Histopathological Picture of Lung Organs Towards Combination of Java Cardamom Seed Extract and Turmeric Rhizome as Anti-Colibacillosis in Broiler Chickens
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
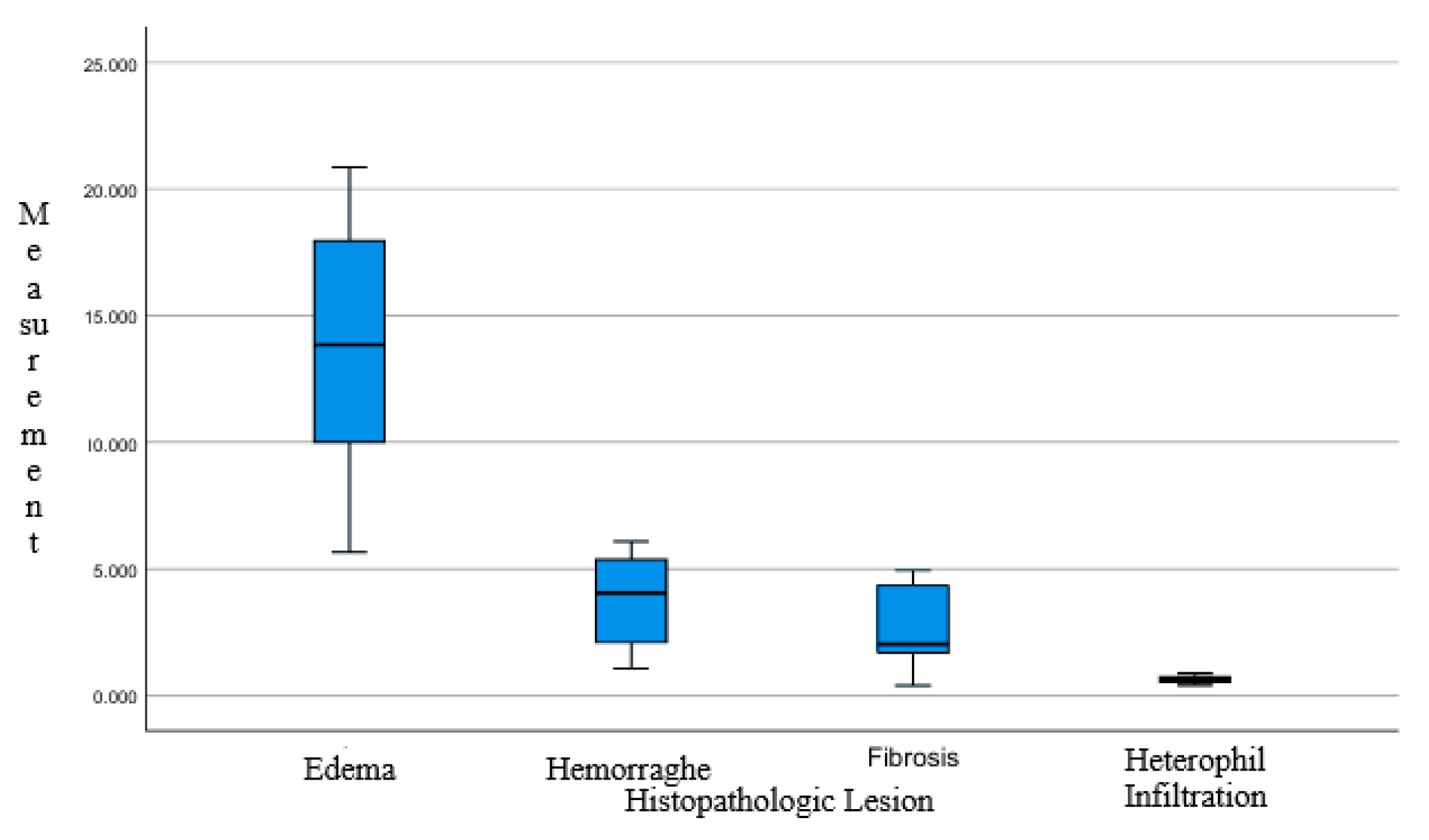
3.1. Histopathological Result
- Hemorrhage in the lungs of chickens is characterized by the extravasation of blood cells from blood vessels into surrounding tissues. This occurs due to damage to the vessel walls and increased vascular permeability.
- Inflammatory cell infiltration occurs when immune cells, including neutrophils and lymphocytes, accumulate in areas of infection or inflammation. This reflects the host’s immune reaction to tissue injury or microbial infiltration.
- Pulmonary edema refers to the accumulation of fluid within lung tissue, which emanate by various aspect such as transportation stress or infection. In stressed broiler chickens, edema can result from elevated vascular pressure, leading to fluid leakage into the interstitial spaces and alveoli.
- Pulmonary fibrosis involves the formation of scar tissue during the healing process following tissue injury. In chickens, fibrosis may develop as a response to chronic infection or repeated inflammation. This condition is marked with deposition of collagen and other extracellular matrix constituents, resulting in reduced lung elasticity and impaired respiratory function.
