3.2. Marginal Hypothesis Tests for Fixed Effects
The fixed effects of season, diet, and sampling time, and their interactions, on biochemical, hematological, and body weight parameters in goats were analyzed using linear mixed models. Significance was determined using F tests, adjusted by the Benjamini–Hochberg (BH) method.
- (a)
Blood parameters
According to
Table 2, season significantly influenced BUN, TP, and ALB (
p < 0.001), reflecting marked seasonal variations. In contrast, diet had no effect (
p > 0.05), suggesting limited sensitivity to dietary changes. All parameters were affected by sampling time (
p < 0.001), demonstrating physiological variability.
Season × sampling time (BUN, ALB; p < 0.001), season × diet (ALB; p < 0.01), and diet × sampling time (BUN; p < 0.05) interactions indicated combined physiological modulations. ALP responded to diet (p < 0.01) and ALT to season (p < 0.001). The season × diet interaction also affected ALP (p < 0.01), and season × sampling impacted ALT (p < 0.001), highlighting synergistic effects of environment and time.
The triple interaction (season × diet × sampling time) was not significant, suggesting that season and sampling are the main modulating factors.
- (b)
Hematological parameters and body weight
Table 3 shows that the season affected all parameters and body weight (
p < 0.001), except for lymphocytes (
p = 0.86). Diet only modified ALP (
p < 0.01). Sampling influenced leukocytes, neutrophils, hemoglobin, hematocrit, MCV, and body weight (
p < 0.01).
The season × diet interaction was significant for all parameters except lymphocytes (p > 0.05), as was season × sampling (p < 0.001), highlighting the importance of periodic monitoring. Diet × sampling affected leukocytes, neutrophils, hemoglobin, and body weight (p < 0.05). The three-way interaction was significant for hemoglobin and MCV (p < 0.001), but not for lymphocytes or body weight (p > 0.05), indicating greater stability of the latter in response to the factors evaluated.
3.3. Comparison of Means According to Climatic Season, Diet, and Sampling Time
- (a)
Blood parameters
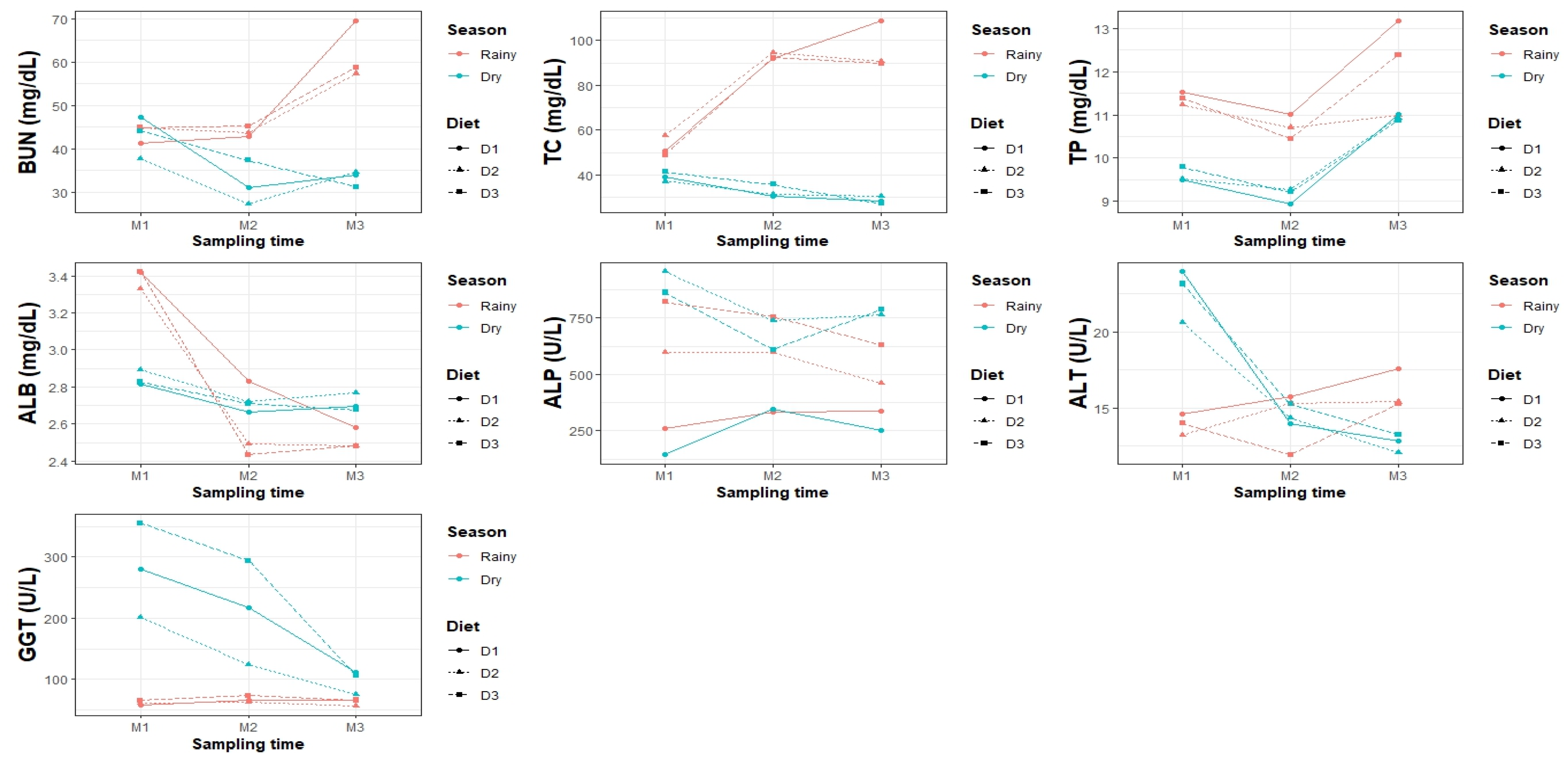
