Finite Element Analysis of Stress Distribution in Canine Lumbar Fractures with Different Pedicle Screw Insertion Angles
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
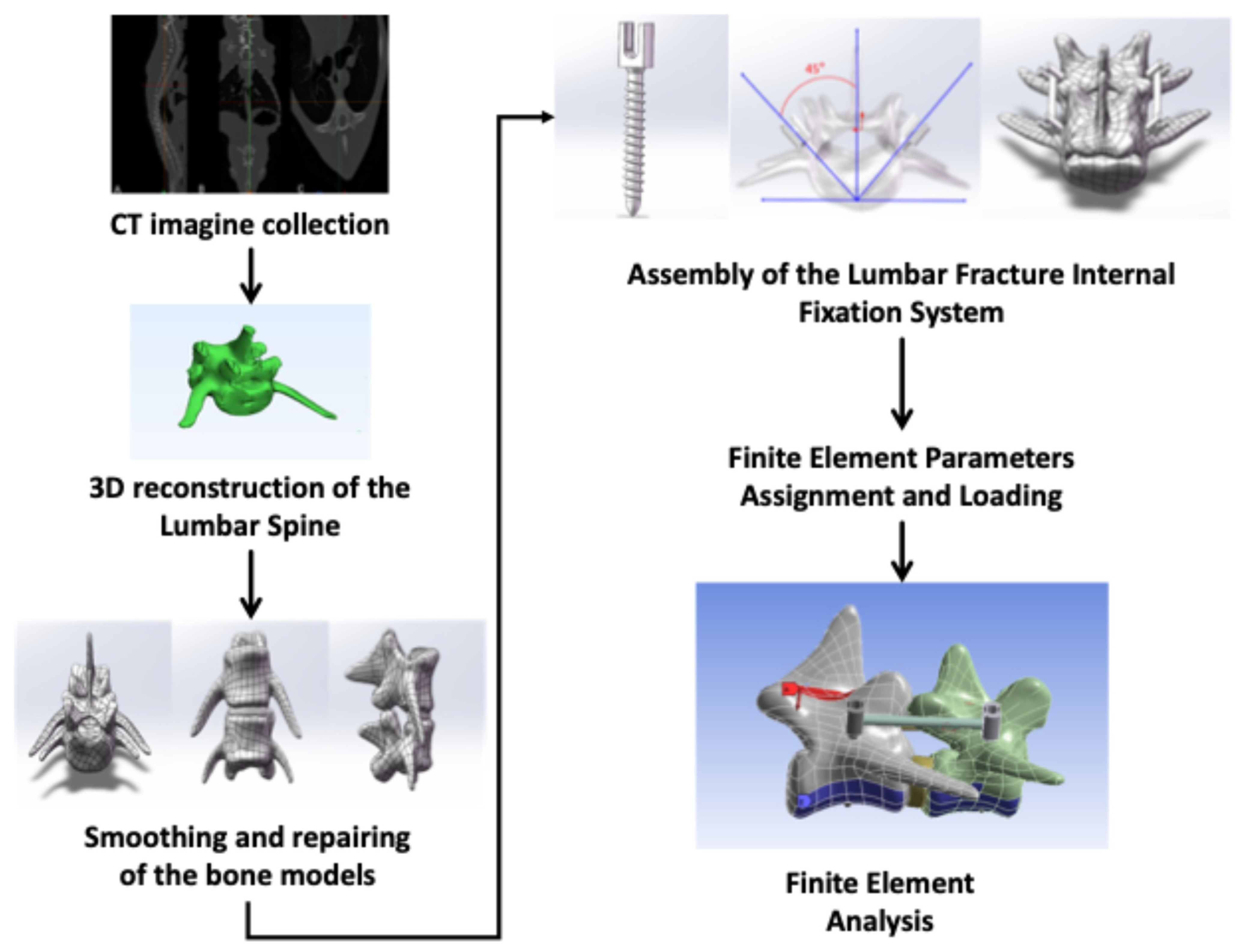
2.2. CT Imagine Collection of Beagle Dog
2.3. Three-Dimensional Reconstruction of the Lumbar Spine
2.4. Smoothing and Repairing of the Bone Models
2.5. Assembly of the Lumbar Fracture Internal Fixation System
2.6. Finite Element Parameter Assignment and Loading
2.7. Finite Element Analysis
3. Results
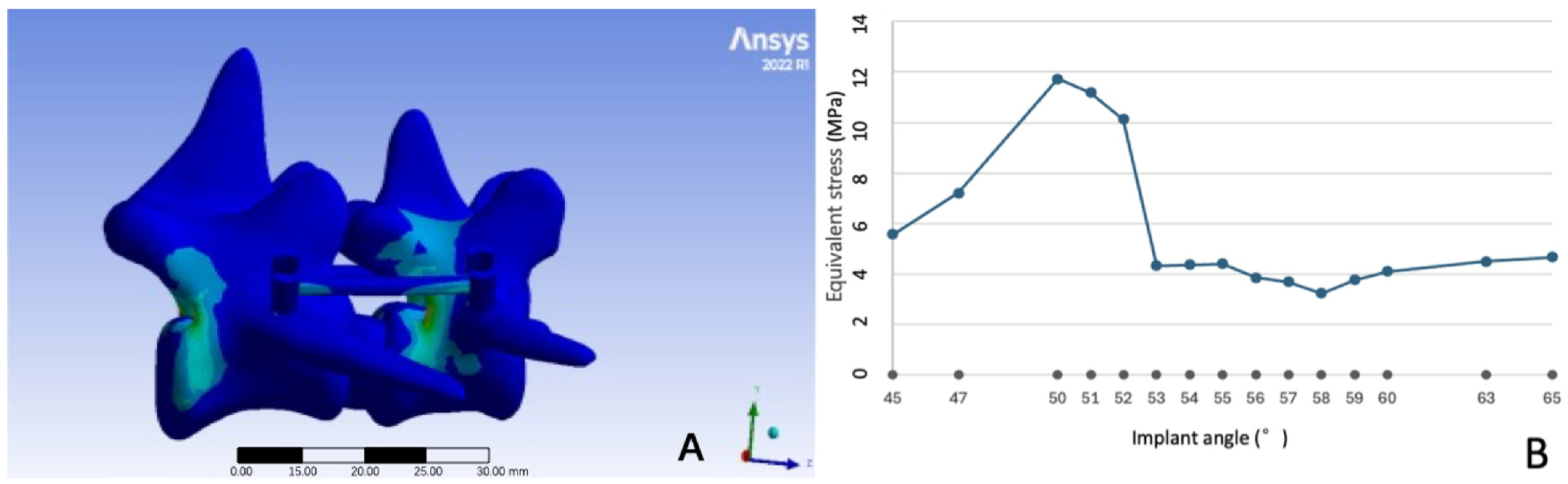
3.1. Model Establishment Results
3.2. Equivalent Stress at Different Pedicle Screw Implant Angles
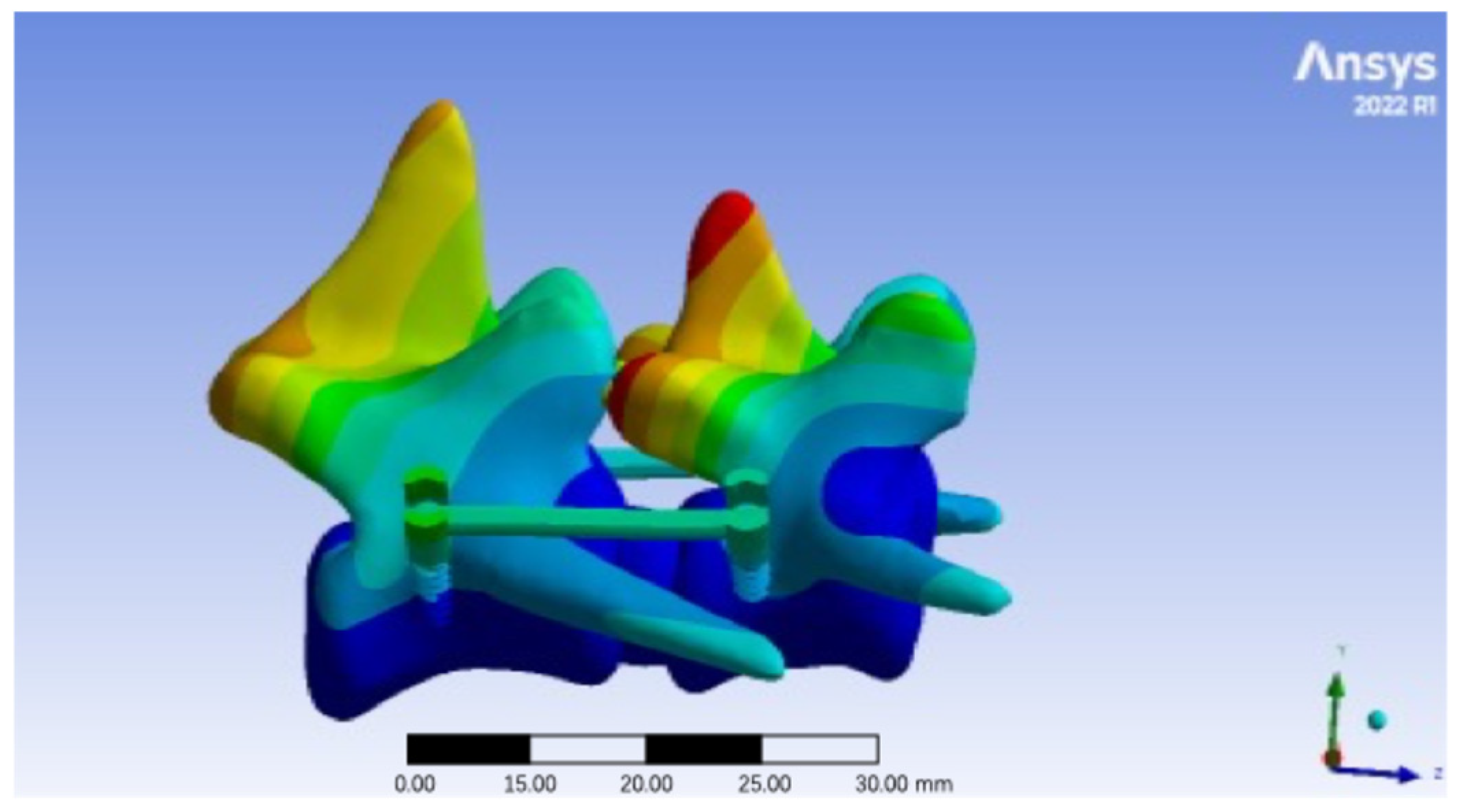
3.3. Total Deformation at Different Pedicle Screw Insertion Angles
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Della Valle, G.; Di Dona, F.; Mennonna, G.; Lamagna, B.; Pasolini, M.; Caterino, C.; Lamagna, F.; Fatone, G. Traumatic lumbosacral joint dislocation in 3 dogs: Clinical presentation, diagnosis, treatment and short-term follow-up. Pak. Vet. J. 2021, 41, 97–101. [Google Scholar]
- Jeffery, N.D. Vertebral fracture and luxation in small animals. Vet. Clin. N. Am. Small Anim. Pract. 2010, 40, 809–828. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, H.S.; Coates, J.R. Diagnosis of and Treatment Options for Disorders of the Spine; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Orgonikova, I.; Brocal, J.; Cherubini, G.; Palus, V. Vertebral fractures and luxations in dogs and cats, part 1: Evaluation of diagnosis and prognosis. Companion Anim. 2021, 26, 1–10. [Google Scholar] [CrossRef]
