A Review of Avian Influenza Virus Exposure Patterns and Risks Among Occupational Populations
Simple Summary
Abstract
1. Introduction
2. Methods
3. AIV Exposure Patterns Among Occupational Populations
3.1. Contact and Respiratory Exposure
3.2. Foodborne Exposure
3.3. Potential Human-to-Human Transmission
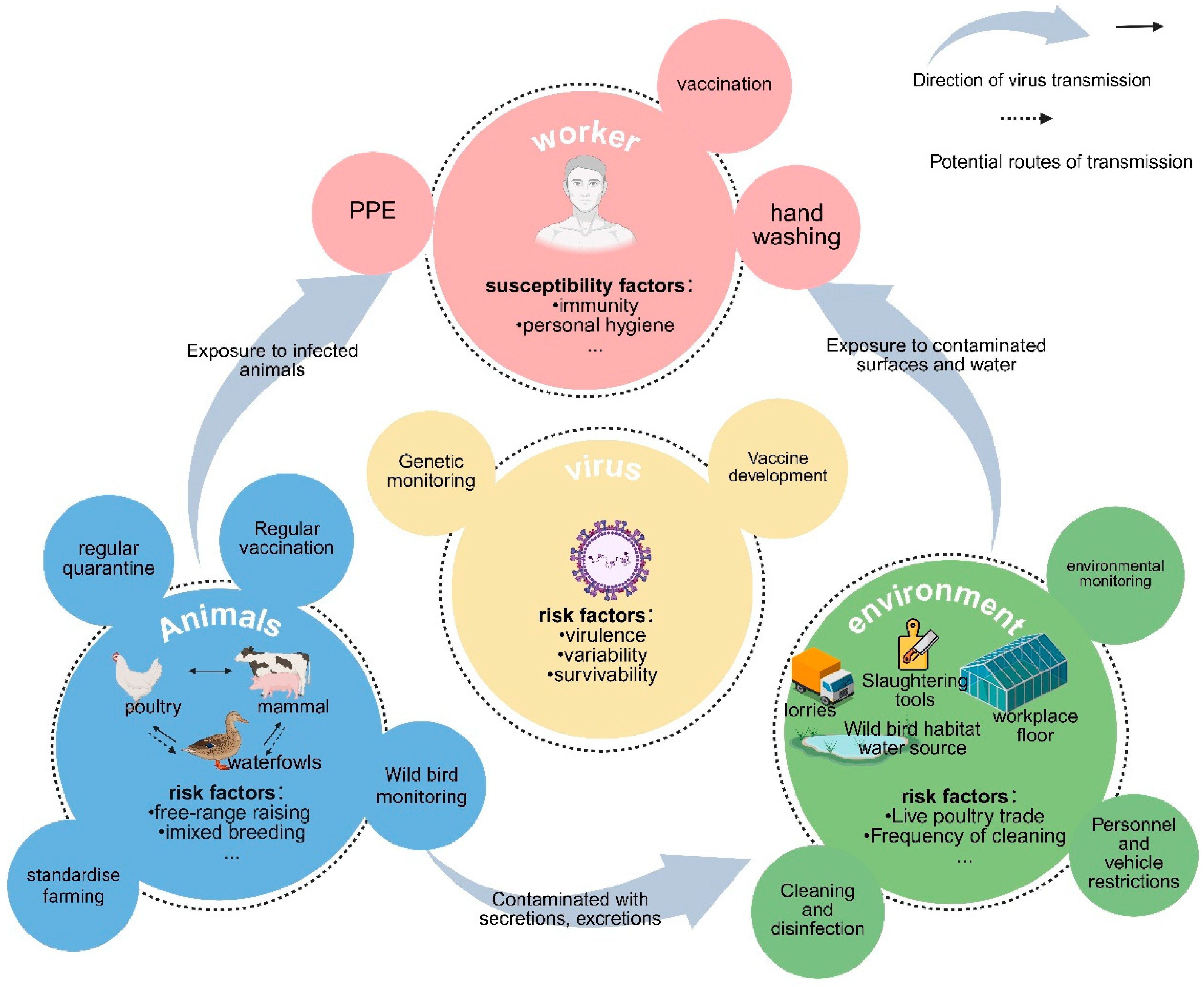
4. Risk Factors for AIV Exposure in Occupational Populations
4.1. Viral Factors
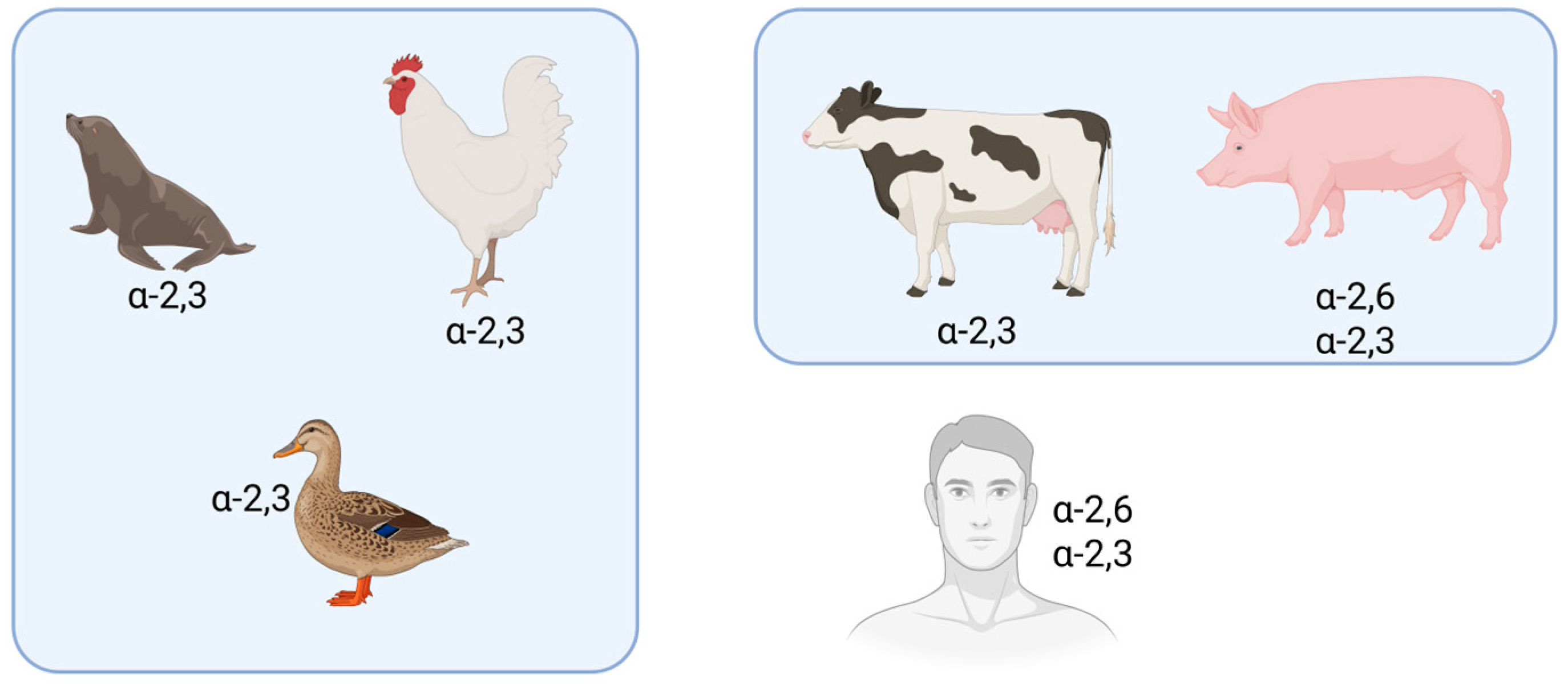
4.1.1. Cross-Species Transmissibility and Virulence of Avian Influenza Viruses
4.1.2. Viral Survival Capability
4.2. Animal Host Factors
4.2.1. Wild Bird Activities
4.2.2. Poultry Trade
4.2.3. Milking Activities
4.3. Environmental Factors
4.3.1. Mechanical Carrying by Personnel and Production Materials
4.3.2. Environmental Contamination Factors
4.4. Susceptible Population Factors
5. Prevention and Control Measures for Occupational AIV Exposure
5.1. Multi-Dimensional Surveillance Network and Response
5.2. Enhanced Environmental Management and Hygiene
5.3. Health Education, Occupational Training, and Worker Symptom Monitoring
6. Conclusions and Prospects
7. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Li, Y.-T.; Linster, M.; Mendenhall, I.H.; Su, Y.C.F.; Smith, G.J.D. Avian Influenza Viruses in Humans: Lessons from Past Outbreaks. Br. Med. Bull. 2019, 132, 81. [Google Scholar] [CrossRef] [PubMed]
- Abdelwhab, E.M.; Mettenleiter, T.C. Zoonotic Animal Influenza Virus and Potential Mixing Vessel Hosts. Viruses 2023, 15, 980. [Google Scholar] [CrossRef] [PubMed]
- WHO’s Western Pacific Regional Office. Avian Influenza Weekly Update 2025. 2024. Available online: https://iris.who.int/handle/10665/380024 (accessed on 16 July 2025).
