Effects of Rumen-Protected Methionine, Choline, and Betaine Supplementation on Ewes’ Pregnancy and Reproductive Outcomes
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Management and Nutritional Treatments
2.2. Sample Analyses
2.2.1. Maternal Progesterone Determination
2.2.2. Maternal PAG Determination
2.2.3. Total Antioxidant Capacity and Oxidative Stress Indicator Measurements in Blood
2.3. Statistical Analysis
3. Results
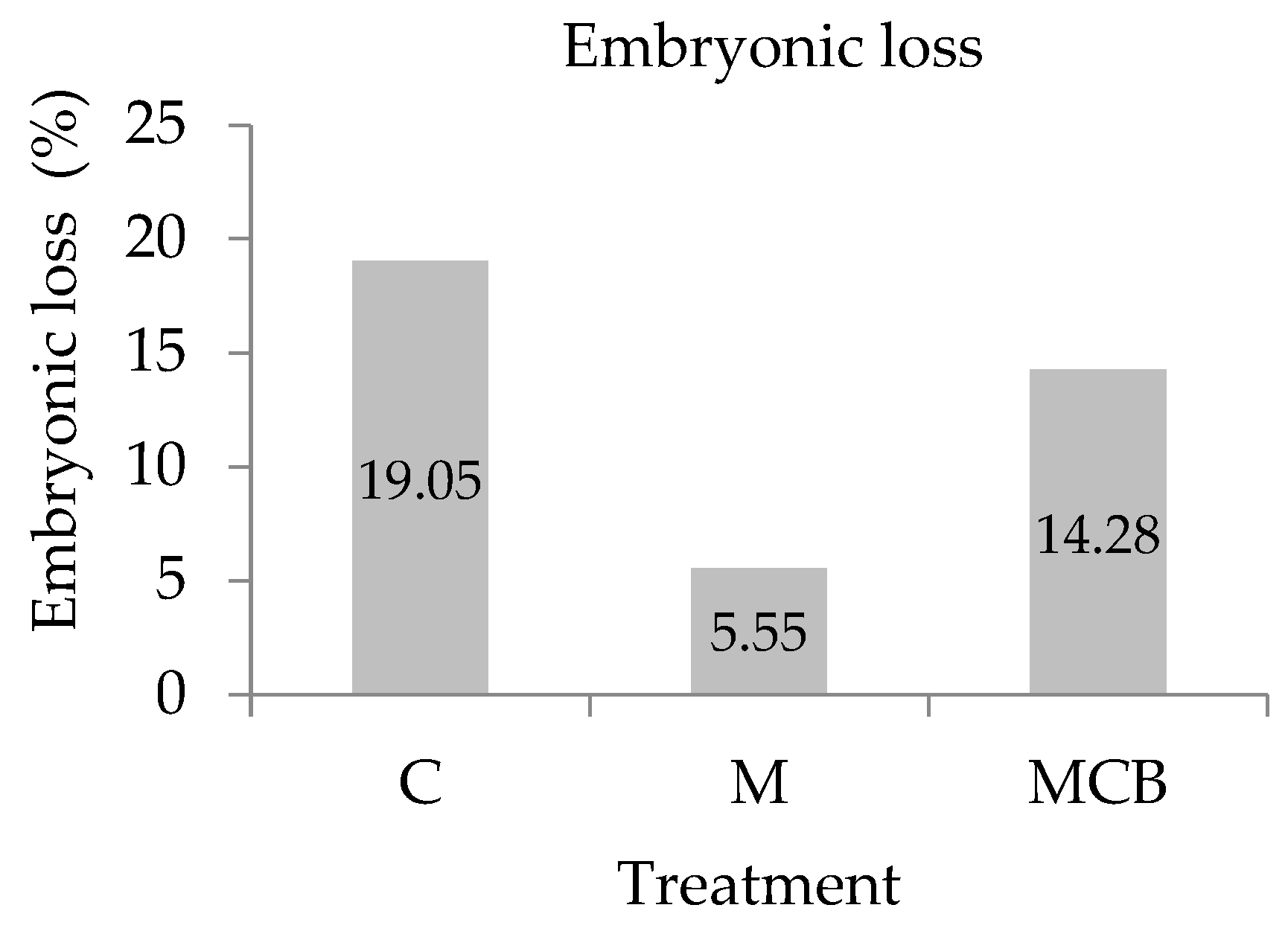
3.1. Pregnancy Rate and Embryonic Loss
3.2. Ewes’ Antioxidant Status Periconceptionally, Prepartum, and at Weaning
3.3. Lambs’ Birth and Weaning Body Weights
3.4. Lambs’ Antioxidant Status at Weaning
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cardoso, F.C.; LeBlanc, S.J.; Murphy, M.R.; Drackley, J.K. Prepartum nutritional strategy affects reproductive performance in dairy cows. J. Dairy Sci. 2013, 96, 5859–5871. [Google Scholar] [CrossRef] [PubMed]
- Chadio, S.; Kotsampasi, B. The role of early life nutrition in programming of reproductive function. J. Devel. Orig. Health Dis. 2014, 5, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Fleming, T.P.; Velazquez, M.A.; Eckert, J.J. Embryos, DOHaD and David Barker. J. Develop. Orig. Health Dis. 2015, 6, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Spencer, T.E.; Johnson, G.A.; Bazer, F.W.; Burghardt, R.C. Implantation mechanisms: Insights from the sheep. Reproduction 2004, 128, 657–668. [Google Scholar] [CrossRef]
- Martínez, Y.; Li, X.; Liu, G.; Bin, P.; Yan, W.; Más, D.; Valdivié, M.; Hu, C.A.A.; Ren, W.; Yin, Y. The role of methionine on metabolism, oxidative stress, and diseases. Amino Acids 2017, 49, 2091–2098. [Google Scholar] [CrossRef]
- Lauinger, L.; Kaiser, P. Sensing and signaling of methionine metabolism. Metabolites 2021, 11, 83. [Google Scholar] [CrossRef]
- Groebner, A.E.; Rubio-Aliaga, I.; Schulke, K.; Reichenbach, H.D.; Daniel, H.; Wolf, E.; Meyer, H.H.; Ulbrich, S.E. Increase of essential amino acids in the bovine uterine lumen during preimplantation development. Reproduction 2011, 141, 685. [Google Scholar] [CrossRef]
- Van den Veyver, I.B. Genetic effects of methylation diets. Ann. Rev. Nutr. 2002, 22, 255–282. [Google Scholar] [CrossRef]
- Martinov, M.V.; Vitvitsky, V.M.; Banerjee, R.; Ataullakhanov, F.I. The logic of the hepatic methionine metabolic cycle. Bioch. Bioph. Acta-Prot. Proteom. 2010, 1804, 89–96. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Da Costa, K.A.; Franklin, P.D.; Alexander, E.A.; Lamont, J.T.; Sheard, N.F.; Beiser, A.L.E.X.A. Choline, an essential nutrient for humans. Fed. Am. Soc. Exp. Biol. J. 1991, 5, 2093–2098. [Google Scholar] [CrossRef]
- Zeisel, S.H. Metabolic crosstalk between choline/1-carbon metabolism and energy homeostasis. Clin. Chem. Lab. Med. 2013, 51, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Kidd, M.T.; Ferket, P.R.; Garlich, J.D. Nutritional and osmoregulatory functions of betaine. World’s Poult. Sci. J. 1997, 53, 125–139. [Google Scholar] [CrossRef]
- Eklund, M.; Bauer, E.; Wamatu, J.; Mosenthin, R. Potential nutritional and physiological functions of betaine in livestock. Nutr. Res. Rev. 2005, 18, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Diskin, M.G.; Morris, D.G. Embryonic and early foetal losses in cattle and other ruminants. Reprod. Dom. Anim. 2008, 43, 260–267. [Google Scholar] [CrossRef]
- Nancarrow, C.D. Mbryonic mortality in the ewe and doe. In Embryonic Mortality in Domestic Species; Zavy, M.T., Geisert, R.D., Eds.; CRC Press: London, UK, 1994; pp. 79–98. [Google Scholar]
- Ikeda, S.; Sugimoto, M.; Kume, S. Importance of methionine metabolism in morula-to-blastocyst transition in bovine preimplantation embryos. J. Reprod. Devel. 2012, 58, 91–97. [Google Scholar] [CrossRef]