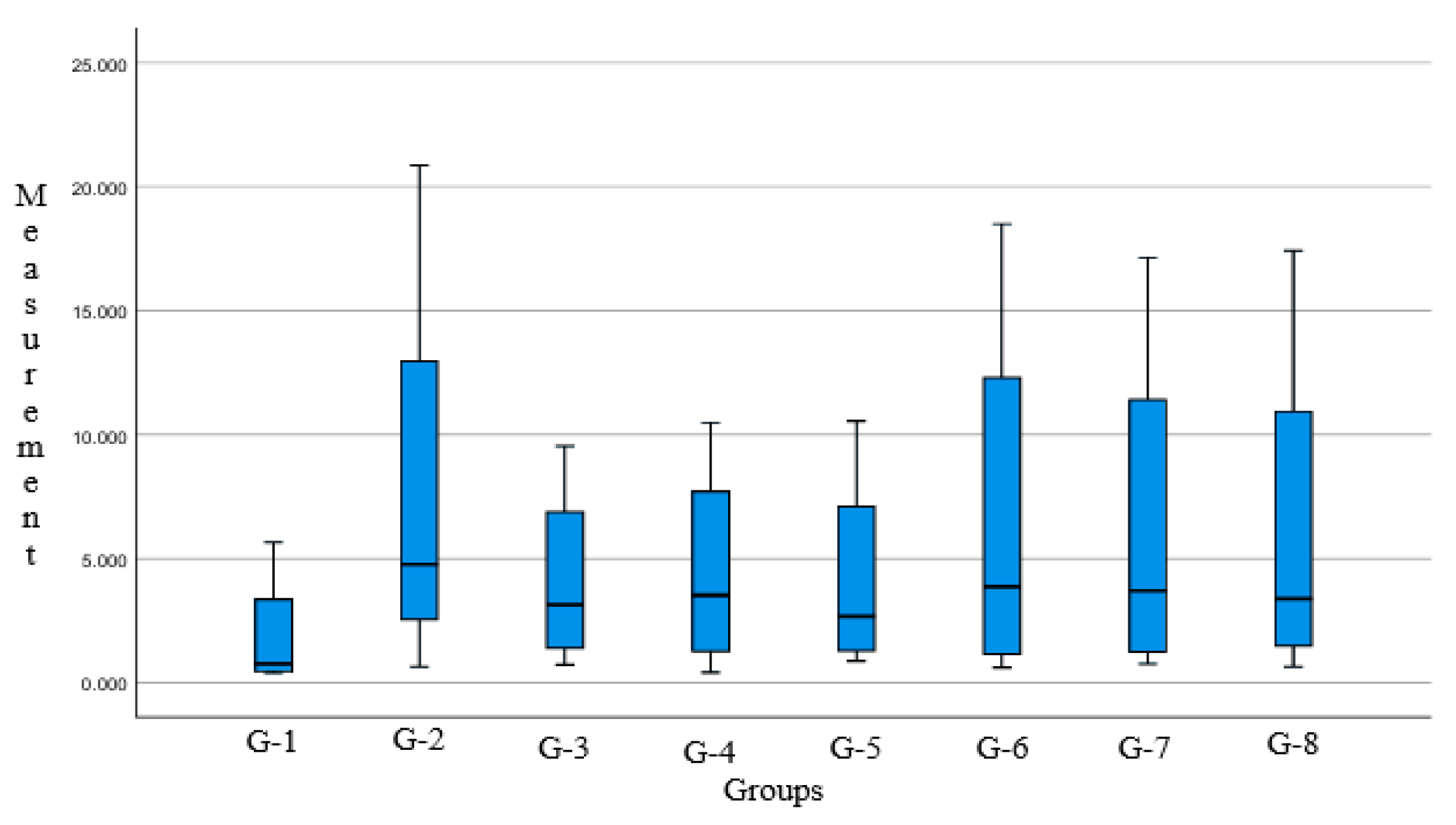
3.2. Data Analysis
4. Discussion
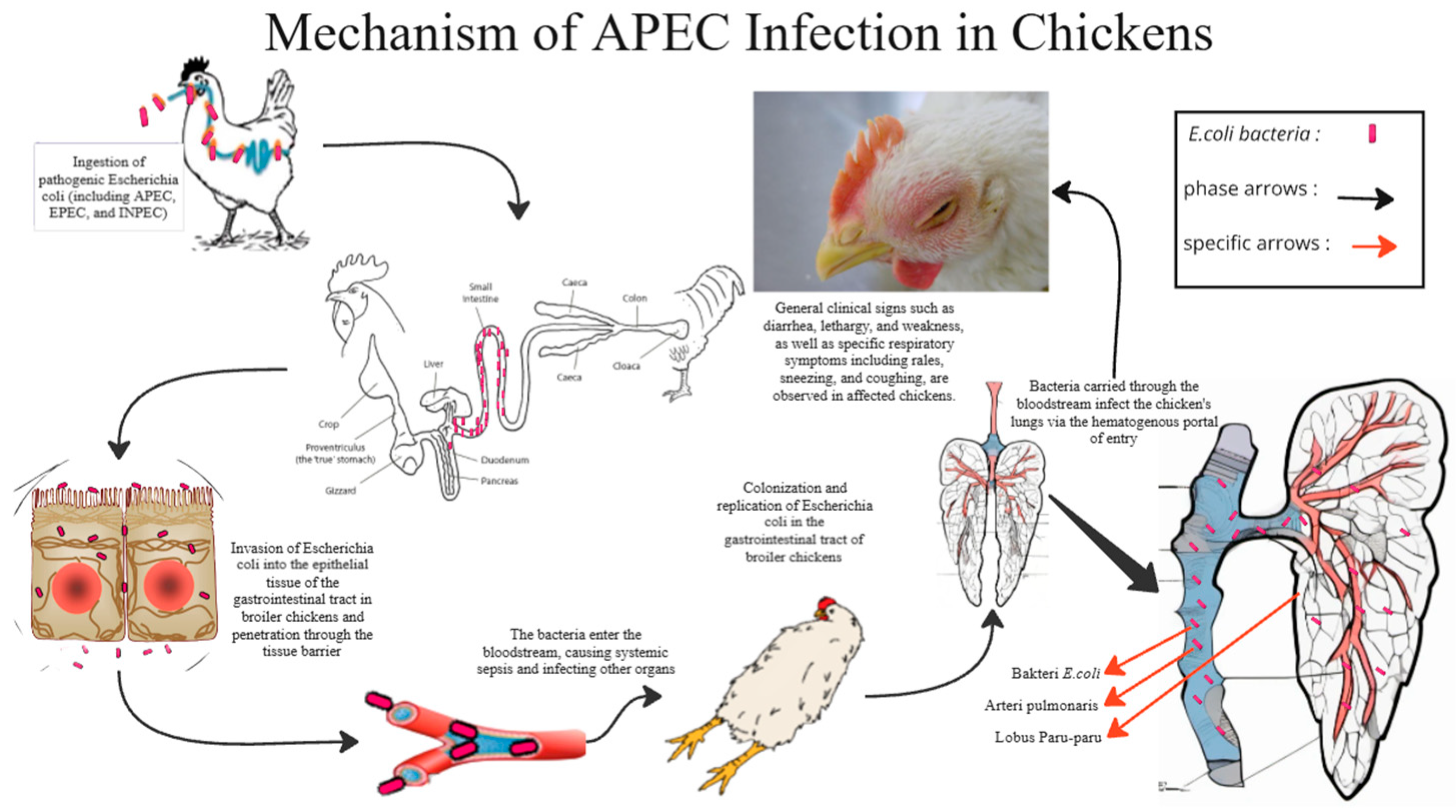
4.1. E. coli Infection
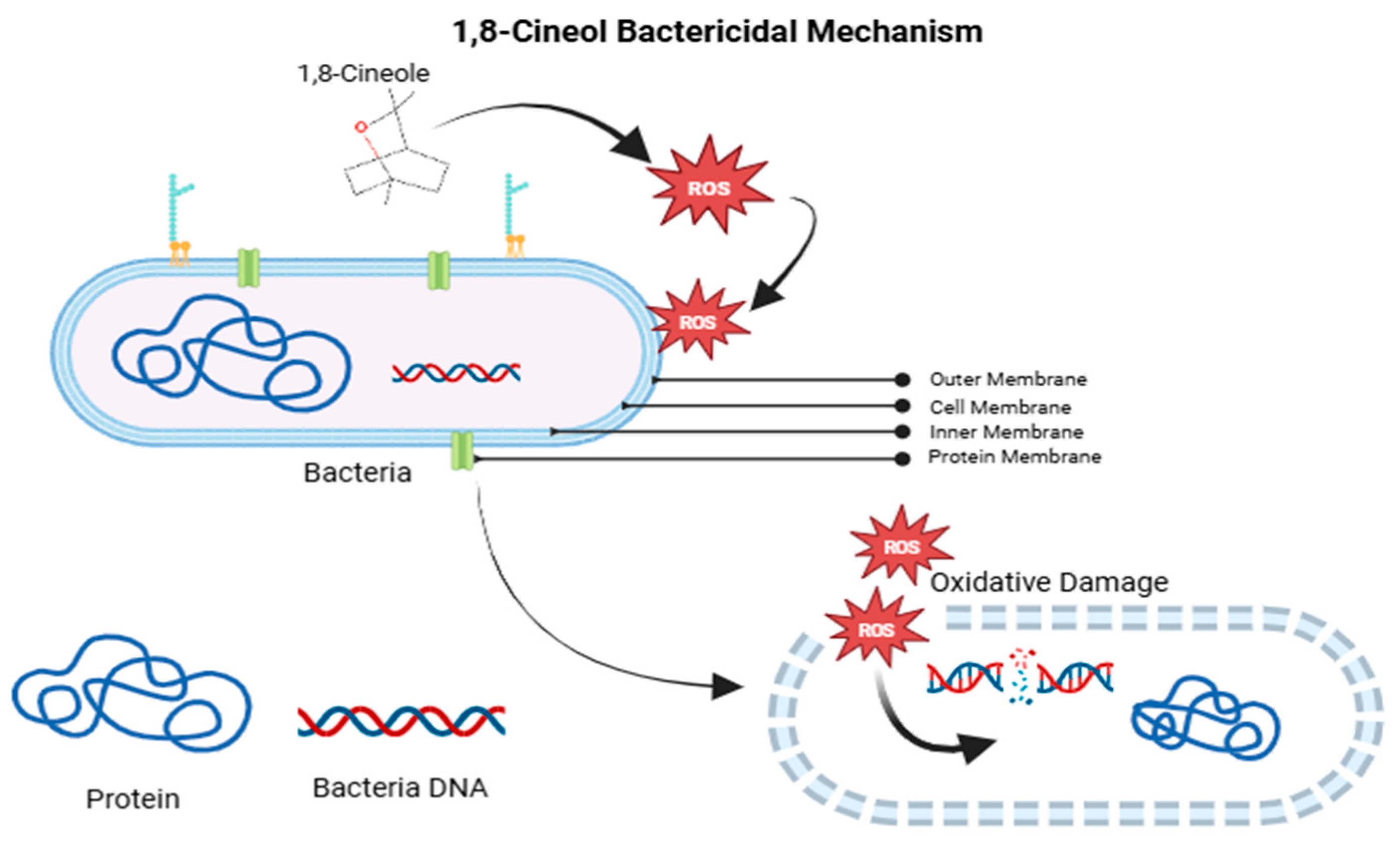
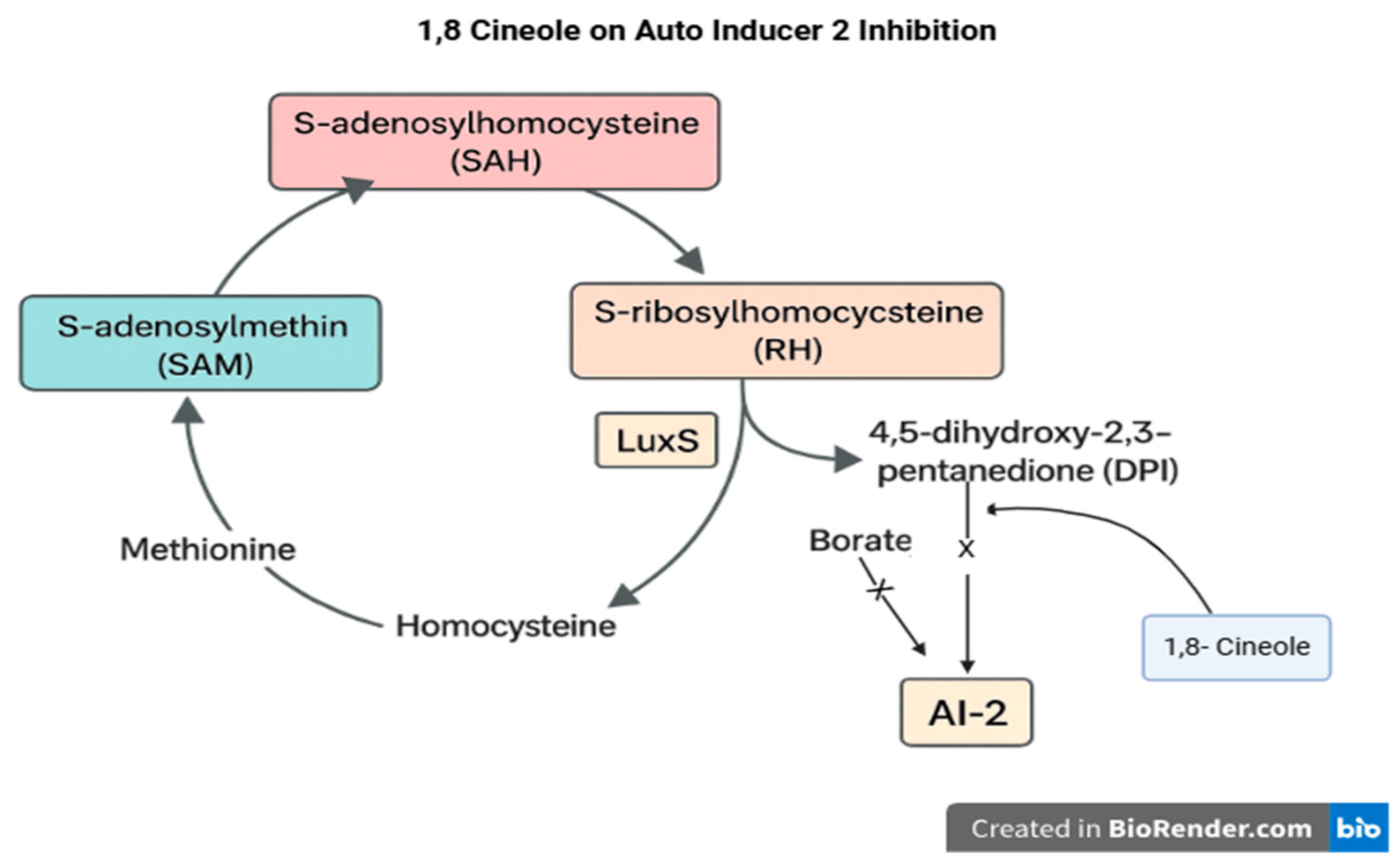
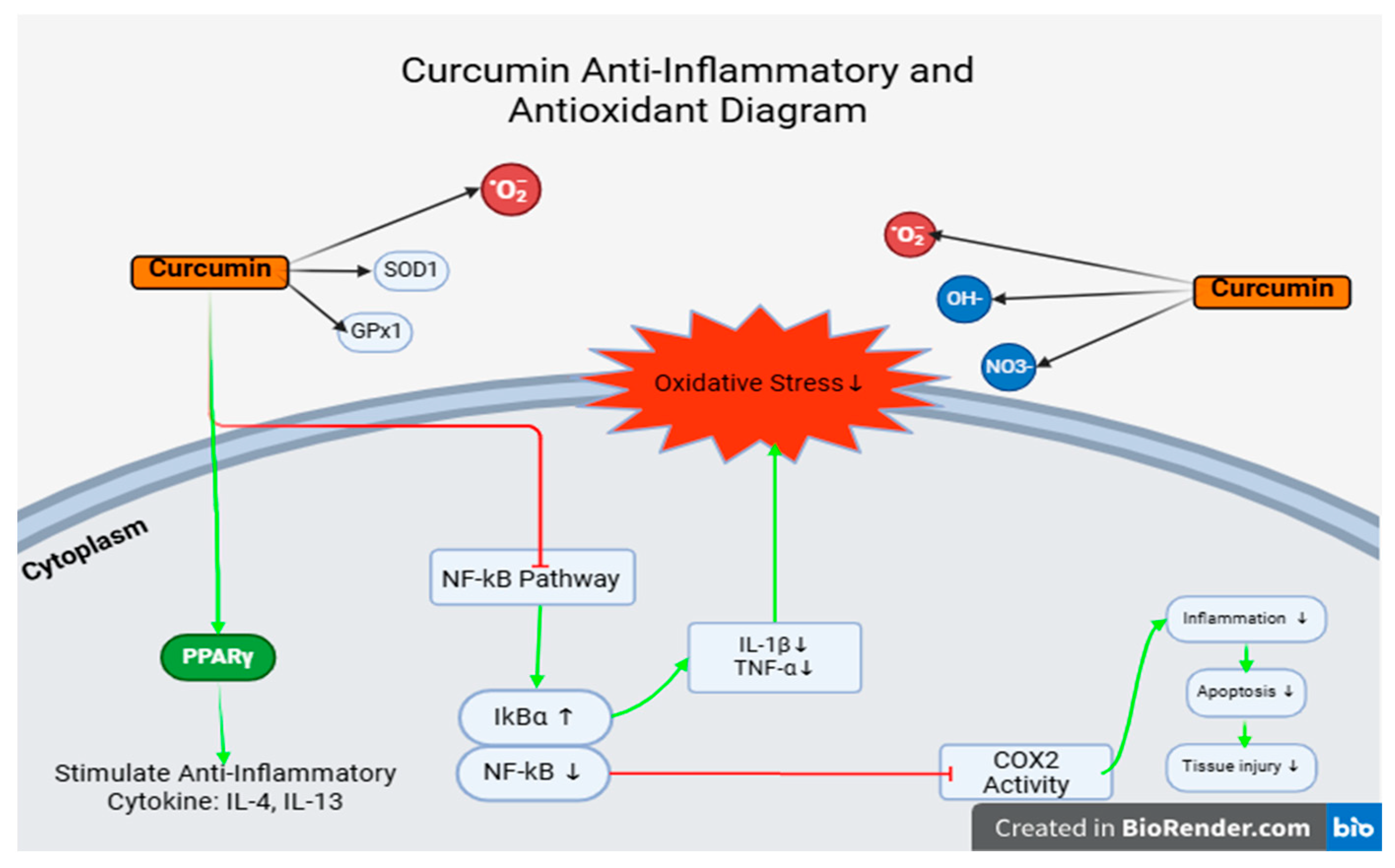
4.2. Javanese Cardamom Extract
- The lesion grading lacked standardization and clear criteria for severity, leading to its exclusion as a variable in the data analysis.
- The histological findings in lung tissue may not accurately represent or correlate with the degree of damage in other organs. Therefore, pulmonary tissue damage should not be assumed to reflect systemic pathological conditions.
- The examination of other organs such as the liver, kidneys, and heart is recommended to obtain a more comprehensive understanding of systemic pathological effects.
- The establishment of a standardized grading system for tissue damage based on lesion severity would enhance the accuracy and consistency of histopathological evaluations.
- Alternative diagnostic techniques may be employed, such as Ziehl–Neelsen staining for bacterial visualization in tissues or immunohistochemistry (IHC) to detect specific antibody responses at the tissue level.
5. Conclusions
- There’s no significant differences were observed in the histomorphometric measurements of broiler chicken lungs treated with a combination of Java cardamom extract and turmeric extract. The range of administered doses did not produce statistically significant effects. One-way ANOVA analysis showed a non-significant p-value [p = 0.922; p > 0.05] between intervention groups and the control group. Based on histopathological evaluations, there were no significant differences in lesion severity or tissue integrity across all groups. Common findings such as pneumocyte inflammation, damage to parabronchial structures (e.g., necrosis, inflammation, and cellular fluid accumulation), and hemorrhage were observed consistently in all microscopic fields across all groups. The infiltration of immune cells was present but not markedly significant in any field of view.