BUN was significantly higher in the rainy season (49.81 ± 0.75 mg/dL) than in the dry season (36.04 ± 0.77 mg/dL). There were no differences between D1 and D3, but D2 showed lower values. Sampling time influenced BUN, with the highest concentration in M3 (47.55 ± 0.96 mg/dL) and the lowest in M2 (37.90 ± 0.92 mg/dL).
In the rainy season, concentrations were homogeneous among diets, whereas in the dry season, D2 had the lowest values. In the season × time interaction, M3 had the highest concentration in the rainy season (61.87 ± 1.30 mg/dL), whereas in the dry season, M2 and M3 had the lowest values.
In the third-order interaction, urea remained high in the rainy diet (48–51 mg/dL), with no differences between diets, while in the dry diet it decreased, being the lowest in D2 (33.23 ± 1.40 mg/dL). In the rainy diet, M3 presented the highest values in D1 and D2 (57.33 ± 2.26 and 58.73 ± 2.26 mg/dL), while M1 and M2 were more homogeneous (44–45 mg/dL). In the dry diet, the lowest values were recorded in D1-M2 (27.33 ± 2.26 mg/dL) and D2-M3 (31.18 ± 2.26 mg/dL). D3 showed similar values between seasons, with lower concentrations in M2 and M3 in the dry season (31.00 ± 2.26 and 33.87 ± 2.26 mg/dL).
- (b)
Biochemical parameters
TP concentration was higher in the rainy season (11.43 ± 0.12 g/dL) than in the dry season (9.90 ± 0.12 g/dL), with no differences between diets (10.44 ± 0.15–10.86 ± 0.15 g/dL). TP decreased over time, reaching its maximum value in M3 (11.56 ± 0.15 g/dL), followed by M1 (10.49 ± 0.15 g/dL) and M2 (9.94 ± 0.15 g/dL).
In the rainy season, D1 showed the highest concentration (11.91 ± 0.21 g/dL) compared to D2 (10.98 ± 0.21 g/dL) and D3 (11.41 ± 0.21 g/dL), with no differences between the latter (p > 0.05). In the dry season, the diets were homogeneous (D1: 9.82 ± 0.21, D2: 9.91 ± 0.21, D3: 9.96 ± 0.22 g/dL).
The season × sampling time interaction revealed that M3 in the rainy season had the highest TP (12.19 ± 0.21 g/dL), while M2 in the dry season had the lowest (9.15 ± 0.21 g/dL). In the diet × sampling time interaction, D1-M3 had the highest value (12.10 ± 0.25 g/dL) and D3-M2 the lowest (9.83 ± 0.25 g/dL). Finally, the rainy-season-D1-M3 had the highest value (13.19 ± 0.36 g/dL) and the dry-season-D1-M2 the lowest (8.95 ± 0.36 g/dL).
