, C.; Vilhena, H.; Santos, J.; Faísca, P.; Dias-Pereira, P.; et al. PD-1, PD-L1, and PD-L2 Expression as Predictive Markers in Rare Feline Mammary Tumors. Vet. Sci. 2025, 12, 731. https://doi.org/10.3390/vetsci12080731
Franco M, Seixas F, Pires MdA, Alves A, Santos A, Marrinhas C, Vilhena H, Santos J, Faísca P, Dias-Pereira P, et al. PD-1, PD-L1, and PD-L2 Expression as Predictive Markers in Rare Feline Mammary Tumors. Veterinary Sciences. 2025; 12(8):731. https://doi.org/10.3390/vetsci12080731
Chicago/Turabian StyleFranco, Maria, Fernanda Seixas, Maria dos Anjos Pires, Anabela Alves, Andreia Santos, Carla Marrinhas, Hugo Vilhena, Joana Santos, Pedro Faísca, Patrícia Dias-Pereira, and et al. 2025. "PD-1, PD-L1, and PD-L2 Expression as Predictive Markers in Rare Feline Mammary Tumors" Veterinary Sciences 12, no. 8: 731. https://doi.org/10.3390/vetsci12080731
APA StyleFranco, M., Seixas, F., Pires, M. d. A., Alves, A., Santos, A., Marrinhas, C., Vilhena, H., Santos, J., Faísca, P., Dias-Pereira, P., Gama, A., Correia, J., & Ferreira, F. (2025). PD-1, PD-L1, and PD-L2 Expression as Predictive Markers in Rare Feline Mammary Tumors. Veterinary Sciences, 12(8), 731. https://doi.org/10.3390/vetsci12080731










