Exportin 1 (XPO1) Expression and Effectiveness of XPO1 Inhibitor Against Canine Lymphoma Cell Lines
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.3. Western Blotting
2.4. Cell Proliferation Assay
2.5. Trypan Blue Exclusion Assay
2.6. Statistical Analyses
3. Results
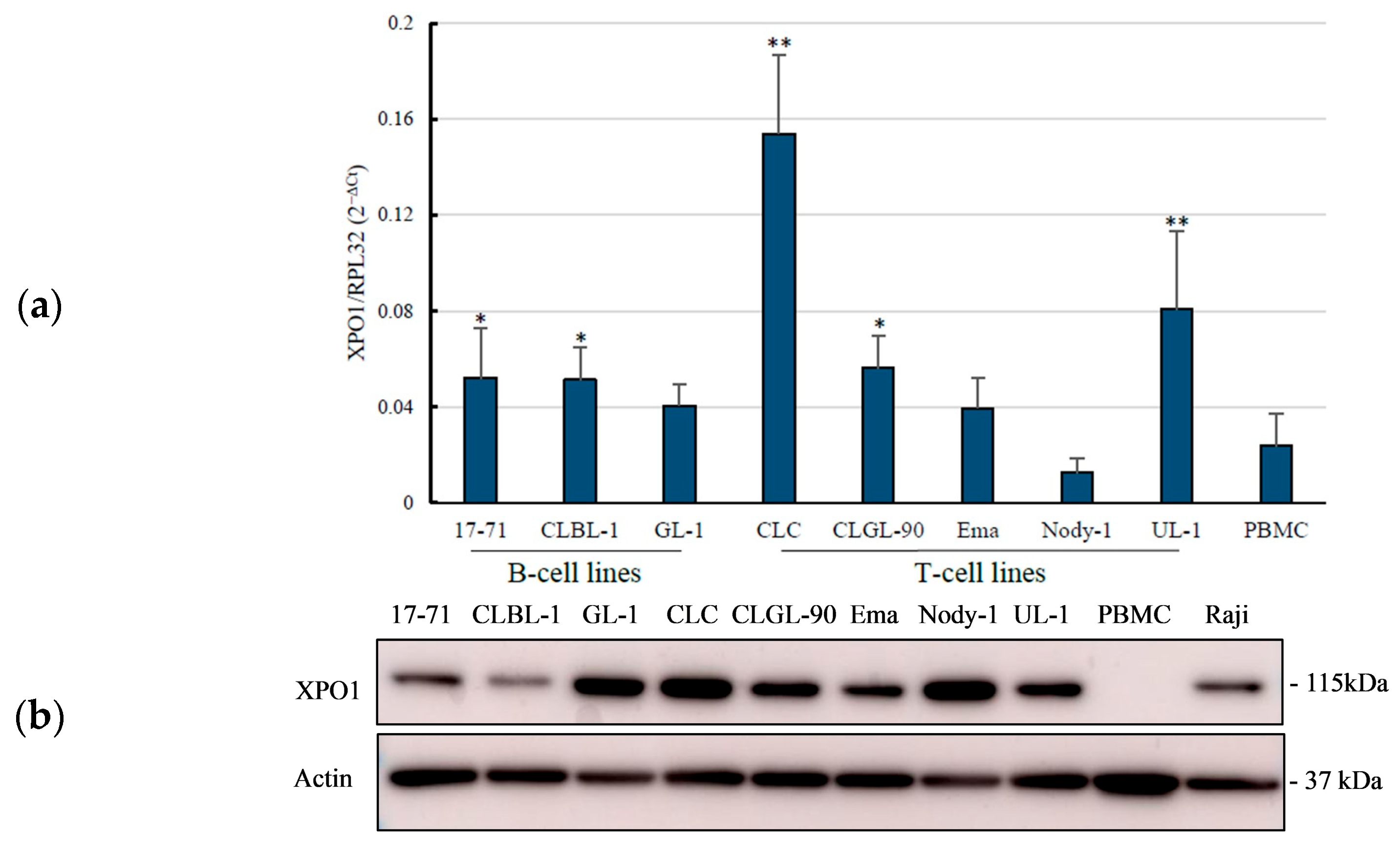
3.1. Overexpression of the XPO1 mRNA in Canine Lymphoma Cell Lines
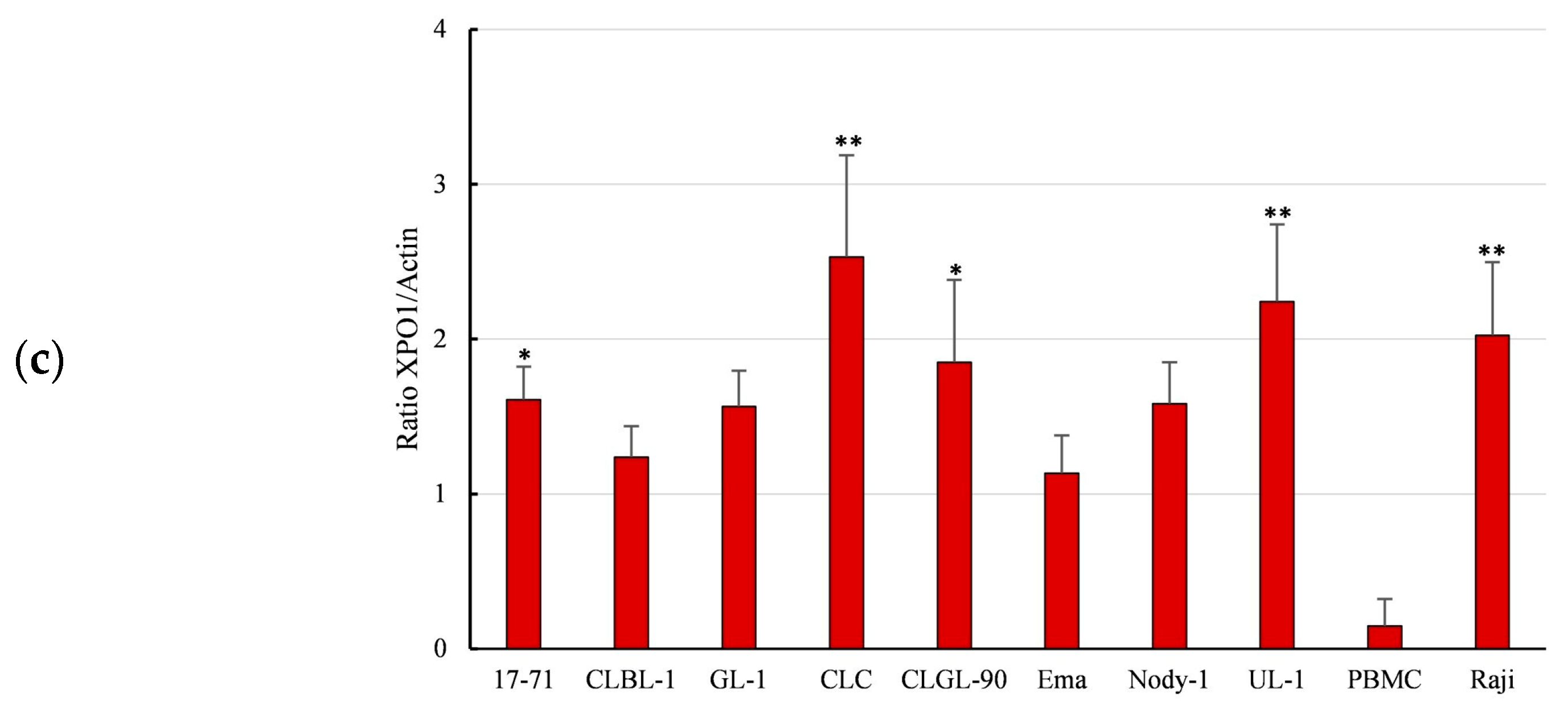
3.2. Overexpression of XPO1 Protein in the Canine Lymphoma Cell Lines
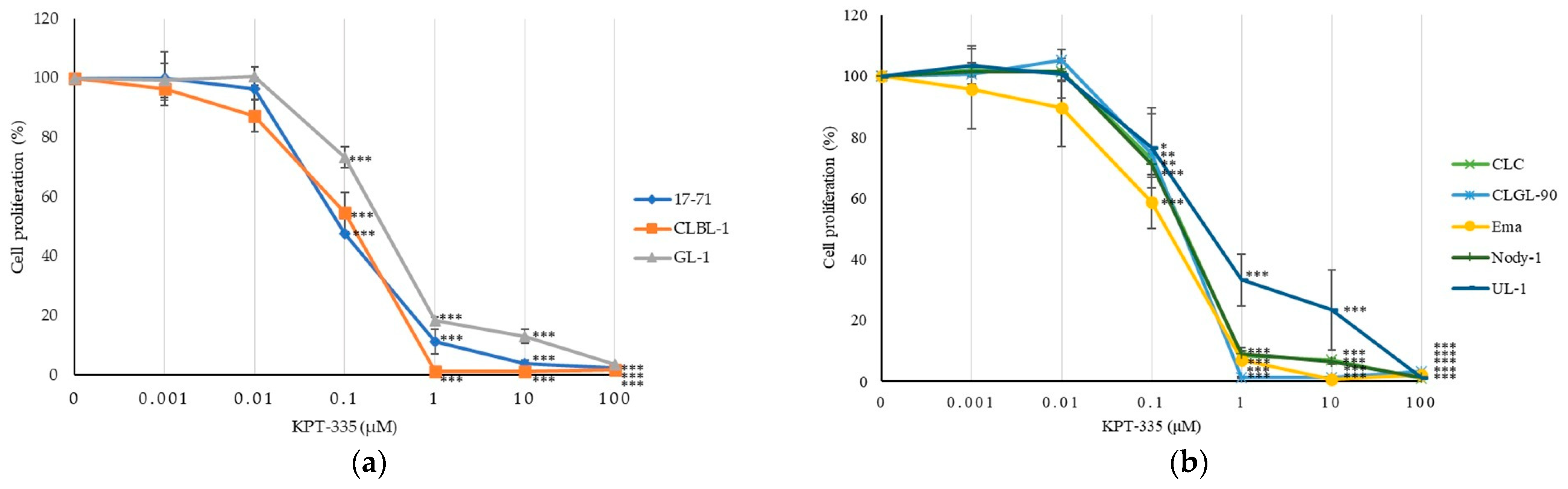
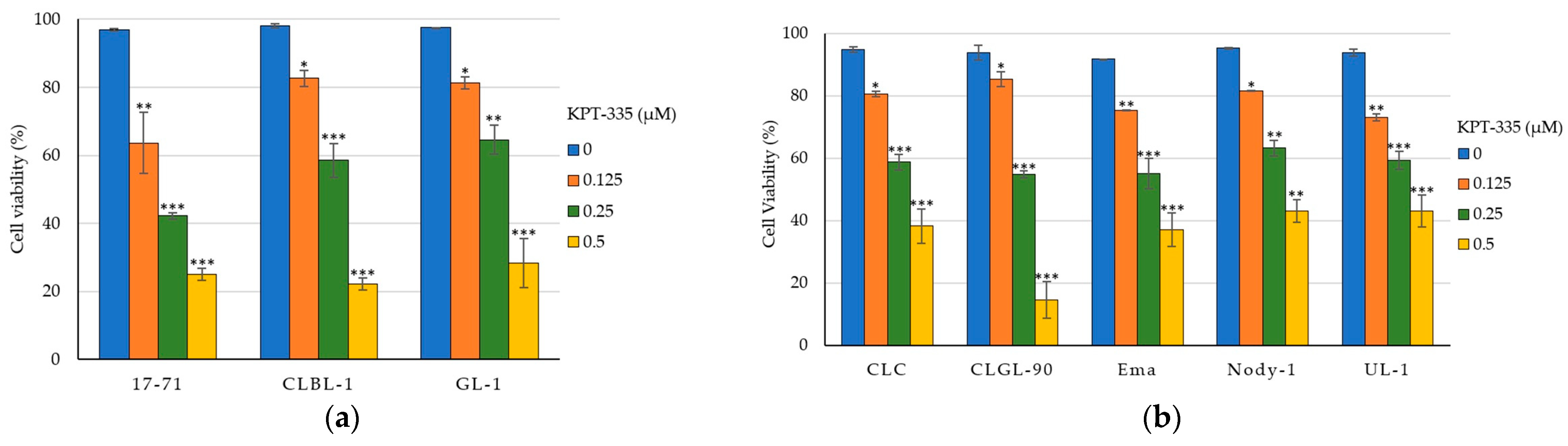
3.3. KPT-335 Inhibits the Proliferation of Canine Lymphoma Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valli, V.E.; Kass, P.H.; Myint, M.S.; Scott, F. Canine lymphomas: Association of classification type, disease stage, tumor subtype, mitotic rate, and treatment with survival. Vet. Pathol. 2012, 50, 738–748. [Google Scholar] [CrossRef]
- Sapierzyński, R.; Kliczkowska-Klarowicz, K.; Jankowska, U.; Jagielski, D. Cytodiagnostics of canine lymphomas—Possibilities and limitations. Pol. J. Vet. Sci. 2016, 19, 433–439. [Google Scholar] [CrossRef]
- Avery, A.C. The genetic and molecular basis for canine models of human leukemia and lymphoma. Front. Oncol. 2020, 10, 23. [Google Scholar] [CrossRef]
- Ponce, F.; Marchal, T.; Magnol, J.P.; Turinelli, V.; Ledieu, D.; Bonnefont, C.; Pastor, M.; Delignette, M.L.; Fournel-Fleury, C. A morphological study of 608 cases of canine malignant lymphoma in France with a focus on comparative similarities between canine and human lymphoma morphology. Vet. Pathol. 2010, 47, 414–433. [Google Scholar] [CrossRef]
- London, C.A.; Bernabe, L.F.; Barnard, S.; Kisseberth, W.C.; Borgatti, A.; Henson, M.; Wilson, H.; Jensen, K.; Ito, D.; Modiano, J.F.; et al. Preclinical evaluation of the novel, orally bioavailable selective inhibitor of nuclear export (SINE) KPT-335 in spontaneous canine cancer: Results of a phase I study. PLoS ONE 2014, 9, e87585. [Google Scholar] [CrossRef]