- Improvement in pathological conditions was noted in Group 3 (G3) and Group 6 (G6). Group 3 received a dose of 0.06 mL/kg BW of Java cardamom extract combined with 400 mg of curcumin extract, while Group 6 received 0.06 mL/kg BW of Java cardamom extract only.
- In practical applications, these results may benefit poultry farms. However, certain concerns should be considered, particularly regarding the concentration of cardamom extract. The compound 1,8-cineole can cause a burning sensation in the digestive tract, potentially discouraging feed consumption due to swallowing difficulties. Excessive concentration may also lead to lesions in the upper digestive tract.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APEC | Avian Pathogenic Escherichia Coli |
| SBM | Stored Biological Material |
| CNF-1 | Cytotoxic Necrotizing Factor 1 |
| DPPH | Diphenyl Picryl Hydrazyl Hydrate |
| DOC | Day-Old Chicken |
| IFN-γ | Interferon Gamma |
| IL-1β | Interleukin 1β |
| IL-4 | Interleukin 4 |
| IL-5 | Interleukin 5 |
| IL-6 | Interleukin 6 |
| IL-10 | Interleukin 10 |
| Ig A | Immunoglobulin A |
| Ig E | Immunoglobulin E |
| Ig G | Immunoglobulin G |
| DNA | Deoxyribonucleic Acid |
| mRNA | Messenger Ribo-Nucleic Acid |
| OVA | Ovalbumin |
| BALF | Broncho-Aveolar Lavage Fluid |
| HO-1 | Heme Oxygenase 1 |
| Th-2 | T Helper 2 |
| ROS | Reactive Oxygen Species |
| COX-2 | Cyclooxygenase-2 |
| iNOS | Inducible Nitric Oxide Synthase |
| AMR | Antimicrobial Resistance |
| LD50 | Lethal Dose 50 |
| H&E | Hematoxylin & Eosin |
References
- Pudjiatmoko. Manual Penyakit; Direktorat Jenderal Peternakan dan Kesehatan Hewan, Kementerian Pertanian: Jakarta, Indonesia, 2020.
- Ronco, T.; Stegger, M.; Pedersen, K.; Olsen, R.H.; Sekse, C.; Nordstoga, A.B.; Pohjanvirta, T.; Lilje, B.; Lyhs, U.; Andersen, P.S. Spread of avian pathogenic Escherichia coli ST117 O78:H4 in Nordic broiler production. BMC Genom. 2017, 18, 13. [Google Scholar] [CrossRef]
- Shah, S.A.; Mir, M.S.; Wani, B.M. Pathological studies on avian pathogenic Escherichia coli infection in broilers. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 68–73. [Google Scholar]
- Wibisono, F.J.; Effendi, M.H.; Wibisono, F.M. Occurrence, antimicrobial resistance, and potential zoonosis risk of avian pathogenic Escherichia coli in Indonesia: A review. Int. J. One Health 2022, 8, 76–85. [Google Scholar] [CrossRef]
- Guabiraba, R.; Schouler, C. Avian colibacillosis: Still many black holes. FEMS Microbiol. Lett. 2015, 362, fnv118. [Google Scholar] [CrossRef]
- Abalaka, S.E.; Junaidu, A.U.; Ibrahim, U.I.; Kaikabo, A.A. Pathological changes associated with an outbreak of colibacillosis in a commercial broiler flock. Vet. World 2017, 15, 95–102. [Google Scholar] [CrossRef]
- Nakamura, K.; Imada, T. Pathology of spontaneous colibacillosis in a broiler flock. Avian Dis. 1985, 29, 592–597. [Google Scholar] [CrossRef]
- Nolan, L.K.; Vaillancourt, J.-P.; Barbieri, N.L.; Logue, C.M. Colibacillosis. In Diseases of Poultry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 770–830. [Google Scholar] [CrossRef]
- Ginns, C.A.; Bragg, R.R.; Bayley, R.I. Development and application of an aerosol challenge method for reproduction of avian colibacillosis. Avian Pathol. 1998, 27, 505–511. [Google Scholar] [CrossRef]
- Rahman, R.T.; Fliss, I.; Biron, E. Insights in the development and uses of alternatives to antibiotic growth promoters in poultry and swine production. Antibiotics 2022, 11, 766. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Meng, Y.; Yin, Y.; Zhang, Y.; Zhang, J.; Zhou, W. Characteristics, pathogenic mechanism, zoonotic potential, drug resistance, and prevention of avian pathogenic Escherichia coli (APEC). Front. Microbiol. 2022, 13, 1049391. [Google Scholar] [CrossRef]
- Chotiah, S.; Damayanti, R. Colibacillosis and antibiotics resistance patterns in broiler. IOP Conf. Ser. Earth Environ. Sci. 2016, 275, 434–440. [Google Scholar]
- Solà-Ginés, M.; Cameron-Veas, K.; Badiola, I.; Dolz, R.; Majo, N.; Dahbi, G.; Viso, S.; Mora, A.; Blanco, J.; Piedra-Carrasco, N.; et al. Diversity of multi-drug resistant avian pathogenic Escherichia coli (APEC) causing outbreaks of colibacillosis in broilers during 2012 in Spain. PLoS ONE 2015, 10, e0143191. [Google Scholar] [CrossRef] [PubMed]