Albumin (ALB) concentration was significantly higher during the rainy season (2.83 ± 0.02 g/dL) compared to the dry season (2.75 ± 0.02 g/dL). Sampling time also had a significant influence, with the highest value in M1 (3.12 ± 0.03 g/dL) and the lowest in M2 (2.64 ± 0.03 g/dL) and M3 (2.61 ± 0.03 g/dL). In the rainy season, D1 showed the highest concentration (2.95 ± 0.04 g/dL), while D2 and D3 presented similar values. In the dry season, D2 and D3 slightly exceeded D1, although without significant differences (p > 0.05). The season × time interaction revealed the highest value in M1–rainy combination (3.39 ± 0.04 g/dL) and the lowest in M3–rainy combination (2.52 ± 0.04 g/dL). In the diet × time combination, M1 presented the highest concentrations for all diets. In the triple interaction, the highest values corresponded to the M1–rainy combination in D3 and D1 (3.42 ± 0.06 g/dL), while the lowest ALB was observed in M2–rainy–D3 (2.43 ± 0.06 g/dL).
Alkaline phosphatase (ALP) activity did not show significant differences between seasons (dry: 606.72 ± 44.95 U/L; rainy: 533.23 ± 43.72 U/L). However, significant differences were observed between diets (p < 0.05), with D3 showing the highest activity (745.03 ± 54.92 U/L), followed by D2 (686.02 ± 54.32 U/L), while D1 showed the lowest activity (278.88 ± 53.65 U/L). Sampling time did not significantly influence the interaction between season and diet. D2 and D3 had the highest values in the dry season (819.92 ± 76.31 and 753.90 ± 76.31 U/L, respectively), while D1 was consistently low. In the rainy season, D3 (736.16 ± 75.42 U/L) and D2 (552.12 ± 76.31 U/L) maintained higher values than D1.
In the diet × sampling interaction, D3 peaked in M1 (842.50 ± 92.37 U/L), whereas D1 showed reduced enzyme activity at all times. The triple interaction showed the highest values in the dry season for D2 and D3 in M1 (955.73 ± 130.63 and 862.93 ± 130.63 U/L, respectively). The lowest values were observed in D1–M1–dry (143.07 ± 130.63 U/L) and D1–M3–rainy (338.93 ± 130.63 U/L), as shown in
Figure 2.
ALT activity was significantly higher in the dry season (16.61 ± 0.33 U/L) than in the rainy season (14.79 ± 0.33 U/L). No significant differences were found between diets.
Regarding the sampling time, M1 presented the highest activity (18.24 ± 0.40 U/L), while M2 and M3 were similar (14.43 ± 0.40 and 14.41 ± 0.40 U/L, respectively).