- Gallastegui, A.; Davies, E.; Zwingenberger, A.; Nykamp, S.; Rishniw, M.; Johnson, P. MRI has limited agreement with CT in the evaluation of vertebral fractures of the canine trauma patient. Vet. Radiol. Ultrasound 2019, 60, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Bali, M.S.; Lang, J.; Jaggy, A.; Spreng, D.; Doherr, M.G.; Forterre, F. Comparative study of vertebral fractures and luxations in dogs and cats. Vet. Comp. Orthop. Traumatol. 2009, 22, 47–53. [Google Scholar] [PubMed]
- Liu, R.; Yang, J.; Zhu, Y.; Zhou, X.; Zhou, Q.; Liang, T.; Wang, H.; Luo, Y.; Xie, Y.; Liu, H. A forecasting model for suitable dental implantation in canine mandibular premolar region based on finite element analysis. BMC Vet. Res. 2024, 20, 353. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Wang, F.; Wang, D.; Li, X.; Wang, Q. 3-D finite element analysis of the influence of synovial condition in sacroiliac joint on the load transmission in human pelvic system. Med. Eng. Phys. 2014, 36, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Zhang, H.; Deng, L.; Li, C.; Zhu, Y.; Ling, S.; Li, D.; Zhou, X.; Liu, H.; Zhong, Z. Biomechanics of Dental Implantation in the Giant Panda (Ailuropoda melanoleuca): A Comparative Study Using Finite Element Analysis. J. Vet. Dent. 2024, 42, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Yin, M.; Ma, J.; Liu, Y.; Chen, G.; Huang, Q.; Zhao, G.; Lu, T.; Yao, S.; Chen, Q.; et al. Design and Application of a Novel Patient-Specific Three-Dimensional Printed Drill Navigational Guiding in Atlantoaxial Pedicle Screw Placement. World Neurosurg. 2018, 114, e1–e10. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Kwak, H.-H.; Woo, H.-M.; Kim, J. Enhancing the Accuracy of Pedicle Screw Placement Using 3D-Printed Screw-Guiding Techniques in the Lumbosacral Region for Small Breed Dogs: A Cadaveric Study. Animals 2024, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- MacArthur, C.J.A.; Sun, D. Guidelines for the ethical review of laboratory animal welfare People’s Republic of China National Standard GB/T 35892-2018. Anim. Models Exp. Med. 2020, 3, 103–113. [Google Scholar]