- Zandvliet, M. Canine lymphoma: A review. Vet. Q. 2016, 36, 76–104. [Google Scholar] [CrossRef]
- Turner, J.G.; Dawson, J.; Sullivan, D.M. Nuclear export of proteins and drug resistance in cancer. Biochem. Pharmacol. 2012, 83, 1021–1032. [Google Scholar] [CrossRef]
- Wang, Y.; Geng, M.; Chen, Q.; Li, Y.; Guo, X.; Shi, P.; He, L.; Xie, S.; Yu, L.; Zhang, H.; et al. Apatinib exerts anti-tumor activity to non-Hodgkin lymphoma by inhibition of the Ras pathway. Eur. J. Pharmacol. 2019, 843, 145–153. [Google Scholar] [CrossRef]
- Sendino, M.; Omaetxebarria, M.J.; Rodriguez, J.A. Hitting a moving target: Inhibition of the nuclear export receptor XPO1/CRM1 as a therapeutic approach in cancer. Cancer Drug Resist. 2018, 1, 139–163. [Google Scholar] [CrossRef]
- Talati, C.; Sweet, K.L. Nuclear transport inhibition in acute myeloid leukemia: Recent advances and future perspectives. Int. J. Hematol. Oncol. 2018, 7, IJH04. [Google Scholar] [CrossRef]
- Benkova, K.; Mihalyova, J.; Hajek, R.; Jelinek, T. Selinexor, selective inhibitor of nuclear export: Unselective bullet for blood cancers. Blood Rev. 2021, 46, 100758. [Google Scholar] [CrossRef]
- Parikh, K.; Cang, S.; Sekhri, A.; Liu, D. Selective inhibitors of nuclear export (SINE)—A novel class of anti-cancer agents. J. Hematol. Oncol. 2014, 7, 78. [Google Scholar] [CrossRef]
- Breit, M.N.; Kisseberth, W.C.; Bear, M.D.; Landesman, Y.; Kashyap, T.; McCauley, D.; Kauffman, M.G.; Shacham, S.; London, C.A. Biologic activity of the novel orally bioavailable selective inhibitor of nuclear export (SINE) KPT-335 against canine melanoma cell lines. BMC Vet. Res. 2014, 10, 160. [Google Scholar] [CrossRef]