In the dry season, D3 (17.22 ± 0.60 U/L) and D1 (16.93 ± 0.57 U/L) showed higher values than D2 (15.67 ± 0.57 U/L). In the rainy season, D1 was the highest (15.98 ± 0.57 U/L). The highest activity was recorded in D1–M1–dry (23.93 ± 0.98 U/L) and D3–M1–dry (23.13 ± 0.98 U/L), while the lowest value was observed in D3–M2–rainy (11.93 ± 0.98 U/L).
- (c)
Hematological parameters and body weight
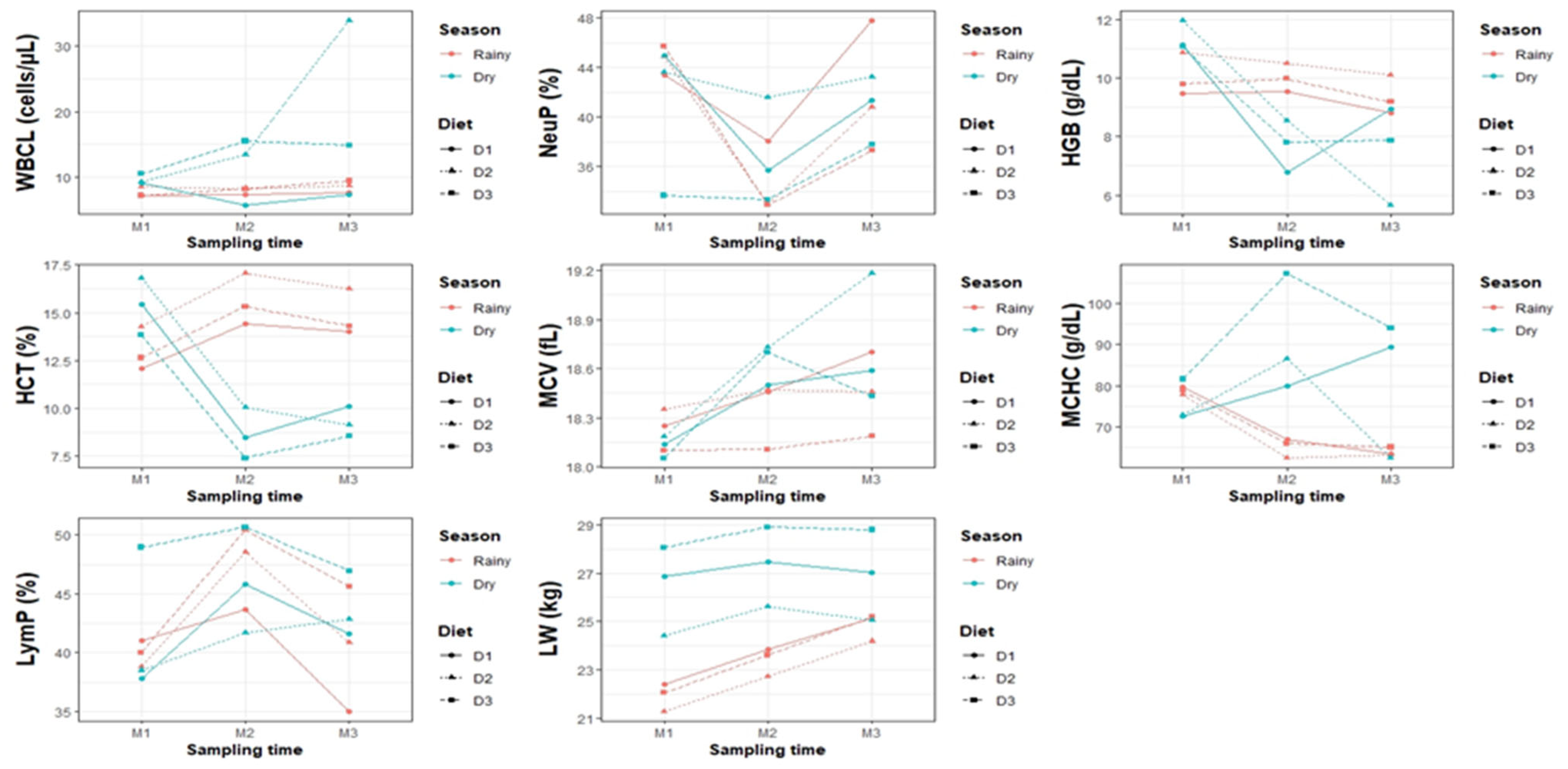
Leukocytes were significantly higher in the dry season (13.31 ± 0.87 × 103/µL) than in the rainy season (8.06 ± 0.90 × 103/µL), suggesting greater immune activation by environmental stress and lower forage availability.
Diet also had a significant effect (p < 0.05): D2 had the highest value (13.70 ± 1.01 × 103/µL), followed by D3 (10.95 ± 1.19) and D1 (7.41 ± 1.05), indicating a possible immunomodulatory effect of the diet ingredients.
Regarding time, M3 recorded the highest count (13.63 ± 1.03), while M1 and M2 showed lower values (8.67 ± 1.17 and 9.76 ± 1.05, respectively).
During the dry season, D2 reached the highest peak (18.89 ± 1.48), while in the rainy season, D1 and D3 had the lowest values (~7.38–8.52), reflecting greater immune demand under heat stress.
The highest value was observed in M3–dry (18.67 ± 1.54), and the lowest (~7.65–8.59) in M1– and M2–rainy, reaffirming the effect of the environment on the immune response.
There were no significant differences in the triple interaction (p > 0.05), except in dry–D2–M3 (33.88 ± 2.77, the highest value) and dry–D1–M2 (5.80 ± 3.25, the lowest), suggesting greater immune activation under conditions of greater environmental stress and better dietary quality.
NeuP values did not show significant differences between seasons, diets, or sampling times (p > 0.05), suggesting a stable immune response. Although not significant, D1 (42.70 ± 2.80%), and D2 (41.33 ± 2.76%) exhibited higher mean concentrations than D3 (36.54 ± 2.93%). Similarly, NeuP showed a reduction trend from M1 (42.43 ± 2.20%) to M2 (36.83 ± 2.05%), suggesting potential variability in immune activation.
Hemoglobin (HGB) was higher in the rainy season (9.84 ± 0.16 g/dL) than in the dry season (8.86 ± 0.15 g/dL; p < 0.05). There were no differences between diets: D2 (9.62 ± 0.22 g/dL), D3 (9.33 ± 0.23 g/dL), and D1 (9.10 ± 0.22 g/dL; p > 0.05).