- Toledo, M.Z.; Baez, G.M.; Garcia-Guerra, A.; Lobos, N.E.; Guenther, J.N.; Trevisol, E.; Luchini, D.; Shaver, R.D.; Wiltbank, M.C. Effect of feeding rumen-protected methionine on productive and reproductive performance of dairy cows. PLoS ONE 2017, 12, e0189117. [Google Scholar] [CrossRef]
- Titi, H.H.; Alnimer, M.A.; Abedal-majed, M.A. Effect of supplemental rumen-protected methionine on reproduction and production of Awassi ewes. It. J. Anim. Sci. 2022, 21, 624–633. [Google Scholar] [CrossRef]
- Batistel, F.; Alharthi, A.S.; Wang, L.; Parys, C.; Pan, Y.X.; Cardoso, F.C.; Loor, J.J. Placentome nutrient transporters and mammalian target of rapamycin signaling proteins are altered by the methionine supply during late gestation in dairy cows and are associated with newborn birth weight. J. Nutr. 2017, 147, 1640–1647. [Google Scholar] [CrossRef]
- Batistel, F.; Arroyo, J.M.; Bellingeri, A.; Wang, L.; Saremi, B.; Parys, C.; Trevisi, E.; Cardoso, F.C.; Loor, J.J. Ethyl-cellulose rumen-protected methionine enhances performance during the periparturient period and early lactation in Holstein dairy cows. J. Dairy Sci. 2017, 100, 7455–7467. [Google Scholar] [CrossRef]
- Alharthi, A.S.; Batistel, F.; Abdelmegeid, M.K.; Lascano, G.; Parys, C.; Helmbrecht, A.; Trevisi, E.; Loor, J.J. Maternal supply of methionine during late-pregnancy enhances rate of Holstein calf development in utero and postnatal growth to a greater extent than colostrum source. J. Anim. Sci. Biotechn. 2018, 9, 83. [Google Scholar] [CrossRef]
- Alharthi, A.S.; Lopreiato, V.; Dai, H.; Bucktrout, R.; Abdelmegeid, M.; Batistel, F.; Parys, C.; Shen, X.; Ballou, M.A.; Trevisi, E.; et al. Short communication: Supply of methionine during late pregnancy enhances whole-blood innate immune response of Holstein calves partly through changes in mRNA abundance in polymorphonuclear leukocytes. J. Dairy Sci. 2019, 102, 10599–10605. [Google Scholar] [CrossRef] [PubMed]
- Jacometo, C.B.; Zhou, Z.; Luchini, D.; Trevisi, E.; Corrêa, M.N.; Loor, J.J. Maternal rumen-protected methionine supplementation and its effect on blood and liver biomarkers of energy metabolism, inflammation, and oxidative stress in neonatal Holstein calves. J. Dairy Sci. 2016, 99, 6753–6763. [Google Scholar] [CrossRef] [PubMed]
- Atmaca, G. Antioxidant effects of sulfur-containing amino acids. Yonsei Med. J. 2004, 45, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, J.T.; Brosnan, M.E. The sulfur-containing amino acids: An overview. J. Nutr. 2006, 136, 1636S–1640S. [Google Scholar] [CrossRef]
- Gaál, T.; Ribiczeyné-Szabó, P.; Stadler, K.; Jakus, J.; Reiczigel, J.; Kövér, P.; Mezes, M.; Sümeghy, L. Free radicals, lipid peroxidation and the antioxidant system in the blood of cows and newborn calves around calving. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2006, 143, 391–396. [Google Scholar] [CrossRef]
- Aydın, I.; Bulbul, T.; Polat, E.S. Serum antioxidant status and adenosine deaminase activity during the gestational period of sheep. Rev. de Médec. Vétér. 2010, 161, 469–474. [Google Scholar]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antiox. Red. Sign. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Abuelo, A.; Hernández, J.; Benedito, J.L.; Castillo, C. Redox biology in transition periods of dairy cattle: Role in the health of periparturient and neonatal animals. Antioxidants 2019, 8, 20. [Google Scholar] [CrossRef]
- Osorio, J.S.; Ji, P.; Drackley, J.K.; Luchini, D.; Loor, J.J. Smartamine M and MetaSmart supplementation during the peripartal period alter hepatic expression of gene networks in 1-carbon metabolism, inflammation, oxidative stress, and the growth hormone–insulin-like growth factor 1 axis pathways. J. Dairy Sci. 2014, 97, 7451–7464. [Google Scholar] [CrossRef]
- Osorio, J.S.; Trevisi, E.R.M.I.N.I.O.; Ji, P.; Drackley, J.K.; Luchini, D.; Bertoni, G.; Loor, J.J. Biomarkers of inflammation, metabolism, and oxidative stress in blood, liver, and milk reveal a better immunometabolic status in peripartal cows supplemented with Smartamine M or MetaSmart. J. Dairy Sci. 2014, 97, 7437–7450. [Google Scholar] [CrossRef]