- Breitbach, J.T.; Louke, D.S.; Tobin, S.J.; Watts, M.R.; Davies, A.E.; Fenger, J.M. The selective inhibitor of nuclear export (SINE) verdinexor exhibits biologic activity against canine osteosarcoma cell lines. Vet. Comp. Oncol. 2021, 19, 362–373. [Google Scholar] [CrossRef]
- Ou, L.; Wang, X.; Cheng, S.; Zhang, M.; Cui, R.; Hu, C.; Liu, S.; Tang, Q.; Peng, Y.; Chai, R.; et al. Verdinexor, a selective inhibitor of nuclear Exportin 1, inhibits the proliferation and migration of esophageal cancer via XPO1/c-Myc/FOSL1 axis. Int. J. Biol. Sci. 2022, 18, 276–291. [Google Scholar] [CrossRef]
- Pan, L.; Cheng, C.; Duan, P.; Chen, K.; Wu, Y.; Wu, Z. XPO1/CRM1 is a promising prognostic indicator for neuroblastoma and represented a therapeutic target by selective inhibitor verdinexor. J. Exp. Clin. Cancer Res. 2021, 40, 255. [Google Scholar] [CrossRef]
- Sadowski, A.R.; Gardner, H.L.; Borgatti, A.; Wilson, H.; Vail, D.M.; Lachowicz, J.; Manley, C.; Turner, A.; Klein, M.K.; Waite, A.; et al. Phase II study of the oral selective inhibitor of nuclear export (SINE) KPT-335 (verdinexor) in dogs with lymphoma. BMC Vet. Res. 2018, 14, 250. [Google Scholar] [CrossRef]
- Maylina, L.; Kambayashi, S.; Baba, K.; Okuda, M. Simultaneous analysis of the p16 gene and protein in canine lymphoma cells and their correlation with pRb phosphorylation. Vet. Sci. 2022, 9, 393. [Google Scholar] [CrossRef]
- Peters, I.R.; Peeters, D.; Helps, C.R.; Day, M.J. Development and application of multiple internal reference (housekeeper) gene assays for accurate normalisation of canine gene expression studies. Vet. Immunol. Immunopathol. 2007, 117, 55–66. [Google Scholar] [CrossRef]
- Balasubramanian, S.K.; Azmi, A.S.; Maciejewski, J. Selective inhibition of nuclear export: A promising approach in the shifting treatment paradigms for hematological neoplasms. Leukemia 2022, 36, 601–612. [Google Scholar] [CrossRef]