- Peng, J.; Yang, J.; Liu, R.; Liu, H.; Zhong, Z.; Peng, G.; Zhang, K.; Zheng, C.; Zhang, M.; Zhou, Z. Evaluating the concept of three-dimensional printing guided endodontics in the dog. Front. Vet. Sci. 2024, 11, 1481612. [Google Scholar] [CrossRef] [PubMed]
- Ford, J.M.; Decker, S.J. Computed tomography slice thickness and its effects on three-dimensional reconstruction of anatomical structures. J. Forensic Radiol. Imaging 2016, 4, 43–46. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Choi, W.S.; Rhyu, K.W. Assessment of pedicle screw pullout strength based on various screw designs and bone densities-an ex vivo biomechanical study. Spine J. Off. J. N. Am. Spine Soc. 2012, 12, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Yang, K. Basic Finite Element Method as Applied to Injury Biomechanics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–730. [Google Scholar]
- Lewis, G.S.; Mischler, D.; Wee, H.; Reid, J.S.; Varga, P. Finite Element Analysis of Fracture Fixation. Curr. Osteoporos. Rep. 2021, 19, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y.; Shimada, M.; Takahashi, F.; Yamaguchi, S.; Hara, Y. Finite element analysis shows minimal stability difference between individualized mini-hemilaminectomy-corpectomy and partial lateral corpectomy in a dog model. Am. J. Vet. Res. 2024, 85, 12. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Zheng, L.; Chen, G.; Wang, X.; Chi, H.; Song, C.; Xi, C.; Yan, J. A novel dynamic fixation system with biodegradable components on lumbar fusion between articular processes in a canine model. J. Eng. Med. 2020, 234, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.-H.; Goel, V.K.; Weinstein, J.N.; Kong, W. Stress analysis of a canine spinal motion segment using the finite element technique. J. Biomech. 1994, 27, 1259–1269. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, M.; Park, S.H.; Wang, H.L. Methods used to assess implant stability: Current status. Int. J. Oral Maxillofac. Implant. 2007, 22, 743–754. [Google Scholar]