- Sun, F.; Cao, Y.; Cai, C.; Li, S.; Yu, C.; Yao, J. Regulation of nutritional metabolism in transition dairy cows: Energy homeostasis and health in response to post-ruminal choline and methionine. PLoS ONE 2016, 11, e0160659. [Google Scholar] [CrossRef]
- Tsiplakou, E.; Mavrommatis, A.; Kalogeropoulos, T.; Chatzikonstantinou, M.; Koutsouli, P.; Sotirakoglou, K.; Labrou, N.; Zervas, G. The effect of dietary supplementation with rumen-protected methionine alone or in combination with rumen-protected choline and betaine on sheep milk and antioxidant capacity. J. Anim. Phys. Anim. Nutr. 2017, 101, 1004–1013. [Google Scholar] [CrossRef]
- Raheja, N.; Kumar, N.; Patel, B.; Lathwal, S.S. Effect of dietary betaine on reproductive performance of Karan Fries cows during hot humid season. Int. J. Current. Microb. Appl. Sci. 2018, 7, 1451–1460. [Google Scholar] [CrossRef]
- Mavrommatis, A.; Mitsiopoulou, C.; Christodoulou, C.; Kariampa, P.; Simoni, M.; Righi, F.; Tsiplakou, E. Effects of supplementing rumen-protected methionine and lysine on milk performance and oxidative status of dairy ewes. Antioxidants 2021, 10, 654. [Google Scholar] [CrossRef]
- Al-Gubory, K.H.; Fowler, P.A.; Garrel, C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Inter. J Biochem. Cell Biol. 2010, 42, 1634–1650. [Google Scholar] [CrossRef]
- European Parliament and of the Council on the Protection of Animals Used for Scientific Purposes Text with EEA Relevance, 2010. Directive 2010/63/EU. Available online: http://data.europa.eu/eli/dir/2010/63/oj (accessed on 15 September 2020).
- National Research Council. Nutrient Requirements of Dairy Cattle Nutrition, Committee on Animal Nutrition, 7th ed.; The National Academies Press: Washington, DC, USA, 2001; 381p, ISBN 0-309-06997-1. [Google Scholar]
- Association of Official Analytical Chemists. Official Methods of Analysis, 15th ed.; Helrich, K., Ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Van Soest, P.V.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 4, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- National Research Council; Committee on Animal Nutrition. Nutrient Requirements of Small Ruminants: Sheep; National Academy Press: Washington, DC, USA, 1985; 112p, ISBN 0-309-59555-X. [Google Scholar]
- Available online: https://guidelines.beefimprovement.org/index.php/Weaning_Weight#Adjusted_Value (accessed on 10 May 2025).
- Nanas, I.; Chouzouris, T.M.; Dadouli, K.; Dovolou, E.; Stamperna, K.; Barbagianni, M.; Valasi, I.; Tsiaras, A.; Amiridis, G.S. A study on stress response and fertility parameters in phenotypically thermotolerant and thermosensitive dairy cows during summer heat stress. Reprod. Dom. Anim. 2020, 55, 1774–1783. [Google Scholar] [CrossRef] [PubMed]
- El Amiri, B.; Delahaut, P.; Colemonts, Y.; De Sousa, N.M.; Beckers, J.F. Investigation of pregnancy-associated glycoproteins (PAGs) by means of an enzymoimmunoassay (ELISA) sandwich kit for pregnancy monitoring in sheep. Opt. Méditer. Série A. Sémin. Méditer. 2014, 108, 299–303. Available online: http://om.ciheam.org/om/pdf/a108/00007646.pdf (accessed on 10 May 2025).
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef]
- Li, P.; Huo, L.; Su, W.; Lu, R.; Deng, C.; Liu, L.; Deng, Y.; Guo, N.; Lu, C.; He, C. Free radical-scavenging capacity, antioxidant activity and phenolic content of Pouzolzia zeylanica. J. Serb. Chem. Soc. 2011, 76, 709–717. [Google Scholar] [CrossRef]
- Nielsen, F.; Mikkelsen, B.B.; Nielsen, J.B.; Andersen, H.R.; Grandjean, P. Plasma malondialdehyde as bio-marker for oxidative stress: Reference interval and effects of life-style factors. Clin. Chem. 1997, 43, 1209–1214. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 13 December 2024).