- Chen, H.; Smith, G.J.D.; Li, K.S.; Wang, J.; Fan, X.H.; Rayner, J.M.; Vijaykrishna, D.; Zhang, J.X.; Zhang, L.J.; Guo, C.T.; et al. Establishment of Multiple Sublineages of H5N1 Influenza Virus in Asia: Implications for Pandemic Control. Proc. Natl. Acad. Sci. USA 2006, 103, 2845–2850. [Google Scholar] [CrossRef] [PubMed]
- Claas, E.C.; Osterhaus, A.D.; Van Beek, R.; De Jong, J.C.; Rimmelzwaan, G.F.; Senne, D.A.; Krauss, S.; Shortridge, K.F.; Webster, R. Human Influenza A H5N1 Virus Related to a Highly Pathogenic Avian Influenza Virus. Lancet 1998, 351, 472–477. [Google Scholar] [CrossRef]
- Krammer, F.; Hermann, E.; Rasmussen, A.L. Highly Pathogenic Avian Influenza H5N1: History, Current Situation, and Outlook. J. Virol. 2025, 99, e02209. [Google Scholar] [CrossRef]
- Kilpatrick, A.M.; Chmura, A.A.; Gibbons, D.W.; Fleischer, R.C.; Marra, P.P.; Daszak, P. Predicting the Global Spread of H5N1 Avian Influenza. Proc. Natl. Acad. Sci. USA 2006, 103, 19368–19373. [Google Scholar] [CrossRef]
- Gutiérrez, R.A.; Naughtin, M.J.; Horm, S.V.; San, S.; Buchy, P. A(H5N1) Virus Evolution in South East Asia. Viruses 2009, 1, 335–361. [Google Scholar] [CrossRef]
- Olsen, B.; Munster, V.J.; Wallensten, A.; Waldenström, J.; Osterhaus, A.D.; Fouchier, R.A. Global Patterns of Influenza a Virus in Wild Birds. Science 2006, 312, 384–388. [Google Scholar] [CrossRef]
- Peacock, T.P.; Moncla, L.; Dudas, G.; VanInsberghe, D.; Sukhova, K.; Lloyd-Smith, J.O.; Worobey, M.; Lowen, A.C.; Nelson, M.I. The Global H5N1 Influenza Panzootic in Mammals. Nature 2025, 637, 304–313. [Google Scholar] [CrossRef]
- Mostafa, A.; Naguib, M.M.; Nogales, A.; Barre, R.S.; Stewart, J.P.; García-Sastre, A.; Martinez-Sobrido, L. Avian Influenza A (H5N1) Virus in Dairy Cattle: Origin, Evolution, and Cross-Species Transmission. mBio 2024, 15, e0254224. [Google Scholar] [CrossRef]
- Lowen, A.C.; Baker, A.L.; Bowman, A.S.; García-Sastre, A.; Hensley, S.E.; Lakdawala, S.S.; Moncla, L.H.; Nelson, M.I.; Pekosz, A.; Poulson, R.L.; et al. Pandemic Risk Stemming from the Bovine H5N1 Outbreak: An Account of the Knowns and Unknowns. J. Virol. 2025, 99, e00052-25. [Google Scholar] [CrossRef]
- Leguia, M.; Garcia-Glaessner, A.; Muñoz-Saavedra, B.; Juarez, D.; Barrera, P.; Calvo-Mac, C.; Jara, J.; Silva, W.; Ploog, K.; Amaro, L.; et al. Highly Pathogenic Avian Influenza A (H5N1) in Marine Mammals and Seabirds in Peru. Nat. Commun. 2023, 14, 5489. [Google Scholar] [CrossRef] [PubMed]
- Rimondi, A.; Vanstreels, R.E.; Olivera, V.; Donini, A.; Lauriente, M.M.; Uhart, M.M. Highly Pathogenic Avian Influenza A(H5N1) Viruses from Multispecies Outbreak, Argentina, August 2023. Emerg. Infect. Dis. 2024, 30, 812. [Google Scholar] [CrossRef]
- Kuiken, T.; Vanstreels, R.E.T.; Banyard, A.; Begeman, L.; Breed, A.C.; Dewar, M.; Fijn, R.; Serafini, P.P.; Uhart, M.; Wille, M. Emergence, Spread, and Impact of High-Pathogenicity Avian Influenza H5 in Wild Birds and Mammals of South America and Antarctica. Conserv. Biol. 2025, e70052. [Google Scholar] [CrossRef] [PubMed]
- Banyard, A.C.; Bennison, A.; Byrne, A.M.P.; Reid, S.M.; Lynton-Jenkins, J.G.; Mollett, B.; Silva, D.D.; Peers-Dent, J.; Finlayson, K.; Hall, R.; et al. Detection and Spread of High Pathogenicity Avian Influenza Virus H5N1 in the Antarctic Region. Nat. Commun. 2024, 15, 7433. [Google Scholar] [CrossRef]
- Ahmadivand, S.; Savage, A.C.N.P.; Palic, D. Biosecurity and Vaccines for Emerging Aquatic Animal RNA Viruses. Viruses 2025, 17, 768. [Google Scholar] [CrossRef]
- Zhou, L.; Liao, Q.; Dong, L.; Huai, Y.; Bai, T.; Xiang, N.; Shu, Y.; Liu, W.; Wang, S.; Qin, P.; et al. Risk Factors for Human Illness with Avian Influenza A (H5N1) Virus Infection in China. J. Infect. Dis. 2009, 199, 1726–1734. [Google Scholar] [CrossRef]
- Catalan Saenz, H.S.; Cruz-Ausejo, L. Preventive, Safety and Control Measures against Avian Influenza A(H5N1) in Occupationally Exposed Groups: A Scoping Review. One Health 2024, 19, 100766. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Harriman, K.; Liu, C.; Kraushaar, V.; Hoover, C.; Shim, K.; Brummitt, S.I.; Limas, J.; Garvey, K.; McNary, J.; et al. Human Cases of Highly Pathogenic Avian Influenza A(H5N1)—California, September-December 2024. MMWR Morb. Mortal. Wkly. Rep. 2025, 74, 127–133. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control; European Union Reference Laboratory for Avian Influenza; Alexakis, L.; Buczkowski, H.; Ducatez, M.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Ståhl, K.; et al. Avian Influenza Overview December 2024-March 2025. EFSA J. 2025, 23, e9352. [Google Scholar]
