Comparison of Intravenous and Oral Meloxicam Pharmacokinetics in Female and Male Saanen Goats
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Study Design
2.4. Analytical Procedure
2.4.1. Instrumentation
2.4.2. Chromatographic Conditions
2.4.3. Preparation of Standard Solution
2.4.4. Sample Preparations and Extraction
2.4.5. Validation of Analytical Method
2.5. Pharmacokinetics and Statistical Analysis of Data
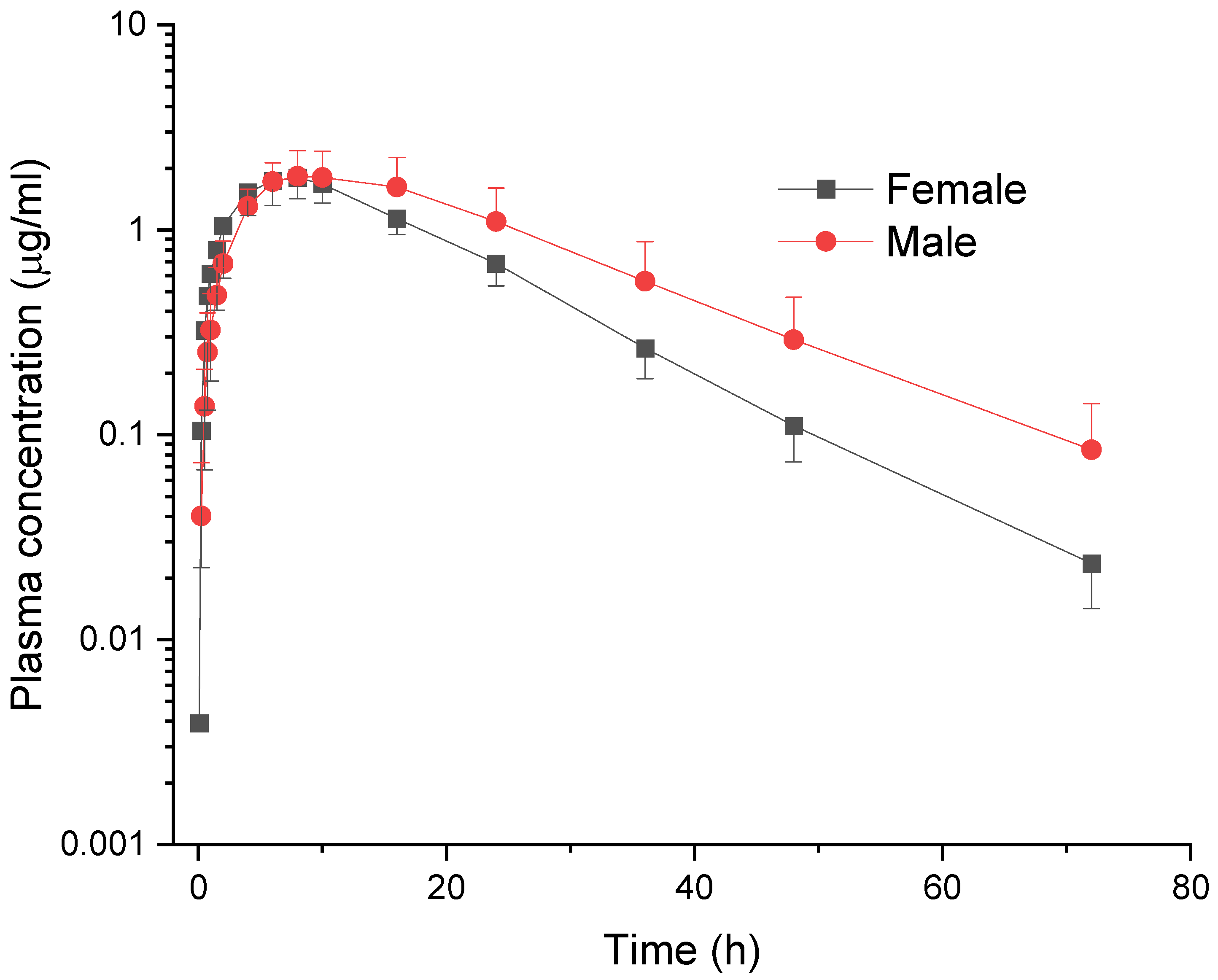
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mathews, K.A. Non-steroidal anti-inflammatory analgesics: A review of current practice. J. Vet. Emerg. Crit. Care 2002, 12, 89–97. [Google Scholar] [CrossRef]
- Curry, S.L.; Cogar, S.M.; Cook, J.L. Nonsteroidal antiinflammatory drugs: A review. J. Am. Anim. Hosp. Assoc. 2005, 41, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Luger, P.; Daneck, K.; Engel, W.; Trummlitz, G.; Wagner, K. Structure and physicochemical properties of meloxicam, a new NSAID. Eur. J. Pharm. Sci. 1996, 4, 175–187. [Google Scholar] [CrossRef]
- Del Tacca, M.; Colucci, R.; Fornai, M.; Blandizzi, C. Efficacy and tolerability of meloxicam, a COX-2 preferential nonsteroidal anti-inflammatory drug: A review. Clin. Drug Investig. 2002, 22, 799–818. [Google Scholar] [CrossRef]
- Gates, B.J.; Nguyen, T.T.; Setter, S.M.; Davies, N.M. Meloxicam: A reappraisal of pharmacokinetics, efficacy and safety. Expert. Opin. Pharmacother. 2005, 6, 2117–2140. [Google Scholar] [CrossRef]
- Furst, D.E. Meloxicam: Selective COX-2 inhibition in clinical practice. Semin. Arthritis Rheum. 1997, 26, 21–27. [Google Scholar] [CrossRef]
- Gorski, J.C.; Vannaprasaht, S.; Hamman, M.A.; Ambrosius, W.T.; Bruce, M.A.; Haehner-Daniels, B.; Hall, S.D. The effect of age, sex, and rifampin administration on intestinal and hepatic cytochrome P450 3A activity. Clin. Pharmacol. Ther. 2003, 74, 275–287. [Google Scholar] [CrossRef]
- Overholser, B.R.; Kays, M.B.; Forrest, A.; Sowinski, K.M. Sex-related differences in the pharmacokinetics of oral ciprofloxacin. J. Clin. Pharmacol. 2004, 44, 1012–1022. [Google Scholar] [CrossRef]
- Carcas, A.; Guerra, P.; Frias, J.; Soto, A.; Fernandez-Aijón, A.; Montuenga, C.; Govantes, C. Gender differences in the disposition of metronidazole. Int. J. Clin. Pharmacol. Ther. 2001, 39, 213–218. [Google Scholar] [CrossRef]
- Konieczna, L.; Chmielewska, A.; Lamparczyk, H. Influence of sex on the pharmacokinetics of ciprofloxacin and ofloxacin. Chemotherapy 2006, 52, 111–121. [Google Scholar] [CrossRef]