- Ardalan, M.; Rezayazdi, K.; Dehghan-Banadaky, M. Effect of rumen-protected choline and methionine on physiological and metabolic disorders and reproductive indices of dairy cows. J. Anim. Physiol. Anim. Nutr. 2010, 94, e259–e265. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Wang, Q.; Kong, C.; Liu, K.; Si, H.; Sui, S. Mechanism of action and the uses betaine in pig production. J. Anim. Phyiol. Anim. Nutr. 2022, 106, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Peñagaricano, F.; Souza, A.H.; Carvalho, P.D.; Driver, A.M.; Gambra, R.; Kropp, J.; Hackbart, K.S.; Luchini, D.; Shaver, R.D.; Wiltbank, M.C.; et al. Effect of maternal methionine supplementation on the transcriptome of bovine preimplantation embryos. PLoS ONE 2013, 8, e72302. [Google Scholar] [CrossRef]
- Acosta, D.A.V.; Denicol, A.C.; Tribulo, P.; Rivelli, M.I.; Skenandore, C.; Zhou, Z.; Luchini, D.; Corrêa, M.N.; Hansen, P.J.; Cardoso, F.C. Effects of rumen-protected methionine and choline supplementation on the preimplantation embryo in Holstein cows. Theriogenology 2016, 85, 1669–1679. [Google Scholar] [CrossRef] [PubMed]
- Acosta, D.A.V.; Rivelli, M.I.; Skenandore, C.; Zhou, Z.; Keisler, D.H.; Luchini, D.; Corrêa, M.N.; Cardoso, F.C. Effects of rumen-protected methionine and choline supplementation on steroidogenic potential of the first postpartum dominant follicle and expression of immune mediators in Holstein cows. Theriogenology 2017, 96, 1–9. [Google Scholar] [CrossRef]
- Arshad, U.; Zenobi, M.G.; Staples, C.R.; Santos, J.E.P. Meta-analysis of the effects of supplemental rumen-protected choline during the transition period on performance and health of parous dairy cows. J. Dairy Sc. 2020, 103, 282–300. [Google Scholar] [CrossRef]
- Alcolea, M.P.; Colom, B.; Lladó, I.; García-Palmer, F.J.; Gianotti, M. Mitochondrial differentiation and oxidative phosphorylation system capacity in rat embryo during placentation period. Reproduction 2007, 134, 147–154. [Google Scholar] [CrossRef]
- Huang, Y.; Wen, J.; Kong, Y.; Zhao, C.; Liu, S.; Liu, Y.; Li, L.; Yang, J.; Zhu, X.; Zhao, B.; et al. Oxidative status in dairy goats: Periparturient variation and changes in subclinical hyperketonemia and hypocalcemia. BMC Vet. Res. 2021, 17, 238. [Google Scholar] [CrossRef]
- Khan, M.Z.; Liu, S.; Ma, Y.; Ma, M.; Ullah, Q.; Khan, I.M.; Wang, J.; Xiao, J.; Chen, T.; Khan, A.; et al. Overview of the effect of rumen-protected limiting amino acids (methionine and lysine) and choline on the immunity, antioxidative, and inflammatory status of periparturient ruminants. Front. Immun. 2023, 13, 1042895. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lei, J.; Hancock, S.; Scanlan, V.; Broomfield, S.; Currie, A.; Thompson, A. Lamb survival, glutathione redox state and immune function of neonates and lambs from periparturient Merino ewes supplemented with rumen-protected methionine. Arch. Anim. Nutr. 2016, 70, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Gutierrez, D.; Hernández-Arteaga, L.E.; Flores-Najera, M.J.; Cuevas-Reyes, V.; Vázquez-García, J.M.; Loredo-Osti, C.; Beltrán-López, S.; Ballesteros-Rodea, G.; Gonzalez-Bulnes, A.; Meza-Herrera, C.A.; et al. Methionine supplementation during pregnancy of goats improves kids’ birth weight, body mass index, and postnatal growth pattern. Biology 2022, 11, 1065. [Google Scholar] [CrossRef] [PubMed]
- Titi, H.H. Effect of long-term rumen-protected methionine supplementation on performance of Shami goats and growth performance of their kids. Anim. Prod. Sci. 2017, 57, 1713–1718. [Google Scholar] [CrossRef]
- Jia, Y.; Song, H.; Gao, G.; Cai, D.; Yang, X.; Zhao, R. Maternal betaine supplementation during gestation enhances expression of mtDNA-encoded genes through D-loop DNA hypomethylation in the skeletal muscle of newborn piglets. J. Agric. Food Chem. 2015, 63, 10152–10160. [Google Scholar] [CrossRef]