Sampling time had a significant influence, with decreasing values from M1 (10.77 ± 0.19 g/dL) to M3 (8.43 ± 0.16 g/dL; p < 0.05). The season × diet interaction showed higher values in the rainy season with D2 (10.50 ± 0.25 g/dL) and lower values in the dry season with D2 (8.74 ± 0.25 g/dL; p < 0.05).
In the season × time interaction, HGB was highest in the dry season during M1 (11.41 ± 0.21 g/dL) and lowest in M3 (7.49 ± 0.21 g/dL; p < 0.05). In diet × time, D2 in M1 reached the maximum (11.43 ± 0.28 g/dL) and in M3 the minimum (7.89 ± 0.28 g/dL; p < 0.05).
The triple interaction reflected the combined effect of environment and nutrition: the highest HGB was recorded in the dry season with D2 in M1 (11.99 ± 0.36 g/dL) and the lowest in the dry season with D2 in M3 (5.67 ± 0.36 g/dL; p < 0.05), evidencing the environmental and dietary impact on blood oxygenation.
MCV varied significantly by season, with higher values in the dry season (18.51 ± 0.06 fL) than in the rainy season (18.34 ± 0.06 fL). However, diet had no significant effect, with similar values between D2 (18.56 ± 0.09 fL), D1 (18.45 ± 0.09 fL), and D3 (18.26 ± 0.09 fL).
Sampling time had a significant influence: M3 (18.59 ± 0.06 fL) and M2 (18.50 ± 0.06 fL) recorded higher values than M1 (18.18 ± 0.06 fL). In the season × diet interaction, D2 in the dry season reached the highest value (18.70 ± 0.10 fL), while D3 in the rainy season had the lowest (18.12 ± 0.11 fL).
In the season × sampling interaction, M3 and M2 in the dry season showed the highest values (18.73 ± 0.07 and 18.66 ± 0.07 fL), while M1 in the same season recorded the lowest (18.13 ± 0.07 fL). For the diet × sampling interaction, D2 in M3 presented the highest value (18.82 ± 0.10 fL) and D3 in M1 the lowest (18.06 ± 0.12 fL).
Finally, in the triple interaction, D2 in M3 during the dry season had the highest MCV (19.18 ± 0.12 fL), while D3 in M1 during the same season had the lowest (18.05 ± 0.12 fL). These results show that season and sampling time significantly affect MCV, whereas diet does not generate marked differences.
LymP did not vary between seasons (
p > 0.05), with similar values in the dry season (43.19 ± 1.68%) and rainy season (42.84 ± 1.72%). There were also no differences by diet (
p > 0.05), although D3 had the highest LymP (47.37 ± 2.68%), followed by D2 (41.68 ± 2.55%) and D1 (39.99 ± 2.57%), as shown in
Figure 3.
The sampling time influenced the values: M2 recorded the highest (45.72 ± 1.84%), while M3 (42.19 ± 1.82%) and M1 (41.13 ± 1.96%) were lower (p > 0.05). In the season × diet interaction, D3 in the dry season reached the highest LymP (48.83 ± 2.86%), while D1 in the rainy season had the lowest (39.88 ± 2.79%).
In the season × sampling interaction, M2 in the rainy season showed the highest value (47.54 ± 2.13%), while M3 in the same season recorded the lowest (40.48 ± 2.13%). For the diet × sampling time interaction, D3 in M2 presented the highest LymP (50.26 ± 3.07%), while D1 in M3 had the lowest (38.02 ± 3.11%).
In the triple interaction, D3 in M2 in the rainy season reached the highest LymP (50.45 ± 3.69%), while D1 in M3 in the same season recorded the lowest (34.97 ± 3.69%).
BW was higher in the dry season (27.02 ± 0.48 kg) than in the rainy season (24.86 ± 0.48 kg; p < 0.05), with a reduction of 2.16 kg under more humid conditions. Diet did not have a significant influence (p > 0.05), although D3 showed the highest value (27.18 ± 0.82 kg), followed by D1 (26.05 ± 0.82 kg) and D2 (24.60 ± 0.82 kg), with a difference of up to 2.58 kg between treatments.
In the season × diet interaction, D3 in the dry season reached the highest weight (29.05 ± 0.83 kg), exceeding D2 in the rainy season (24.13 ± 0.83 kg) by 4.92 kg, which registered the lowest value. In the dry season, D1 (26.95 ± 0.83 kg) showed no differences with D3 (25.32 ± 0.83 kg). The same way, D1 was similar between season.