- Abeykoon, J.P.; Paludo, J.; Nowakowski, K.E.; Stenson, M.J.; King, R.L.; Wellik, L.E.; Wu, X.; Witzig, T.E. The effect of CRM1 inhibition on human non-Hodgkin lymphoma cells. Blood Cancer. J. 2019, 9, 24. [Google Scholar] [CrossRef]
- Lapalombella, R.; Sun, Q.; Williams, K.; Tangeman, L.; Jha, S.; Zhong, Y.; Goettl, V.; Mahoney, E.; Berglund, C.; Gupta, S.; et al. Selective inhibitors of nuclear export show that CRM1/XPO1 is a target in chronic lymphocytic leukemia. Blood 2012, 120, 4621–4634. [Google Scholar] [CrossRef]
- Nie, D.; Xiao, X.; Chen, J.; Xie, S.; Xiao, J.; Yang, W.; Liu, H.; Wang, J.; Ma, L.; Du, Y.; et al. Prognostic and therapeutic significance of XPO1 in T-cell lymphoma. Exp. Cell Res. 2022, 416, 113180. [Google Scholar] [CrossRef]
- Aladhraei, M.; Al-Thobbani, A.K.; Poungvarin, N.; Suwannalert, P. Association of XPO1 overexpression with NF-κB and Ki67 in colorectal cancer. Asian Pac. J. Cancer. Prev. 2019, 20, 3747–3754. [Google Scholar] [CrossRef]
- Conforti, F.; Zhang, X.; Rao, G.; De Pas, T.; Yonemori, Y.; Rodriguez, J.A.; McCutcheon, J.N.; Rahhal, R.; Alberobello, A.T.; Wang, Y.; et al. Therapeutic effects of XPO1 inhibition in thymic epithelial tumors. Cancer Res. 2017, 77, 5614–5627. [Google Scholar] [CrossRef]
- Sexton, R.; Mahdi, Z.; Chaudhury, R.; Beydoun, R.; Aboukameel, A.; Khan, H.Y.; Baloglu, E.; Senapedis, W.; Landesman, Y.; Tesfaye, A.; et al. Targeting nuclear exporter protein XPO1/CRM1 in gastric cancer. Int. J. Mol. Sci. 2019, 20, 4826. [Google Scholar] [CrossRef]
- Yang, J.; Bill, M.A.; Young, G.S.; Perle, K.L.; Landesman, Y.; Shacham, S.; Kauffman, M.; Senapedis, W.; Kahsyap, T.; Saint-Martin, J.R.; et al. Novel small molecule XPO1/CRM1 inhibitors induce nuclear accumulation of TP53, phosphorylated MAPK and apoptosis in human melanoma cells. PLoS ONE 2014, 9, e102983. [Google Scholar] [CrossRef]