- Gao, Q.K.; Ma, C.; Kong, X.; Yin, F.G.; Han, Q.; Yin, Y.L.; Wang, Z.B. Effects of dietary betaine supplementation on reproductive performance, colostrum composition and plasma metabolite and reproductive hormone contents of bama mini pigs. Chin. J. Anim. Nutr. 2020, 32, 646–653. [Google Scholar] [CrossRef]
- Song, M.T.; Zhu, Q.; Ma, W.Q.; Gao, Q.K.; Yin, F.G.; Han, Q.; Kong, X.F. Effects of dietary betaine addition on blood routine Indexes and organ growth of bama mini-pigs. Chin. J. Anim. Nutr. 2020, 32, 1908–1915. [Google Scholar] [CrossRef]
- Silva, G.M.; Chalk, C.D.; Ranches, J.; Schulmeister, T.M.; Henry, D.D.; DiLorenzo, N.; Arthington, J.D.; Moriel, P.; Lancaster, P.A. Effect of rumen-protected methionine supplementation to beef cows during the periconception period on performance of cows, calves, and subsequent offspring. Animals 2021, 15, 100055. [Google Scholar] [CrossRef]
- Estrada-Cortés, E.; Ortiz, W.; Rabaglino, M.B.; Block, J.; Rae, O.; Jannaman, E.A.; Xiao, Y.; Hansen, P.J. Choline acts during preimplantation development of the bovine embryo to program postnatal growth and alter muscle DNA methylation. FASEB J. 2021, 35, e21926. [Google Scholar] [CrossRef]
- Oster, M.; Nuchchanart, W.; Trakooljul, N.; Murani, E.; Zeyner, A.; Wirthgen, E.; Hoeflich, A.; Ponsuksili, S.; Wimmers, K. Methylating micronutrient supplementation during pregnancy influences foetal hepatic gene expression and IGF signalling and increases foetal weight. Eur. J. Nutr. 2016, 55, 1717–1727. [Google Scholar] [CrossRef]
- Suárez-Suárez, N.E.; Lee-Rangel, H.A.; Lizarazo-Chaparro, A.C.; Mendoza-Martínez, G.D.; Espinosa-Reyes, G.; Hernández-García, P.A.; García-López, J.C.; Martínez-García, J.A.; Álvarez-Fuentes, G.; Roque-Jiménez, J.A. Effect of the supplementation using an herbal mixture as a choline source during early gestation in Rambouillet ewes. Animals 2023, 13, 645. [Google Scholar] [CrossRef]
- Jacometo, C.B.; Alharthi, A.S.; Zhou, Z.; Luchini, D.; Loor, J.J. Maternal supply of methionine during late pregnancy is associated with changes in immune function and abundance of microRNA and mRNA in Holstein calf polymorphonuclear leukocytes. J. Dairy Sci. 2018, 101, 8146–8158. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, F.; Castoldi, A.; Nicastro, R.; Reghellin, V.; Lombardi, L.; Airoldi, C.; Falletta, E.; Maffioli, E.; Scarcia, P.; Palmieri, L.; et al. Methionine supplementation stimulates mitochondrial respiration. Bioch. Bioph. Acta-Mol. Cell Res. 2018, 1865, 1901–1913. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, G.; Degen, A.; Ji, K.; Jiao, D.; Liang, Y.; Xiao, L.; Long, R.; Zhou, J. Effect of feed level and supplementary rumen protected lysine and methionine on growth performance, rumen fermentation, blood metabolites and nitrogen balance in growing Tan lambs fed low protein diets. Anim. Feed Sci. Technol. 2021, 279, 115024. [Google Scholar] [CrossRef]
- Batistel, F.; Alharthi, A.S.; Yambao, R.R.; Elolimy, A.A.; Pan, Y.X.; Parys, C.; Loor, J.J. Methionine supply during late-gestation triggers offspring sex-specific divergent changes in metabolic and epigenetic signatures in bovine placenta. J. Nutr. 2019, 149, 6–17. [Google Scholar] [CrossRef]
- Brougham, B.J.; Weaver, A.C.; Swinbourne, A.M.; Cottrell, J.J.; Kelly, J.M.; Kleemann, D.O.; van Wettere, W.H. Supplementing dietary betaine during late gestation increases plasma betaine and methionine concentrations in pregnant Merino ewes but not neonatal lambs. Small Rumin. Res. 2024, 232, 107226. [Google Scholar] [CrossRef]
- McCarthy, N.; Weaver, A.C.; Agenbag, B.; Flinn, T.; Brougham, B.J.; Swinbourne, A.M.; Kelly, J.M.; Kleemann, D.O.; Gatford, K.K.; van Wettere, W.H. Maternal lysine, methionine and choline supplementation in twin-bearing Merino ewes during mid-to-late gestation does not alter pregnancy outcomes or progeny growth and survival. Liv. Sci. 2021, 251, 104620. [Google Scholar] [CrossRef]