- Zhou, Y. Cross-Species Transmission and Human Adaptive Mutation of H7N9 Avian Influenza Virus. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2021. [Google Scholar]
- Lowen, A.C.; Bowman, A.S.; Runstadler, J.A.; Baker, A.L.; García-Sastre, A.; Hensley, S.E.; Lakdawala, S.S.; Moncla, L.H.; Nelson, M.; Pekosz, A.; et al. Controlling Bird Flu Is Urgent—For Dairy, Wildlife, Poultry, Pets, and People. J. Am. Vet. Med. Assoc. 2025, 1, 1–4. [Google Scholar] [CrossRef]
- Ahmed, S.S.U.; Ersbøll, A.K.; Biswas, P.K.; Christensen, J.P.; Hannan, A.S.M.A.; Toft, N. Ecological Determinants of Highly Pathogenic Avian Influenza (H5N1) Outbreaks in Bangladesh. PLoS ONE 2012, 7, e33938. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.; Pfeiffer, D.U. Risk Factor Modelling of the Spatio-Temporal Patterns of Highly Pathogenic Avian Influenza (HPAIV) H5N1: A Review. Spat. Spatiotemporal Epidemiol. 2012, 3, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Kurscheid, J.; Stevenson, M.; Durr, P.A.; Toribio, J.A.; Kurscheid, S.; Ambarawati, I.G.; Abdurrahman, M.; Fenwick, S. Social Network Analysis of the Movement of Poultry to and from Live Bird Markets in Bali and Lombok, Indonesia. Transbound. Emerg. Dis. 2017, 64, 2023–2033. [Google Scholar] [CrossRef]
- Roche, S.E.; Cogger, N.; Garner, M.G.; Putra, A.A.G.; Toribio, J.-A.L.M.L. Assessing the Risk of Highly Pathogenic Avian Influenza H5N1 Transmission through Poultry Movements in Bali, Indonesia. Prev. Vet. Med. 2014, 113, 599–607. [Google Scholar] [CrossRef]
- Zhang, J.; Jing, W.; Zhang, W.; Jin, Z. Avian Influenza A (H7N9) Model Based on Poultry Transport Network in China. Comput. Math. Methods Med. 2018, 2018, 7383170. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.M.; Verhagen, J.H.; Mostafa, A.; Wille, M.; Li, R.; Graaf, A.; Järhult, J.D.; Ellström, P.; Zohari, S.; Lundkvist, Å.; et al. Global Patterns of Avian Influenza A (H7): Virus Evolution and Zoonotic Threats. FEMS Microbiol. Rev. 2019, 43, 608. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Zhang, Y. Analysis and Control of Risk Factors for Introduction of Highly Pathogenic Avian Influenza in Poultry Farms. China Anim. Health Insp. 2018, 35, 29–34. [Google Scholar]
- Islam, S.S.; Akwar, H.; Hossain, M.; Sufian, A.; Hasan, Z.; Chakma, S.; Meeyam, T.; Chaisowwong, W.; Punyapornwithaya, V.; Debnath, N.C.; et al. Qualitative Risk Assessment of Transmission Pathways of Highly Pathogenic Avian Influenza (HPAI) Virus at Live Poultry Markets in Dhaka City, Bangladesh. Zoonoses Public Health 2020, 67, 658–672. [Google Scholar] [CrossRef]
- Xiao, C.; Xu, J.; Lan, Y.; Huang, Z.; Zhou, L.; Guo, Y.; Li, X.; Yang, L.; Gao, G.F.; Wang, D.; et al. Five Independent Cases of Human Infection with Avian Influenza H5N6—Sichuan Province, China, 2021. China CDC Weekly 2021, 3, 751. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, L. Surveillance of avian influenza virus and serological analysis of occupationally exposure population in Yangzhou City from 2019 to 2021. J. Mol. Diagn. Ther. 2023, 15, 536–540. [Google Scholar]
- Fu, X.; Huang, W. Surveillance and analysis of serology and external environmental Avian influenza virus among occupationally exposed population in Ezhou City, Hubei Province, from 2014 to 2021. J. Med. Pest. Control 2023, 39, 329–333. [Google Scholar]
- Castillo, A.; Fasce, R.; Parra, B.; Andrade, W.; Covarrubias, P.; Hueche, A.; Campano, C.; Tambley, C.; Rojas, M.; Araya, M.; et al. The First Case of Human Infection with H5N1 Avian Influenza A Virus in Chile. J. Travel. Med. 2023, 30, taad083. [Google Scholar] [CrossRef]
- Horwood, P.F.; Horm, S.V.; Yann, S.; Tok, S.; Chan, M.; Suttie, A.; Y, P.; Rith, S.; Siegers, J.Y.; San, S.; et al. Aerosol Exposure of Live Bird Market Workers to Viable Influenza A/H5N1 and A/H9N2 Viruses, Cambodia. Zoonoses Public Health 2022, 70, 171. [Google Scholar] [CrossRef]
- Van Kerkhove, M.D.; Ly, S.; Holl, D.; Guitian, J.; Mangtani, P.; Ghani, A.C.; Vong, S. Frequency and Patterns of Contact with Domestic Poultry and Potential Risk of H5N1 Transmission to Humans Living in Rural Cambodia. Influenza Other Respir. Viruses 2008, 2, 155–163. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, F.; Pang, Z. Establishment and application of risk assessment model for avian influenza A (H5N1) virus infection in poultry workers. Chin. J. Public Health 2016, 32, 1732–1736. [Google Scholar]
- Wang, Y. Investigation of Occupational Exposure to Avian Influenza in Jiangsu Province and Study on the Neutralizaiton of Human Monoclonal Neutralizing Antibody Against H7N9. Ph.D. Thesis, Southeast University China, Nanjing, China, 2023. [Google Scholar]