- Corum, O.; Uney, K.; Durna Corum, D.; Coskun, D.; Akin, F.; Oguz, H.; Elmas, M. Effect of Gender on the pharmacokinetics of meloxicam in sheep. J. Vet. Pharmacol. Ther. 2025. [Google Scholar] [CrossRef]
- Gokbulut, C.; Bilgili, A.; Hanedan, B.; Aksit, D.; Aksoy, A.M.; Turgut, C. Sex-related plasma disposition of ivermectin following pour-on administration in goats. Vet. Parasitol. 2009, 162, 342–345. [Google Scholar] [CrossRef]
- Moyer, A.M.; Matey, E.T.; Miller, V.M. Individualized medicine: Sex, hormones, genetics, and adverse drug reactions. Pharmacol. Res. Perspect. 2019, 7, e00541. [Google Scholar] [CrossRef] [PubMed]
- Bosch, E.; Sommer, I.; Touw, D. The influence of female sex and estrogens on drug pharmacokinetics: What is the evidence? Expert. Opin. Drug Metab. Toxicol. 2025, 21, 637–647. [Google Scholar] [CrossRef] [PubMed]
- de la Puente, R.; Diez, R.; Diez, M.J.; Fernandez, N.; Sahagun, A.M.; Rodriguez, J.M.; Garcia, J.J.; Lopez, C. Pharmacokinetics of meloxicam in different animal species: A comprehensive review. Vet. Sci. 2024, 11, 519. [Google Scholar] [CrossRef] [PubMed]
- Karademir, U.; Erdogan, H.; Boyacioglu, M.; Kum, C.; Sekkin, S.; Bilgen, M. Pharmacokinetics of meloxicam in adult goats: A comparative study of subcutaneous, oral and intravenous administration. N. Z. Vet. J. 2016, 64, 165–168. [Google Scholar] [CrossRef]
- Stock, M.L.; Coetzee, J.F.; KuKanich, B.; Smith, B.I. Pharmacokinetics of intravenously and orally administered meloxicam in sheep. Am. J. Vet. Res. 2013, 74, 779–783. [Google Scholar] [CrossRef]
- Woodland, A.N.; Van der Saag, D.; Kimble, B.; White, P.J.; Govendir, M.; Lomax, S. Plasma pharmacokinetic profile and efficacy of meloxicam administered subcutaneously and intramuscularly to sheep. PLoS ONE 2019, 14, e0215842. [Google Scholar] [CrossRef]
- Kreuder, A.J.; Coetzee, J.F.; Wulf, L.W.; Schleining, J.A.; KuKanich, B.; Layman, L.L.; Plummer, P.J. Bioavailability and pharmacokinetics of oral meloxicam in llamas. BMC Vet. Res. 2012, 8, 85. [Google Scholar] [CrossRef]
- Bae, J.-W.; Kim, M.-J.; Jang, C.-G.; Lee, S.-Y. Determination of meloxicam in human plasma using a HPLC method with UV detection and its application to a pharmacokinetic study. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 859, 69–73. [Google Scholar] [CrossRef]
- International Conference on Harmonization (ICH). Technical Requirements for the Registration of Pharmaceutical for Human Use, Validation of Analytical Procedures; Text and Methodology Q2 (R1). 2005. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-q2r1-validation-analytical-procedures-text-methodology-step-5-first-version_en.pdf (accessed on 23 June 2025).