- Al-Qaisi, M.A.; Titi, H.H. Effect of rumen-protected methionine on production and composition of early lactating Shami goats milk and growth performance of their kids. Arch. Anim. Breed. 2014, 57, 1–11. [Google Scholar] [CrossRef]
| Treatment | |||
|---|---|---|---|
| C | M | MCB | |
| Ingredient Composition (g/kg) | |||
| Corn grain | 300 | 300 | 300 |
| Barley grain | 200 | 200 | 200 |
| Wheat grain | 200 | 200 | 200 |
| Soyameal | 100 | 100 | 100 |
| Sunflower meal | 175 | 164 | 157 |
| Mineral and vitamin premix | 25 | 25 | 25 |
| Methionine formula 1 | 0 | 11 | 0 |
| Methionine, choline, and betaine mix 2 | 0 | 0 | 18 |
| Chemical Composition (g/kg) 3 | |||
| Dry matter (DM) | 875 | 864 | 857 |
| Crude protein (CP) | 151 | 148 | 145 |
| Crude fat (CF) | 19.4 | 19.2 | 19.1 |
| Neutral detergent fiber (om) | 162 | 157 | 154 |
| Acid detergent fiber (om) | 81.5 | 77.9 | 75.5 |
| Ash | 26.8 | 26.0 | 25.5 |
| Net energy of lactation (MJ NEL/kg) | 6.32 | 6.26 | 6.21 |
| Treatment | |||
|---|---|---|---|
| C | M | MCB | |
| Ingredient Composition (g/kg) | |||
| Corn grain | 300 | 300 | 300 |
| Barley grain | 200 | 200 | 200 |
| Wheat grain | 200 | 200 | 200 |
| Soyameal | 100 | 100 | 100 |
| Sunflower meal | 175 | 165 | 159 |
| Mineral and vitamin premix | 25 | 25 | 25 |
| Methionine formula 1 | 0 | 10 | 0 |
| Methionine, choline, and betaine mix 2 | 0 | 0 | 16 |
| Chemical Composition (g/kg) 3 | |||
| Dry matter (DM) | 875 | 866 | 860 |
| Crude protein (CP) | 151 | 148 | 146 |
| Crude fat (CF) | 19.4 | 19.2 | 19.1 |
| Neutral detergent fiber (om) | 162 | 158 | 155 |
| Acid detergent fiber (om) | 81.5 | 78.5 | 76.7 |
| Ash | 26.8 | 26.1 | 25.7 |
| Net energy of lactation (MJ NEL/kg) | 6.32 | 6.26 | 6.23 |
| Treatment | FRAP −14 (μmol Ascorbic Acid) | FRAP 0 (μmol Ascorbic Acid) | FRAP + 14 (μmol Ascorbic Acid) | ABTS -14 (% Inhibition) | ABTS 0 (% Inhibition) | ABTS + 14 (% Inhibition) | MDA −14 (μM) | MDA 0 (μM) | MDA +14 (μM) |
|---|---|---|---|---|---|---|---|---|---|
| C | 0.66 ± 0.18 | 0.57 ± 0.14 | 0.55 ± 0.16 | 41.66 ± 4.34 | 40.24 ± 3.63 | 37.53 ± 4.59 | 0.58 ± 0.08 | 0.60 ± 0.13 | 0.57 ± 0.20 |
| M | 0.69 ± 0.20 | 0.62 ± 0.19 | 0.59 ± 0.24 | 41.76 ± 3.12 | 37.67 ± 4.57 | 35.79 ± 4.83 | 0.57 ± 0.10 | 0.54 ± 0.06 | 0.51 ± 0.09 |
| MCB | 0.71 ± 0.20 | 0.69 ± 0.13 | 0.79 ± 0.16 | 40.85 ± 3.74 | 41.30 ± 2.56 | 31.95 ± 5.16 | 0.46 ± 0.04 | 0.54 ± 0.22 | 0.42 ± 0.07 |
| Treatment | FRAP Prepartum (μmol Ascorbic Acid) | FRAP Weaning (μmol Ascorbic Acid) | ABTS Prepartum (% Inhibition) | ABTS Weaning (% Inhibition) | MDA Prepartum (μM) | MDA Weaning (μM) |
|---|---|---|---|---|---|---|
| C-C | 1.08 ± 0.12 | 0.94 ± 0.21 | 43.23 ± 5.70 | 43.98 ± 5.51 | 0.53 ± 0.14 | 0.69 ± 0.11 |
| C-MCB | 0.88 ± 0.07 | 0.99 ± 0.16 | 42.29 ± 6.76 | 38.17 ± 2.88 | 0.66 ± 0.18 | 0.73 ± 0.09 |
| C-M | 0.87 ± 0.10 | 0.96 ± 0.14 | 43.36 ± 2.83 | 41.99 ± 2.06 | 0.62 ± 0.20 | 0.66 ± 0.08 |
| M-C | 1.09 ± 0.18 | 1.08 ± 0.22 | 40.92 ± 6.30 | 47.99 ± 6.41 | 0.53 ± 0.17 | 0.66 ± 0.07 |
| M-M | 0.97 ± 0.14 | 1.10 ± 0.15 | 42.59 ± 6.25 | 43.40 ± 8.39 | 0.75 ± 0.27 | 0.79 ± 0.14 |
| MCB-C | 1.05 ± 0.12 | 0.86 ± 0.11 | 41.80 ± 8.34 | 37.34 ± 3.44 | 0.72 ± 0.22 | 0.62 ± 0.05 |
| MCB-MCB | 0.93 ± 0.07 | 0.96 ± 0.22 | 42.95 ± 4.15 | 39.11 ± 8.84 | 0.74 ± 0.12 | 0.70 ± 0.17 |
| ABTS (% Inhibition) | FRAP (μmol Ascorbic Acid) | MDA (μM) | ||||
|---|---|---|---|---|---|---|
| Periconceptional | Chisq | Pr (>Chisq) | Chisq | Pr (>Chisq) | Chisq | Pr (>Chisq) |
| (Intercept) | 58.31 | 0.00 * | 85.97 | <2.0 × 10−16 * | 15.77 | 0.00 * |
| Treatment | 5.94 | 0.051 | 7.15 | 0.027 * | 4.02 | 0.13 |
| Time | 7.70 | 0.005 * | 0.00 | 0.98 | 379,300.00 | <2.2 × 10−16 * |