- Le Sage, V.; Campbell, A.J.; Reed, D.S.; Duprex, W.P.; Lakdawala, S.S. Persistence of Influenza H5N1 and H1N1 Viruses in Unpasteurized Milk on Milking Unit Surfaces. Emerg. Infect. Dis. 2024, 30, 1721–1723. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Fu, W. Epidemiological investigation and disposal of the first human case of H5N6 avian influenza death in Yiyang County, Jiangxi Province. Anhui J. Prev. Med. 2024, 30, 314–316, 343. [Google Scholar]
- Abdelwhab, E.M.; Grund, C.; Aly, M.M.; Beer, M.; Harder, T.C.; Hafez, H.M. Multiple Dose Vaccination with Heterologous H5N2 Vaccine: Immune Response and Protection against Variant Clade 2.2.1 Highly Pathogenic Avian Influenza H5N1 in Broiler Breeder Chickens. Vaccine 2011, 29, 6219–6225. [Google Scholar] [CrossRef]
- Caserta, L.C.; Frye, E.A.; Butt, S.L.; Laverack, M.; Nooruzzaman, M.; Covaleda, L.M.; Thompson, A.C.; Koscielny, M.P.; Cronk, B.; Johnson, A.; et al. Spillover of Highly Pathogenic Avian Influenza H5N1 Virus to Dairy Cattle. Nature 2024, 634, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Rosenke, K.; Griffin, A.; Kaiser, F.; Altynova, E.; Mukesh, R.; Bushmaker, T.; Flagg, M.; Tipih, T.; Goldin, K.; Wickenhagen, A.; et al. Pathogenesis of Bovine H5N1 Clade 2.3.4.4b Infection in Macaques. Nature 2025, 640, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Saied, A.A.; El-Saeed, B.A. Infectiousness of Raw (Unpasteurised) Milk from Influenza H5N1-Infected Cows beyond the USA. Lancet Microbe 2025, 101107. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, E.; Bao, C.; Xiang, N.; Wu, J.; Wu, S.; Shi, J.; Wang, X.; Zheng, Y.; Zhang, Y.; et al. Clusters of Human Infection and Human-to-Human Transmission of Avian Influenza A(H7N9) Virus, 2013–2017. Emerg. Infect. Dis. 2018, 24, 397. [Google Scholar] [CrossRef]
- Philippon, D.A.; Wu, P.; Cowling, B.J.; Lau, E.H. Avian Influenza Human Infections at the Human-Animal Interface. J. Infect. Dis. 2020, 222, 528–537. [Google Scholar] [CrossRef]
- Herfst, S.; Schrauwen, E.J.; Linster, M.; Chutinimitkul, S.; De Wit, E.; Munster, V.J.; Sorrell, E.M.; Bestebroer, T.M.; Burke, D.F.; Smith, D.J.; et al. Airborne Transmission of Influenza A/H5N1 Virus between Ferrets. Science 2012, 336, 1534–1541. [Google Scholar] [CrossRef]
- Zhu, W.; Yang, L.; Han, X.; Tan, M.; Zou, S.; Li, X.; Huang, W.; Zeng, X.; Wang, D. Origin, Pathogenicity, and Transmissibility of a Human Isolated Influenza A(H10N3) Virus from China. Emerg. Microbes Infect. 2025, 14, 2432364. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Hutter, C.R.; Markin, A.; Thomas, M.; Lantz, K.; Killian, M.L.; Janzen, G.M.; Vijendran, S.; Wagle, S.; Inderski, B.; et al. Emergence and Interstate Spread of Highly Pathogenic Avian Influenza A(H5N1) in Dairy Cattle in the United States. Science 2025, 388, eadq0900. [Google Scholar] [CrossRef]
- Neumann, G.; Kawaoka, Y. Host Range Restriction and Pathogenicity in the Context of Influenza Pandemic. Emerg. Infect. Dis. 2006, 12, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Medina, R.A.; García-Sastre, A. Influenza A Viruses: New Research Developments. Nat. Rev. Microbiol. 2011, 9, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-H.; Zhu, X. A Single Mutation in Bovine Influenza H5N1 Hemagglutinin Switches Specificity to Human Receptors. Science 2024, 386, 1128–1134. [Google Scholar] [CrossRef]
- Zhang, R.; Dong, X.; Wang, D.; Feng, L.; Zhou, L.; Ren, R.; Greene, C.; Song, Y.; Millman, A.J.; Azziz-Baumgartner, E.; et al. One Hundred Years of Influenza Since the 1918 Pandemic—Is China Prepared Today? China CDC Weekly 2019, 1, 56. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasan, C.C.; Li, F.; Wang, D. Emerging Threats of Highly Pathogenic Avian Influenza A (H5N1) in US Dairy Cattle: Understanding Cross-Species Transmission Dynamics in Mammalian Hosts. Viruses 2024, 16, 1703. [Google Scholar] [CrossRef]
- Webster, R.G.; Yakhno, M.; Hinshaw, V.S.; Bean, W.J.; Murti, K.G. Intestinal Influenza: Replication and Characterization of Influenza Viruses in Ducks. Virology 1978, 84, 268–278. [Google Scholar] [CrossRef]
- Sharp, G.B.; Kawaoka, Y.; Jones, D.J.; Bean, W.J.; Pryor, S.P.; Hinshaw, V.; Webster, R.G. Coinfection of Wild Ducks by Influenza A Viruses: Distribution Patterns and Biological Significance. J. Virol. 1997, 71, 6128–6135. [Google Scholar] [CrossRef]
- Islam, A.; Amin, E.; Munro, S.; Hossain, M.E.; Islam, S.; Hassan, M.M.; Mamun, A.A.; Samad, M.A.; Shirin, T.; Rahman, M.Z.; et al. Potential Risk Zones and Climatic Factors Influencing the Occurrence and Persistence of Avian Influenza Viruses in the Environment of Live Bird Markets in Bangladesh. One Health 2023, 17, 100644. [Google Scholar] [CrossRef]