- Bogan, J.; Benoit, E.; Delatour, P. Pharmacokinetics of oxfendazole in goats: A comparison with sheep. J. Vet. Pharmacol. Ther. 1987, 10, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Aksit, D.; Yalinkilinc, H.S.; Sekkin, S.; Boyacioğlu, M.; Cirak, V.Y.; Ayaz, E.; Gokbulut, C. Comparative pharmacokinetics and bioavailability of albendazole sulfoxide in sheep and goats, and dose-dependent plasma disposition in goats. BMC Vet. Res. 2015, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Gokbulut, C.; Yalinkilinc, H.S.; Aksit, D.; Veneziano, V. Comparative pharmacokinetics of levamisole-oxyclozanide combination in sheep and goats following per os administration. Can. J. Vet. Res. 2014, 78, 316–320. [Google Scholar] [PubMed]
- Fadel, C.; Łebkowska-Wieruszewska, B.; Serih, F.; Lisowski, A.; Poapolathep, A.; Giorgi, M. Comparative pharmacokinetic evaluation of metronidazole in sheep and goats. Res. Vet. Sci. 2025, 184, 105507. [Google Scholar] [CrossRef]
- Fleischmann, R.; Iqbal, I.; Slobodin, G. Meloxicam. Expert. Opin. Pharmacother. 2002, 3, 1501–1512. [Google Scholar] [CrossRef]
- Toutain, P.; Autefage, A.; Legrand, C.; Alvinerie, M. Plasma concentrations and therapeutic efficacy of phenylbutazone and flunixin meglumine in the horse: Pharmacokinetic/pharmacodynamic modelling. J. Vet. Pharmacol. Ther. 1994, 17, 459–469. [Google Scholar] [CrossRef]
- Lees, P.; Landoni, M.F.; Giraudel, J.; Toutain, P.-L. Pharmacodynamics and pharmacokinetics of nonsteroidal anti-inflammatory drugs in species of veterinary interest. J. Vet. Pharmacol. Ther. 2004, 27, 479–490. [Google Scholar] [CrossRef]
- Landoni, M.; Cunningham, F.; Lees, P. Pharmacokinetics and pharmacodynamics of ketoprofen in calves applying PK/PD modelling. J. Vet. Pharmacol. Ther. 1995, 18, 315–324. [Google Scholar] [CrossRef]
- Witkamp, R.; Lohuis, J.; Nijmeijer, S.; Kolker, H.; Noordhoek, J.; Van Miert, A. Species-and sex-related differences in the plasma clearance and metabolite formation of antipyrine. A comparative study in four animal species: Cattle, goat, rat and rabbit. Xenobiotica 1991, 21, 1483–1492. [Google Scholar] [CrossRef]
- Witkamp, R.; Nijmeijer, S.; Kolker, H.; Noordhoek, J.; van Miert, A.S. Effect of gonadal hormones on the plasma clearance and metabolite formation of antipyrine in the dwarf goat. J. Vet. Pharmacol. Ther. 1993, 16, 164–173. [Google Scholar] [CrossRef]
- Gleiter, C.; Gundert-Remy, U. Gender differences in pharmacokinetics. Eur. J. Drug Metab. Pharmacokinet. 1996, 21, 123–128. [Google Scholar] [CrossRef]
- Scandlyn, M.J.; Stuart, E.C.; Rosengren, R.J. Sex-specific differences in CYP450 isoforms in humans. Expert. Opin. Drug Metab. Toxicol. 2008, 4, 413–424. [Google Scholar] [CrossRef]
- Schwartz, J.B. The influence of sex on pharmacokinetics. Clin. Pharmacokinet. 2003, 42, 107–121. [Google Scholar] [CrossRef]