| Baseline measurement | 0.55 | 0.46 | 0.51 | 0.48 | 2.04 | 0.15 |
| Treatment:time | 14.32 | 0.0007 * | 1.12 | 0.57 | 1.69 | 0.43 |
| Prepartum–Weaning | Chisq | Pr (>Chisq) | Chisq | Pr (>Chisq) | Chisq | Pr (>Chisq) |
| (Intercept) | 35.59 | 0.00 * | 244.13 | <2.0 × 10−16 * | 2.92 | 0.09 |
| Treatment | 1.22 | 0.98 | 16.77 | 0.01 * | 15.99 | 0.01 * |
| Time | 0.28 | 0.59 | 2.90 | 0.08 | 3.60 | 0.057 |
| Baseline measurement | 0.00 | 0.99 | 0.94 | 0.33 | 0.47 | 0.49 |
| Treatment:time | 7.63 | 0.27 | 12.02 | 0.06 | 10.56 | 0.10 |
| ABTS (% Inhibition) | FRAP (μmol Ascorbic Acid) | MDA (μM) | ||||
|---|---|---|---|---|---|---|
| Effect | SE | Effect | SE | Effect | SE | |
| Periconceptional | ||||||
| M | −2.647 * | 1.39 | 0.063 | 0.05 | −0.058 | 0.08 |
| MCB | 1.438 | −1.57 | 0.162 *** | 0.06 | −0.265 | 0.13 |
| Time (+14) | −3.001 *** | 1.08 | 0.001 | 0.04 | −0.190 *** | 0.0003 |
| M:time (+14) | 1.125 | 1.90 | −0.023 | 0.07 | 0.106 | 0.10 |
| MCB:time (+14) | −7.085 *** | 2.08 | 0.073 | 0.08 | 0.13 | 0.17 |
| Prepartum–Weaning | ||||||
| C-M | 1.107 | 2.56 | −0.187 *** | 0.07 | 0.076 | 0.09 |
| C-MCB | −1.145 | 2.67 | −0.194 *** | 0.07 | −0.052 | 0.17 |
| M-C | −1.795 | 2.76 | 0.048 | 0.07 | 0.199 | 0.13 |
| M-M | −0.518 | 2.77 | −0.095 | 0.07 | 0.341 *** | 0.10 |
| MCB-C | −0.811 | 3.10 | −0.026 | 0.08 | 0.287 | 0.18 |
| MCB-MCB | −0.73 | 2.68 | −0.114 | 0.07 | 0.293 ** | 0.12 |
| Time (weaning) | 1.111 | 2.08 | −0.105 * | 0.06 | 0.171 * | 0.09 |
| C-MCB:time (weaning) | −4.857 | 3.46 | 0.253 ** | 0.10 | 0.259 | 0.24 |
| C-M:time (weaning) | −1.623 | 3.32 | 0.190 * | 0.09 | −0.133 | 0.14 |
| MCB-C:time (weaning) | −4.233 | 3.95 | −0.027 | 0.11 | −0.271 | 0.24 |
| MCB-MCB:time (weaning) | −4.477 | 3.46 | 0.109 | 0.10 | −0.398 ** | 0.16 |
| M-C:time (weaning) | 4.171 | 3.632 | 0.055 | 0.107 | −0.178 | 0.17 |
| M-M:time (weaning) | −0.298 | 3.52 | 0.236 ** | 0.104 | −0.251 * | 0.14 |
| Baseline periconceptional | 0.084 | 0.11 | −0.06 | 0.08 | −0.573 | 0.40 |
| Baseline prepartum–weaning | 0.003 | 0.16 | −0.084 | 0.08 | 0.241 | 0.35 |
| Constant periconceptional | 36.798 *** | 4.819 | 0.593 *** | 0.064 | 0.940 *** | 0.237 |
| Constant prepartum–weaning | 42.995 *** | 7.207 | 1.126 *** | 0.072 | 0.377 * | 0.22 |
| Treatment | Birth Weight (Kg) | Weaning Weight (Kg) | Growth Rate (g/day) |
|---|---|---|---|
| C-C | 3.35 ± 0.45 | 12.31 ± 2.95 | 0.197 ± 0.061 |
| C-M | 4.31 ± 0.29 | 13.43 ± 3.51 | 0.201 ± 0.075 |
| C-MCB | 4.11 ± 0.63 | 12.99 ± 2.70 | 0.198 ± 0.059 |
| M-C | 4.08 ± 0.57 | 13.74 ± 2.81 | 0.218 ± 0.064 |
| M-M | 3.83 ± 0.74 | 14.16 ± 2.21 | 0.226 ± 0.048 |
| MCB-C | 3.89 ± 0.93 | 14.69 ± 1.87 | 0.230 ± 0.037 |
| MCB-MCB | 3.87 ± 0.84 | 12.10 ± 2.47 | 0.184 ± 0.053 |
| Dependent Variable | Birth Weight (Kg) | Weaning Weight (Kg) | Growth Rate (g/day) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Chisq | Df | Pr (>Chisq) | Chisq | Df | Pr (>Chisq) | Chisq | Df | Pr (>Chisq) | |
| (Intercept) | 357.78 | 1 | <0.000 *** | 249.07 | 1 | <2.0 × 10−16 *** | 141.38 | 1 | <2.0 × 10−16 *** |
| Sex | 11.46 | 1 | 0.00 *** | 4.75 | 1 | 0.02915 * | 3.18 | 1 | 0.07451 |
| Treatment | 16.05 | 6 | 0.01 ** | 7.25 | 6 | 0.29778 | 5.58 | 6 | 0.471 |
| Dependent Variable | Birth Weight (Kg) | Weaning Weight (Kg) | Growth Rate (g/day) | ||
|---|---|---|---|---|---|
| Sex | Male | Coef | 0.31 *** | 1.3 ** | 0.023 |
| SE | 0.09 | 0.50 | 0.012 | ||
| Treatment | C-M | Coef | 0.91 *** | 1.49 | 0.01 |
| SE | 0.27 | 1.2 | 0.025 | ||
| C-MCB | Coef | 0.88 *** | 0.7 | −0.0005 | |
| SE | 0.27 | 1.10 | 0.02 | ||
| M-C | Coef | 0.61 ** | 1.61 | 0.024 | |
| SE | 0.28 | 1.27 | 0.026 | ||
| M-M | Coef | 0.60 ** | 1.83 | 0.028 | |
| SE | 0.26 | 1.07 | 0.022 | ||
| MCB-C | Coef | 0.51 | 2.69 | 0.04 | |
| SE | 0.33 | 1.75 | 0.037 | ||