- Lowen, A.C.; Mubareka, S.; Steel, J.; Palese, P. Influenza Virus Transmission Is Dependent on Relative Humidity and Temperature. PLoS Pathog. 2007, 3, e151. [Google Scholar] [CrossRef]
- Lau, S.Y.; Chen, E.; Wang, M.; Cheng, W.; Zee, B.C.; Han, X.; Yu, Z.; Sun, R.; Chong, K.C.; Wang, X. Association between Meteorological Factors, Spatiotemporal Effects, and Prevalence of Influenza A Subtype H7 in Environmental Samples in Zhejiang Province, China. Sci. Total Environ. 2019, 663, 793–803. [Google Scholar] [CrossRef]
- Charostad, J.; Rezaei Zadeh Rukerd, M.; Mahmoudvand, S.; Bashash, D.; Hashemi, S.M.A.; Nakhaie, M.; Zandi, K. A Comprehensive Review of Highly Pathogenic Avian Influenza (HPAI) H5N1: An Imminent Threat at Doorstep. Travel. Med. Infect. Dis. 2023, 55, 102638. [Google Scholar] [CrossRef]
- Sun, L.; Ward, M.P.; Li, R.; Xia, C.; Lynn, H.; Hu, Y.; Xiong, C.; Zhang, Z. Global Spatial Risk Pattern of Highly Pathogenic Avian Influenza H5N1 Virus in Wild Birds: A Knowledge-Fusion Based Approach. Prev. Vet. Med. 2018, 152, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Toribio, J.A.; Scott, A.B.; Groves, P.; Barnes, B.; Glass, K.; Moloney, B.; Black, A.; Hernandez-Jover, M. Assessing the Probability of Introduction and Spread of Avian Influenza (AI) Virus in Commercial Australian Poultry Operations Using an Expert Opinion Elicitation. PLoS ONE 2018, 13, e0193730. [Google Scholar] [CrossRef] [PubMed]
- Galletti, G.; Santi, A.; Guberti, V.; Paternoster, G.; Licata, E.; Loli Piccolomini, L.; Procopio, A.; Tamba, M. A Method to Identify the Areas at Risk for the Introduction of Avian Influenza Virus into Poultry Flocks through Direct Contact with Wild Ducks. Transbound. Emerg. Dis. 2018, 65, 1033–1038. [Google Scholar] [CrossRef]
- Cui, P.; Zeng, X.; Li, X.; Li, Y.; Shi, J.; Zhao, C.; Qu, Z.; Wang, Y.; Guo, J.; Gu, W.; et al. Genetic and Biological Characteristics of the Globally Circulating H5N8 Avian Influenza Viruses and the Protective Efficacy Offered by the Poultry Vaccine Currently Used in China. Sci. China Life Sci. 2022, 65, 795–808. [Google Scholar] [CrossRef]
- Xue, F.; Peng, Y. Distribution of different HA subtypes of low pathogenic avian influenza viruses in domestic ducks in East China, 2002–2005. Chin. J. Zoonoses 2006, 888–889, 854. [Google Scholar]
- Verhagen, J.H.; Fouchier, R.A.M.; Lewis, N. Highly Pathogenic Avian Influenza Viruses at the Wild–Domestic Bird Interface in Europe: Future Directions for Research and Surveillance. Viruses 2021, 13, 212. [Google Scholar] [CrossRef] [PubMed]
- Globig, A.; Staubach, C.; Beer, M.; Köppen, U.; Fiedler, W.; Nieburg, M.; Wilking, H.; Starick, E.; Teifke, J.P.; Werner, O.; et al. Epidemiological and Ornithological Aspects of Outbreaks of Highly Pathogenic Avian Influenza Virus H5N1 of Asian Lineage in Wild Birds in Germany, 2006 and 2007. Transbound. Emerg. Dis. 2009, 56, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, B.; Chen, Q.; Chen, J.; Chen, Z. Characterization of an H10N8 Influenza Virus Isolated from Dongting Lake Wetland. Virol. J. 2011, 8, 42. [Google Scholar] [CrossRef]
- Yao, M. Occurrence and lransmission Echanism of Avian Influenza Virus (H9N2 Subtype)Aerosol and Its Infection Characteristics to the SPF Chickens. Ph.D. Thesis, Shandong Agricultural University, Tai’an, China, 2010. [Google Scholar]
- Yoo, D.; Chun, B.C.; Hong, K.; Kim, J. Risk Prediction of Three Different Subtypes of Highly Pathogenic Avian Influenza Outbreaks in Poultry Farms: Based on Spatial Characteristics of Infected Premises in South Korea. Front. Vet. Sci. 2022, 9, 897763. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-Y.; Lau, E.H.; Yuan, J.; Lu, M.-L.; Xie, C.-J.; Li, K.-B.; Ma, X.-W.; Chen, J.-D.; Liu, Y.-H.; Cao, L.; et al. Transmission Risk of Avian Influenza Virus along Poultry Supply Chains in Guangdong, China. J. Infect. 2019, 79, 43–48. [Google Scholar] [CrossRef]
- Chen, Y.; He, R. Risk Factor Survey on Live Poultry Infection with Influenza Virus in Live Poultry Wholesale Markets in Guangzhou City. China Acad. J. Electron. Publ. House 2018, 35, 5–8. [Google Scholar]
- Chaudhry, M.; Rashid, H.B.; Angot, A.; Thrusfield, M.; Bronsvoort, B.M.D.; Capua, I.; Cattoli, G.; Welburn, S.C.; Eisler, M.C. Risk Factors for Avian Influenza H9 Infection of Chickens in Live Bird Retail Stalls of Lahore District, Pakistan 2009–2010. Sci. Rep. 2018, 8, 5634. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. H5 Bird Flu: Current Situation. 2025. Available online: https://www.cdc.gov/ (accessed on 16 July 2025).