- Aberkane, C.; Salmi, D.; Bounar-Kechih, S.; Taha, M.; Bendali, F.; Mouffok, C.; Tazir, M. Antimicrobial resistance pattern of avian pathogenic Escherichia coli with detection of extended-spectrum β-lactamase-producing isolates in broilers in east Algeria. Vet. World 2023, 16, 449–454. [Google Scholar] [CrossRef]
- Silalahi, M. Bioaktivitas Amomum compactum Soland ex Maton dan perspektif konservasinya. J. Pro-Life 2017, 4, 320–328. [Google Scholar]
- Yudhani, R.D.; Nugroho, R.A.; Fitriani, L.D.; Rachmawati, E. Acute toxicity test of Amomum cardamomum (kapulaga) seed extract on hepatic transaminase enzyme in Winstar rats. Indones. J. Clin. Pharm. 2020, 9, 288. [Google Scholar] [CrossRef]
- Hartady, T.; Balia, R.L.; Syamsunarno, M.R.A.A.; Jasni, S.; Priosoeryanto, B.P. Bioactivity of Amomum compactum Soland ex Maton (Java cardamom) as a natural antibacterial. Syst. Rev. Pharm. 2020, 11, 384–387. [Google Scholar]
- Sukandar, D.; Wulandari, E.; Lestari, P. Aktivitas antibakteri ekstrak biji kapulaga (Amomum compactum Sol. ex. Maton). J. Kim. Terap. Indones. 2016, 17, 119–129. [Google Scholar] [CrossRef]
- Praditha, A.; Hartady, T.; Atik, N. The using of Java cardamom (Amomum compactum) seeds as anti-inflammatory and antibiotic growth promoter alternative for production animals: A literature study. Indones. Med. Vet. 2020, 9, 959–969. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin. Exp. Pharmacol. Physiol. 2012, 39, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.A.; Kitts, D.D. Turmeric and its bioactive constituents trigger cell signaling mechanisms that protect against diabetes and cardiovascular diseases. Mol. Cell. Biochem. 2021, 476, 3785–3814. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Huebbe, P.; Ernst, I.M.; Wagner, A.E.; Rimbach, G. Curcumin—From molecule to biological function. Angew. Chem. Int. Ed. 2012, 51, 5308–5332. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Huang, C.; Huang, H.; Zhao, Y.; Khan, M.R.U.; Zhao, H.; Huang, L. Antibacterial mechanism of curcumin: A review. Chem. Biodivers. 2020, 17, e2000171. [Google Scholar] [CrossRef]
- Suryani, A.E.; Karimy, M.F.; Istiqomah, L.; Sofyan, A.; Herdian, H.; Wibowo, M.H. Prevalensi kolibasilosis pada ayam broiler yang diinfeksi Escherichia coli dengan pemberian bioaditif, probiotik, dan antibiotik. Widyariset 2014, 17, 233–244. [Google Scholar]
- Zur, G.; Klement, E. Use of ImageJ software for histomorphometric evaluation of normal and severely affected canine ear canals. Can. J. Vet. Res. 2015, 79, 316–322. [Google Scholar]
- Majeed, H.; Keikhosravi, A.; Kandel, M.E.; Nguyen, T.H.; Liu, Y.; Kajdacsy-Balla, A.; Tangella, K.; Eliceiri, K.W.; Popescu, G. Quantitative Histopathology of Stained Tissues using Color Spatial Light Interference Microscopy (cSLIM). Sci. Rep. 2019, 9, 14679. [Google Scholar] [CrossRef]
- Khairullah, A.R.; Rahman, M.T.; Omar, A.R.; Ariff, A.; Son, R.; Abdul-Aziz, S. Avian pathogenic Escherichia coli: Epidemiology, virulence and pathogenesis, diagnosis, pathophysiology, transmission, vaccination, and control. Vet. World 2024, 17, 2747–2762. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Park, J.H. More about the basic assumptions of t-test: Normality and sample size. Korean J. Anesthesiol. 2019, 72, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Kauppinen, A.; Paterno, J.J.; Blasiak, J.; Salminen, A.; Kaarniranta, K. Inflammation and its role in age-related macular degeneration. Cell. Mol. Life Sci. 2016, 73, 1765–1786. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Chen, X.; Wang, X.; Li, W. Review of respiratory syndromes in poultry: Pathogens, prevention, and control measures. Vet. Res. 2025, 56, 101. [Google Scholar] [CrossRef]