- Camus, V.; Miloudi, H.; Taly, A.; Sola, B.; Jardin, F. XPO1 in B cell hematological malignancies: From recurrent somatic mutations to targeted therapy. J. Hematol. Oncol. 2017, 10, 47. [Google Scholar] [CrossRef]
- Miloudi, H.; Bohers, É.; Guillonneau, F.; Taly, A.; Gibouin, V.C.; Viailly, P.; Jego, G.; Grumolato, L.; Jardin, F.; Sola, B. XPO1E571K mutation modifies exportin 1 localisation and interactome in B-cell lymphoma. Cancers 2020, 12, 2829. [Google Scholar] [CrossRef]
- Nagasaka, M.; Asad, M.F.B.; Al Hallak, M.N.; Uddin, M.H.; Sukari, A.; Baca, Y.; Xiu, J.; Magee, D.; Mamdani, H.; Uprety, D.; et al. Impact of XPO1 mutations on survival outcomes in metastatic non-small cell lung cancer (NSCLC). Lung Cancer 2021, 160, 92–98. [Google Scholar] [CrossRef]
- Taylor, J.; Sendino, M.; Gorelick, A.N.; Pastore, A.; Chang, M.T.; Penson, A.V.; Gavrila, E.I.; Stewart, C.; Melnik, E.M.; Chavez, F.H.; et al. Altered nuclear export signal recognition as a driver of oncogenesis. Cancer Discov. 2019, 9, 1452–1467. [Google Scholar] [CrossRef]
- Walker, J.S.; Hing, Z.A.; Harrington, B.; Baumhardt, J.; Ozer, H.G.; Lehman, A.; Glacopelli, B.; Beaver, L.; Williams, K.; Skinner, J.N.; et al. Recurrent XPO1 mutations alter pathogenesis of chronic lymphocytic leukemia. J. Hematol. Oncol. 2021, 14, 17. [Google Scholar] [CrossRef]
- Grayton, J.E.; Miller, T.; Wilson-Robles, H. In vitro evaluation of selective inhibitors of nuclear export (SINE) drugs KPT-185 and KPT-335 against canine mammary carcinoma and transitional cell carcinoma tumor initiating cells. Vet. Comp. Oncol. 2017, 15, 1455–1467. [Google Scholar] [CrossRef]