- Marshall, K.E.; Drehoff, C.C.; Alden, N.; Montoya, S.; Stringer, G.; Kohnen, A.; Mellis, A.; Ellington, S.; Singleton, J.; Reed, C.; et al. Personal Protective Equipment Use by Dairy Farmworkers Exposed to Cows Infected with Highly Pathogenic Avian Influenza A(H5N1) Viruses—Colorado, 2024. Morb. Mortal. Wkly. Rep. 2024, 73, 999. [Google Scholar] [CrossRef]
- Ssematimba, A.; Hagenaars, T.J.; de Wit, J.J.; Ruiterkamp, F.; Fabri, T.H.; Stegeman, J.A.; de Jong, M.C.M. Avian Influenza Transmission Risks: Analysis of Biosecurity Measures and Contact Structure in Dutch Poultry Farming. Prev. Vet. Med. 2013, 109, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Patyk, K.A.; Fields, V.L.; Beam, A.L.; Branan, M.A.; McGuigan, R.E.; Green, A.; Torchetti, M.K.; Lantz, K.; Freifeld, A.; Marshall, K.; et al. Investigation of Risk Factors for Introduction of Highly Pathogenic Avian Influenza H5N1 Infection among Commercial Turkey Operations in the United States, 2022: A Case-Control Study. Front. Vet. Sci. 2023, 10, 1229071. [Google Scholar] [CrossRef] [PubMed]
- Van Kerkhove, M.D.; Vong, S.; Guitian, J.; Holl, D.; Mangtani, P.; San, S.; Ghani, A.C. Poultry Movement Networks in Cambodia: Implications for Surveillance and Control of Highly Pathogenic Avian Influenza (HPAI/H5N1). Vaccine 2009, 27, 6345–6352. [Google Scholar] [CrossRef]
- Zhou, S. Research on the Outbreak Transmission and Evolution of H5 Highly Pathogenic Avian Influenza Virus. Ph.D. Thesis, Tsinghua University, Beijing, China, 2017. [Google Scholar]
- Campbell, A.J.; Brizuela, K.; Lakdawala, S.S. mGem: Transmission and Exposure Risks of Dairy Cow H5N1 Influenza Virus. mBio 2025, 16, e02944-24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Fu, H. Surveillance of zoonotic pathogens in poultry and environment in Changsha live poultry wholesale market from 2022 to 2023. Mod. Prev. Med. 2024, 51, 2088–2093. [Google Scholar]
- Wang, Y.; Li, P.; Wu, Y.; Sun, X.; Yu, K.; Yu, C.; Qin, A. The Risk Factors for Avian Influenza on Poultry Farms: A Meta-Analysis. Prev. Vet. Med. 2014, 117, 1–6. [Google Scholar] [CrossRef]
- Li, P. Study on the Risks of Avian Influenza in the Poyang Lake Region. Master’s Thesis, Jiangxi Normal University, Nanchang, China, 2009. [Google Scholar]
- Islam, A.; Islam, S.; Amin, E.; Shano, S.; Samad, M.A.; Shirin, T.; Hassan, M.M.; Flora, M.S. Assessment of Poultry Rearing Practices and Risk Factors of H5N1 and H9N2 Virus Circulating among Backyard Chickens and Ducks in Rural Communities. PLoS ONE 2022, 17, e0275852. [Google Scholar] [CrossRef]
- Zhou, L.; Ren, R.; Ou, J.; Kang, M.; Wang, X.; Havers, F.; Huo, X.; Liu, X.; Sun, Q.; He, Y.; et al. Risk Factors for Influenza A(H7N9) Disease in China, a Matched Case Control Study, October 2014 to April 2015. Open Forum Infect. Dis. 2016, 3, ofw182. [Google Scholar] [CrossRef]
- Garg, S.; Reinhart, K.; Couture, A.; Kniss, K.; Davis, C.T.; Kirby, M.K.; Murray, E.L.; Zhu, S.; Kraushaar, V.; Wadford, D.A.; et al. Highly Pathogenic Avian Influenza A(H5N1) Virus Infections in Humans. N. Engl. J. Med. 2025, 392, 843–854. [Google Scholar] [CrossRef]
- Sun, X.; Belser, J.A.; Li, Z.-N.; Brock, N.; Pulit-Penaloza, J.A.; Kieran, T.J.; Pappas, C.; Zeng, H.; Chang, J.C.; Carney, P.J.; et al. Effect of Prior Influenza A(H1N1)Pdm09 Virus Infection on Pathogenesis and Transmission of Human Influenza A(H5N1) Clade 2.3.4.4b Virus in Ferret Model. Emerg. Infect. Dis. 2025, 31, 458–466. [Google Scholar] [CrossRef]
- Islam, A.; Islam, M.; Dutta, P.; Rahman, M.A.; Al Mamun, A.; Khan, A.D.; Samad, M.A.; Hassan, M.M.; Rahman, M.Z.; Shirin, T. Association of Biosecurity and Hygiene Practices with Avian Influenza A/H5 and A/H9 Virus Infections in Turkey Farms. Front. Vet. Sci. 2024, 11, 1319618. [Google Scholar] [CrossRef]