- Meibohm, B.; Beierle, I.; Derendorf, H. How important are gender differences in pharmacokinetics? Clin. Pharmacokinet. 2002, 41, 329–342. [Google Scholar] [CrossRef]
- Soldin, O.P.; Mattison, D.R. Sex differences in pharmacokinetics and pharmacodynamics. Clin. Pharmacokinet. 2009, 48, 143–157. [Google Scholar] [CrossRef]
- Spoletini, I.; Vitale, C.; Malorni, W.; Rosano, G.M. Sex differences in drug effects: Interaction with sex hormones in adult life. Handb. Exp. Pharmacol. 2012, 214, 91–105. [Google Scholar]

| Parameters | Meloxicam |
|---|---|
| LOD (µg/mL) | 0.0031 |
| LOQ (µg/mL) | 0.01 |
| Range of linearity (µg/mL) | 0.01–10.00 |
| Linearity (r2) | 0.9987–1.0000 |
| Recovery (%) | 85.85 (2.91) |
| Coefficient of variation (%) | 4.51 (0.73) |
| Parameters | IV Administration | PO Administration | ||
|---|---|---|---|---|
| Female | Male | Female | Male | |
| T1/2λz (h) a | 10.09 ± 0.97 | 11.72 ± 0.74 * | 9.87 ± 0.85 | 13.10 ± 2.01 * |
| C0 (µg/mL) | 3.76 ± 0.82 | 3.47 ± 0.39 | - | - |
| Tmax (h) | - | - | 7.33 ± 1.03 | 8.33 ± 1.51 |
| Cmax (µg/mL) | - | - | 1.87 ± 0.38 | 1.90 ± 0.50 |
| AUClast (µg.h/mL) | 25.35 ± 6.65 | 26.07 ± 4.14 | 39.24 ± 7.36 | 53.60 ± 21.12 |
| AUC0–∞ (µg.h/mL) | 25.57 ± 6.76 | 26.43 ± 4.30 | 39.59 ± 7.45 | 55.36 ± 22.38 |
| Vz (mL/kg) | 302.08 ± 66.15 | 325.47 ± 36.97 | - | - |
| Cl (mL/h/kg) | 20.69 ± 5.20 | 19.30 ± 2.83 | - | - |
| AUMClast (µg.h2/mL) | 319.66 ± 113.72 | 351.23 ± 82.09 | 654.61 ± 141.85 | 1147.71 ± 556.79 |
| AUMC0–∞ (µg.h2/mL) | 338.73 ± 124.71 | 383.57 ± 97.28 | 684.96 ± 153.68 | 1311.62 ± 685.47 |
| MRTlast (h) a | 12.22 ± 1.50 | 13.30 ± 0.97 | 16.55 ± 1.52 | 20.35 ± 2.55 * |
| MRT0–∞ (h) a | 12.77 ± 1.74 | 14.27 ± 1.26 | 17.12 ± 1.73 | 22.18 ± 3.47 * |
| Vss (L/kg) | 263.27 ± 52.26 | 274.50 ± 24.70 | - | - |
| F (%) | - | - | 77.43 | 104.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozdemir Kutahya, Z.; Aslan Akyol, B.; Mamuk, S.; Piner Benli, P.; Gokbulut, C. Comparison of Intravenous and Oral Meloxicam Pharmacokinetics in Female and Male Saanen Goats. Vet. Sci. 2025, 12, 686. https://doi.org/10.3390/vetsci12080686
Ozdemir Kutahya Z, Aslan Akyol B, Mamuk S, Piner Benli P, Gokbulut C. Comparison of Intravenous and Oral Meloxicam Pharmacokinetics in Female and Male Saanen Goats. Veterinary Sciences. 2025; 12(8):686. https://doi.org/10.3390/vetsci12080686
Chicago/Turabian StyleOzdemir Kutahya, Zeynep, Busra Aslan Akyol, Selen Mamuk, Petek Piner Benli, and Cengiz Gokbulut. 2025. "Comparison of Intravenous and Oral Meloxicam Pharmacokinetics in Female and Male Saanen Goats" Veterinary Sciences 12, no. 8: 686. https://doi.org/10.3390/vetsci12080686
APA StyleOzdemir Kutahya, Z., Aslan Akyol, B., Mamuk, S., Piner Benli, P., & Gokbulut, C. (2025). Comparison of Intravenous and Oral Meloxicam Pharmacokinetics in Female and Male Saanen Goats. Veterinary Sciences, 12(8), 686. https://doi.org/10.3390/vetsci12080686