- Crochiere, M.L.; Hannus, S.; Hansen, K.; Becker, F.; Baloglu, E.; Lee, M.; Kauffman, M.; Shacham, S.; Landesman, Y. XPO1 target occupancy measurements confirm the Selinexor recommended phase 2 dose. Oncotarget 2017, 8, 110503–110516. [Google Scholar] [CrossRef]
- Chakravarti, N.; Boles, A.; Burzinski, R.; Sindaco, P.; Isabelle, C.; McConnell, K.; Mishra, M.; Porcu, P. XPO1 blockade with KPT-330 promotes apoptosis in cutaneous T-cell lymphoma by activating the p53–p21 and p27 pathways. Sci. Rep. 2024, 14, 9305. [Google Scholar] [CrossRef]
- Fujiwara-Igarashi, A.; Goto-Koshino, Y.; Sato, M.; Maeda, S.; Igarashi, H.; Takahashi, M.; Fujino, Y.; Ohno, K.; Tsujimoto, H. Prognostic significance of the expression levels of the p16, p15, and p14 genes in dogs with high-grade lymphoma. Vet. J. 2014, 199, 236–244. [Google Scholar] [CrossRef]
- Modiano, J.F.; Breen, M.; Valli, V.E.O.; Wojcieszyn, J.W.; Cutter, G.R. Predictive value of p16 or Rb inactivation in a model of naturally occurring canine non-Hodgkin’s lymphoma. Leukemia 2007, 21, 184–187. [Google Scholar] [CrossRef]
- Steplewski, Z.; Jeglum, K.A.; Rosales, C.; Weintraub, N. Canine lymphoma-associated antigens defined by murine monoclonal antibodies. Cancer Immunol. Immunother. 1987, 24, 197–201. [Google Scholar] [CrossRef]
- Suter, S.E.; Chein, M.B.; von Messling, V.; Yip, B.; Cattaneo, R.; Vernau, W.; Madewell, B.R.; London, C.A. In vitro canine distemper virus infection of canine lymphoid cells: A prelude to oncolytic therapy for lymphoma. Clin. Cancer Res. 2005, 11, 1579–1587. [Google Scholar] [CrossRef]
- Seiser, E.L.; Thomas, R.; Richards, K.L.; Kathryn Kelley, M.; Moore, P.; Suter, S.E.; Breen, M. Reading between the lines: Molecular characterization of five widely used canine lymphoid tumour cell lines. Vet. Comp. Oncol. 2011, 11, 30–50. [Google Scholar] [CrossRef]