- Bartlett, M.L.; Palese, P.; Davis, M.F.; Vermund, S.H.; Bréchot, C.; Evans, J.D.; Sauer, L.M.; Osterhaus, A.; Pekosz, A.; Nelson, M.; et al. Enhancing the Response to Avian Influenza in the US and Globally. Lancet Reg. Health-Am. 2025, 46, 101100. [Google Scholar] [CrossRef]
- Leung, Y.H.C.; Lau, E.H.Y.; Zhang, L.J.; Guan, Y.; Cowling, B.J.; Peiris, J.S.M. Avian Influenza and Ban on Overnight Poultry Storage in Live Poultry Markets, Hong Kong. Emerg. Infect. Dis. 2012, 18, 1339–1341. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.; Liu, H.; Guo, F.; Doi, S.A.; Smith, C.; Clements, A.C.; Edwards, J.; Huang, B.; Soares Magalhães, R.J. Effectiveness of Market-Level Biosecurity at Reducing Exposure of Poultry and Humans to Avian Influenza: A Systematic Review and Meta-Analysis. J. Infect. Dis. 2018, 218, 1861–1875. [Google Scholar] [CrossRef]
- Yu, H.; Wu, J.T.; Cowling, B.J.; Liao, Q.; Fang, V.J.; Zhou, S.; Wu, P.; Zhou, H.; Lau, E.H.; Guo, D.; et al. Effect of Closure of Live Poultry Markets on Poultry-to-Person Transmission of Avian Influenza A H7N9 Virus: An Ecological Study. Lancet 2014, 383, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, X.; Zhao, W.; Yang, L.; Wang, Z.; Bi, H. Environmental Factors and Spatiotemporal Distribution Characteristics of the Global Outbreaks of the Highly Pathogenic Avian Influenza H5N1. Env. Sci. Pollut. Res. Int. 2022, 29, 44175–44185. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, M.; Webby, R.; Swayne, D.; Rashid, H.B.; DeBeauchamp, J.; Killmaster, L.; Criado, M.F.; Lee, D.-H.; Webb, A.; Yousaf, S.; et al. Avian Influenza at Animal-human Interface: One-health Challenge in Live Poultry Retail Stalls of Chakwal, Pakistan. Influenza Other Respir. Viruses 2020, 14, 257. [Google Scholar] [CrossRef]
- Tenzin, T.; Wangdi, C.; Rai, P.B. Biosecurity Survey in Relation to the Risk of HPAI Outbreaks in Backyard Poultry Holdings in Thimphu City Area, Bhutan. BMC Vet. Res. 2017, 13, 113. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Deng, G.; Cui, P.; Zeng, X.; Li, B.; Wang, D.; He, X.; Yan, C.; Zhang, Y.; Li, J.; et al. Evolution of H7N9 Highly Pathogenic Avian Influenza Virus in the Context of Vaccination. Emerg. Microbes Infect. 2024, 13, 2343912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Huang, C.; Mo, Y.; Wu, Z.; Li, H.; Huang, Y.; Liu, F.; Gao, L. A Detected Case of Avian Influenza H9N2 from Influenza-Like Illness Surveillance—Hunan Province, 2020. China CDC Wkly. 2020, 2, 700. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Ren, R.; Bai, W.; Li, Z.; Zhang, J.; Liu, Y.; Sun, R.; Wang, F.; Li, D.; Li, C.; et al. A Review of Avian Influenza Virus Exposure Patterns and Risks Among Occupational Populations. Vet. Sci. 2025, 12, 704. https://doi.org/10.3390/vetsci12080704
Li H, Ren R, Bai W, Li Z, Zhang J, Liu Y, Sun R, Wang F, Li D, Li C, et al. A Review of Avian Influenza Virus Exposure Patterns and Risks Among Occupational Populations. Veterinary Sciences. 2025; 12(8):704. https://doi.org/10.3390/vetsci12080704
Chicago/Turabian StyleLi, Huimin, Ruiqi Ren, Wenqing Bai, Zhaohe Li, Jiayi Zhang, Yao Liu, Rui Sun, Fei Wang, Dan Li, Chao Li, and et al. 2025. "A Review of Avian Influenza Virus Exposure Patterns and Risks Among Occupational Populations" Veterinary Sciences 12, no. 8: 704. https://doi.org/10.3390/vetsci12080704
APA StyleLi, H., Ren, R., Bai, W., Li, Z., Zhang, J., Liu, Y., Sun, R., Wang, F., Li, D., Li, C., Shi, G., & Zhou, L. (2025). A Review of Avian Influenza Virus Exposure Patterns and Risks Among Occupational Populations. Veterinary Sciences, 12(8), 704. https://doi.org/10.3390/vetsci12080704