- Wu, X.-Y.; Wang, F.-Y.; Chen, H.-X.; Dong, H.-L.; Zhao, Z.-Q.; Si, L.-F. Chronic heat stress induces lung injury in broiler chickens by disrupting the pulmonary blood-air barrier and activating TLRs/NF-κB signaling pathway. Poult. Sci. 2023, 102, 103066. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Zeng, J.; Wang, X.; Drlica, K.; Zhao, X. Post-stress bacterial cell death mediated by reactive oxygen species. Proc. Natl. Acad. Sci. USA 2019, 116, 10064–10071. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bassler, B.L. Bacterial quorum sensing in complex and dynamically changing environments. Nat. Rev. Microbiol. 2019, 17, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Afre, S.; Gilbert, E.S. LuxS influences Escherichia coli biofilm formation through autoinducer-2-dependent and autoinducer-2-independent modalities. FEMS Microbiol. Ecol. 2013, 83, 778–791. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.K.; Park, S.-W.; Chae, S.-W.; Song, J.-J. The LuxS/AI-2 quorum-sensing system of Streptococcus pneumoniae is required to cause disease, and to regulate virulence- and metabolism-related genes in a rat model of middle ear infection. Front. Cell. Infect. Microbiol. 2018, 8, 138. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-A.; Lee, S.; Lee, H.-J.; Park, H.-S.; Choi, J.-W. Anti-asthmatic effects of an Amomum compactum extract on an ovalbumin (OVA)-induced murine asthma model. Biosci. Biotechnol. Biochem. 2010, 74, 1814–1818. [Google Scholar] [CrossRef]
- Shahcheraghi, S.H.; Naghavi, F.S.; Moini, A.; Mohammadi, M.; Ghasemi, A.; Fallahnezhad, M.; Eslami, M.; Mohammadi, H. Nrf2 regulation by curcumin: Molecular aspects for therapeutic prospects. Molecules 2022, 27, 167. [Google Scholar] [CrossRef]
- Xu, D.; Tian, W.; Shen, H. Curcumin prevents induced drug resistance: A novel function? Chin. J. Cancer Res. 2011, 23, 218–223. [Google Scholar] [CrossRef]


| Group | Treatment [24] |
|---|---|
| 1. Negative Control | 0.5 mL of Isotonic saline solution orally daily. |
| 2. Positive Control | 0.5 mL of E. coli mixed suspension with an infection dose of CFU/mL/BW, then received 0.5 mL of Isotonic saline solution orally daily |
| 3. | 0.5 mL of E. coli bacterial suspension with an infection dose of CFU/mL/BW + a mixture of 0.06 mL/kg BW of cardamom essential oil and 400 mg/kg feed/day of curcumin orally daily |
| 4. | 0.5 mL of E. coli bacterial suspension with an infection dose of CFU/mL/BW + a mixture of 1 mL/kg BW of cardamom essential oil and 400 mg/kg feed/day of curcumin orally every day |
| 5. | 0.5 mL of E. coli bacterial suspension with an infection dose of CFU/mL/BW + 0.06 mL/kg BW of cardamom essential oil only orally every day |
| 6. | 0.5 mL of E. coli bacterial suspension with an infectious dose of CFU/mL/BW + 1 mL/kg BW of cardamom essential oil orally every day |
| 7. | 0.5 mL of E. coli bacterial suspension with an infectious dose of CFU/mL/BW + 400 mg/kg feed/day of curcumin orally every day |
| 8. | 0.5 mL of E. coli bacterial suspension with an infectious dose of CFU/mL/BW + ciprofloxacin antibiotic 1 g/2 L of drinking water |
| Group | Histopathological Lesions [% Area] | |||
|---|---|---|---|---|
| Edema | Hemorrhage | Fibrosis | Heterophil Infiltration | |
| G-1 | 5.662 | 1.060 | 0.394 | 0.469 |
| G-2 | 20.852 | 5.073 | 4.457 | 0.650 |
| G-3 | 9.539 | 2.062 | 4.211 | 0.721 |
| G-4 | 10.483 | 2.121 | 4.930 | 0.407 |
| G-5 | 10.545 | 3.652 | 1.687 | 0.881 |
| G-6 | 18.496 | 6.080 | 2.132 | 0.612 |
| G-7 | 17.145 | 5.655 | 1.746 | 0.751 |
| G-8 | 17.415 | 4.440 | 2.326 | 0.640 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hartady, T.; Ghozali, M.; Parsonodihardjo, C. Histopathological Picture of Lung Organs Towards Combination of Java Cardamom Seed Extract and Turmeric Rhizome as Anti-Colibacillosis in Broiler Chickens. Vet. Sci. 2025, 12, 726. https://doi.org/10.3390/vetsci12080726
Hartady T, Ghozali M, Parsonodihardjo C. Histopathological Picture of Lung Organs Towards Combination of Java Cardamom Seed Extract and Turmeric Rhizome as Anti-Colibacillosis in Broiler Chickens. Veterinary Sciences. 2025; 12(8):726. https://doi.org/10.3390/vetsci12080726
Chicago/Turabian StyleHartady, Tyagita, Mohammad Ghozali, and Charles Parsonodihardjo. 2025. "Histopathological Picture of Lung Organs Towards Combination of Java Cardamom Seed Extract and Turmeric Rhizome as Anti-Colibacillosis in Broiler Chickens" Veterinary Sciences 12, no. 8: 726. https://doi.org/10.3390/vetsci12080726
APA StyleHartady, T., Ghozali, M., & Parsonodihardjo, C. (2025). Histopathological Picture of Lung Organs Towards Combination of Java Cardamom Seed Extract and Turmeric Rhizome as Anti-Colibacillosis in Broiler Chickens. Veterinary Sciences, 12(8), 726. https://doi.org/10.3390/vetsci12080726