- Rütgen, B.C.; Hammer, S.E.; Gerner, W.; Christian, M.; de Arespacochaga, A.G.; Willmann, M.; Kleiter, M.; Schwendenwein, I.; Saalmüller, A. Establishment and characterization of a novel canine B-cell line derived from a spontaneously occurring diffuse large cell lymphoma. Leuk. Res. 2010, 34, 932–938. [Google Scholar] [CrossRef]
- Bonnefont-Rebeix, C.; Fournel-Fleury, C.; Ponce, F.; Belluco, S.; Watrelot, D.; Bouteille, S.E.; Rapiteau, S.; Razanajaona-Doll, D.; Pin, J.J.; Leroux, C.; et al. Characterization of a novel canine T-cell line established from a spontaneously occurring aggressive T-cell lymphoma with large granular cell morphology. Immunobiology 2016, 221, 12–22. [Google Scholar] [CrossRef]
- Pawlak, A.; Ziolo, E.; Kutkowska, J.; Blazejczyk, A.; Wietrzyk, J.; Krupa, A.; Hildebrand, W.; Dziegiel, P.; Dzimira, S.; Obminska-Mrukowicz, B.; et al. A novel canine B-cell leukaemia cell line. Establishment, characterisation and sensitivity to chemotherapeutics. Vet. Comp. Oncol. 2017, 15, 1218–1231. [Google Scholar] [CrossRef]
- Kojima, K.; Fujino, Y.; Goto-Koshino, Y.; Ohno, K.; Tsujimoto, H. Analyses on activation of NF-κB and effect of bortezomib in canine neoplastic lymphoid cell lines. J. Vet. Med. Sci. 2013, 75, 727–731. [Google Scholar] [CrossRef]
- Tomiyasu, H.; Goto-Koshino, Y.; Fujino, Y.; Ohno, K.; Tsujimoto, H. Epigenetic regulation of the ABCB1 gene in drug-sensitive and drug-resistant lymphoid tumour cell lines obtained from canine patients. Vet. J. 2014, 199, 103–109. [Google Scholar] [CrossRef]
- Nakaichi, M.; Taura, Y.; Kanki, M.; Mamba, K.; Momoi, Y.; Tsujimoto, H.; Nakama, S. Establishment and characterization of a new canine B-cell leukemia cell line. J. Vet. Med. Sci. 1996, 58, 469–471. [Google Scholar] [CrossRef]