| MCB-MCB | Coef | 0.56 ** | −0.26 | −0.01 | |
| SE | 0.27 | 1.07 | 0.022 | ||
| Constant | Coef | 3.28 *** | 11.81 *** | 0.188 *** | |
| SE | 0.17 | 0.74 | 0.015 | ||
| Treatment | ABTS (% Inhibition) | FRAP (μmol Ascorbic Acid) | MDA (μM) |
|---|---|---|---|
| C-C | 39.7 ± 6.14 | 1.09 ± 0.20 | 0.86 ± 0.19 |
| C-MCB | 43.0 ± 8.36 | 1.24 ± 0.23 | 1.57 ± 0.40 |
| C-M | 43.8 ± 5.91 | 1.14 ± 0.37 | 1.22 ± 0.52 |
| M-C | 34.8 ± 4.27 | 1.01 ± 0.15 | 1.30 ± 0.06 |
| M-M | 45.5 ± 5.38 | 1.16 ± 0.24 | 1.09 ± 0.09 |
| MCB-C | 41.9 ± 0.83 | 0.82 ± 0.09 | 1.35 ± 0.11 |
| MCB-MCB | 43.7 ± 4.42 | 1.03 ± 0.12 | 1.25 ± 0.19 |
| Dependent Variable | ABTS (% Inhibition) | FRAP (μmol Ascorbic Acid) | MDA (μM) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Chisq | Df | Pr (>Chisq) | Chisq | Df | Pr (>Chisq) | Chisq | Df | Pr (>Chisq) | |
| (Intercept) | 464.65 | 1 | <2.0 × 10−16 *** | 233.55 | 1 | <0.00 *** | 47.73 | 1 | 4.89 × 10−12 *** |
| Sex | 0.23 | 1 | 0.63 | 3.34 | 1 | 0.07 | 1.47 | 1 | 0.23 |
| Treatment | 14.51 | 6 | 0.02 * | 7.06 | 6 | 0.32 | 16.85 | 6 | 0.009 ** |
| Dependent Variable | ABTS (% Inhibition) | FRAP (μmol Ascorbic Acid) | MDA (μM) | ||
|---|---|---|---|---|---|
| Sex | Male | Coef | 0.78 | −0.11 | −0.16 |
| SE | 1.61 | 0.06 | 0.14 | ||
| Treatment | C-M | Coef | 3.94 | 0.08 | 0.41 ** |
| SE | 2.71 | 0.12 | 0.19 | ||
| C-MCB | Coef | 3.33 | 0.12 | 0.71 *** | |
| SE | 2.69 | 0.11 | 0.18 | ||
| M-C | Coef | −5.01 | −0.06 | 0.29 | |
| SE | 3.15 | 0.13 | 0.24 | ||
| M-M | Coef | 5.84 ** | 0.06 | 0.45 ** | |
| SE | 2.60 | 0.11 | 0.20 | ||
| MCB-C | Coef | 2.11 | −0.28 | 0.44 | |
| SE | −4.49 | 0.18 | 0.36 | ||
| MCB-MCB | Coef | 3.91 | −0.07 | 0.50 ** | |
| SE | 2.42 | 0.11 | 0.25 | ||
| Constant | Coef | 39.36 *** | 1.15 *** | 0.91 *** | |
| SE | 1.83 | 0.08 | 0.13 | ||
| ABTS (% Inhibition) | FRAP (μmol Ascorbic Acid) | MDA (μM) | Birth Weight (Kg) | Weaning Weight (Kg) | Growth Rate (g/day) | |
|---|---|---|---|---|---|---|
| ABTS | 1.00 | 0.22 | 0.13 | 0.28 | −0.09 | −0.16 |
| FRAP | 1.00 | 0.16 | −0.10 | −0.32 | −0.30 | |
| MDA | 1.00 | 0.18 | −0.28 | −0.32 | ||
| Birth weight | 1.00 | 0.12 | −0.11 | |||
| Weaning weight | 1.00 | 0.97 | ||||
| Growth rate | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotsampasi, B.; Tsiplakou, E.; Karatzia, M.-A.; Oikonomou, S.; Mitsiopoulou, C.; Kalogiannis, D.; Dovolou, E.; Lymperopoulos, A.; Sotirakoglou, K.; Anastasiadou, M.; et al. Effects of Rumen-Protected Methionine, Choline, and Betaine Supplementation on Ewes’ Pregnancy and Reproductive Outcomes. Vet. Sci. 2025, 12, 723. https://doi.org/10.3390/vetsci12080723
Kotsampasi B, Tsiplakou E, Karatzia M-A, Oikonomou S, Mitsiopoulou C, Kalogiannis D, Dovolou E, Lymperopoulos A, Sotirakoglou K, Anastasiadou M, et al. Effects of Rumen-Protected Methionine, Choline, and Betaine Supplementation on Ewes’ Pregnancy and Reproductive Outcomes. Veterinary Sciences. 2025; 12(8):723. https://doi.org/10.3390/vetsci12080723
Chicago/Turabian StyleKotsampasi, Basiliki, Eleni Tsiplakou, Maria-Anastasia Karatzia, Stavroula Oikonomou, Christina Mitsiopoulou, Dimitris Kalogiannis, Eleni Dovolou, Aristotelis Lymperopoulos, Kyriaki Sotirakoglou, Maria Anastasiadou, and et al. 2025. "Effects of Rumen-Protected Methionine, Choline, and Betaine Supplementation on Ewes’ Pregnancy and Reproductive Outcomes" Veterinary Sciences 12, no. 8: 723. https://doi.org/10.3390/vetsci12080723
APA StyleKotsampasi, B., Tsiplakou, E., Karatzia, M.-A., Oikonomou, S., Mitsiopoulou, C., Kalogiannis, D., Dovolou, E., Lymperopoulos, A., Sotirakoglou, K., Anastasiadou, M., Zervas, G., & Chadio, S. (2025). Effects of Rumen-Protected Methionine, Choline, and Betaine Supplementation on Ewes’ Pregnancy and Reproductive Outcomes. Veterinary Sciences, 12(8), 723. https://doi.org/10.3390/vetsci12080723