- Umeki, S.; Ema, Y.; Suzuki, R.; Kubo, M.; Hayashi, T.; Okamura, Y.; Yamazaki, J.; Tsujimoto, H.; Tani, K.; Hiraoka, H.; et al. Establishment of five canine lymphoma cell lines and tumor formation in a xenotransplantation model. J. Vet. Med. Sci. 2013, 75, 467–474. [Google Scholar] [CrossRef]
- Yamazaki, J.; Baba, K.; Goto-Koshino, Y.; Setoguchi-Mukai, A.; Fujino, Y.; Ohno, K.; Tsujimoto, H. Quantitative assessment of minimal residual disease (MRD) in canine lymphoma by using real-time polymerase chain reaction. Vet. Immunol. Immunopathol. 2008, 126, 321–331. [Google Scholar] [CrossRef]
| Cell Group | Cell Lines | KPT-335 IC50 (nM ± SD) |
|---|---|---|
| B-cell lines | 17-71 | 89.8 ± 2.07 |
| CLBL-1 | 108.6 ± 30.54 | |
| GL-1 | 294.3 ± 25.57 | |
| T-cell lines | CLC | 224.7 ± 78.38 |
| CLGL-90 | 215 ± 50.96 | |
| Ema | 147.8 ± 49.69 | |
| Nody-1 | 220.5 ± 27.34 | |
| UL-1 | 418 ± 78.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Primarizky, H.; Kambayashi, S.; Baba, K.; Tani, K.; Okuda, M. Exportin 1 (XPO1) Expression and Effectiveness of XPO1 Inhibitor Against Canine Lymphoma Cell Lines. Vet. Sci. 2025, 12, 700. https://doi.org/10.3390/vetsci12080700
Primarizky H, Kambayashi S, Baba K, Tani K, Okuda M. Exportin 1 (XPO1) Expression and Effectiveness of XPO1 Inhibitor Against Canine Lymphoma Cell Lines. Veterinary Sciences. 2025; 12(8):700. https://doi.org/10.3390/vetsci12080700
Chicago/Turabian StylePrimarizky, Hardany, Satoshi Kambayashi, Kenji Baba, Kenji Tani, and Masaru Okuda. 2025. "Exportin 1 (XPO1) Expression and Effectiveness of XPO1 Inhibitor Against Canine Lymphoma Cell Lines" Veterinary Sciences 12, no. 8: 700. https://doi.org/10.3390/vetsci12080700
APA StylePrimarizky, H., Kambayashi, S., Baba, K., Tani, K., & Okuda, M. (2025). Exportin 1 (XPO1) Expression and Effectiveness of XPO1 Inhibitor Against Canine Lymphoma Cell Lines. Veterinary Sciences, 12(8), 700. https://doi.org/10.3390/vetsci12080700