- Bahl, S.; Suwas, S.; Chatterjee, K. Comprehensive review on alloy design, processing, and performance of β Titanium alloys as biomedical materials. Int. Mater. Rev. 2020, 66, 114–139. [Google Scholar] [CrossRef]
- Li, W.; Zhang, P.; Gan, F. Biomechanical characterization of bilateral pedicle screw internal fixation combinations on lumbar vertebrae. Acta Bioeng. Biomech. 2023, 25, 43–51. [Google Scholar] [CrossRef]
- Giorgio, I.; dell’Isola, F.; Andreaus, U.; Misra, A. An orthotropic continuum model with substructure evolution for describing bone remodeling: An interpretation of the primary mechanism behind Wolff’s law. Biomech. Model. Mechanobiol. 2023, 22, 2135–2152. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Kang, J.H. Mechanical Properties of Compact Bone Defined by the Stress-Strain Curve Measured Using Uniaxial Tensile Test: A Concise Review and Practical Guide. Materials 2021, 14, 4224. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Monazzah, S.; Bigham-Sadegh, A. Bone injury and fracture healing biology. Biomed. Environ. Sci. 2015, 28, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Hoenig, T.; Ackerman, K.E.; Beck, B.R.; Bouxsein, M.L.; Burr, D.B.; Hollander, K.; Popp, K.L.; Rolvien, T.; Tenforde, A.S.; Warden, S.J. Bone stress injuries. Nat. Rev. Dis. Primers 2022, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Meng, B.; Bunch, J.; Burton, D.; Wang, J. Lumbar interbody fusion: Recent advances in surgical techniques and bone healing strategies. Eur. Spine J. 2021, 30, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, Y.; Yang, S.D.; Wang, T.; Wang, H.; Liu, F.Y.; Ding, W.Y. Unilateral versus bilateral pedicle screw fixation with posterior lumbar interbody fusion for lumbar degenerative diseases: A meta-analysis. Medicine 2017, 96, e6882. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Rong, L.; Feng, F.; Liu, B.; Xu, Y.; Wang, Q.; Chen, R.; Xie, P. Unilateral pedicle screw fixation through a tubular retractor via the Wiltse approach compared with conventional bilateral pedicle screw fixation for single-segment degenerative lumbar instability: A prospective randomized study. J. Neurosurg. Spine 2014, 20, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Voss, K.; Galeandro, L.; Wiestner, T.; Haessig, M.; Montavon, P.M. Relationships of body weight, body size, subject velocity, and vertical ground reaction forces in trotting dogs. Vet. Surg. 2010, 39, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Shea, T.M.; Laun, J.; Gonzalez-Blohm, S.A.; Doulgeris, J.J.; Lee III, W.E.; Aghayev, K.; Vrionis, F.D. Designs and techniques that improve the pullout strength of pedicle screws in osteoporotic vertebrae: Current status. BioMed Res. Int. 2014, 2014, 748393. [Google Scholar] [CrossRef] [PubMed]
- Colombo, C.; Libonati, F.; Rinaudo, L.; Bellazzi, M.; Ulivieri, F.M.; Vergani, L. A new finite element based parameter to predict bone fracture. PLoS ONE 2019, 14, e0225905. [Google Scholar] [CrossRef] [PubMed]
- Van den Munckhof, S.; Zadpoor, A.A. How accurately can we predict the fracture load of the proximal femur using finite element models? Clin. Biomech. 2014, 29, 373–380. [Google Scholar] [CrossRef] [PubMed]
| Material | Elastic Modulus (MPa) | Poisson’s Ratio |
|---|---|---|
| Cortical Bone | 12,000 | 0.30 |
| Cancellous Bone | 132 | 0.20 |
| Nucleus Pulposus | 1 | 0.49 |
| Annulus Fibrosus | 4.2 | 0.45 |
| Articular Cartilage | 11 | 0.40 |
| Endplate | 23.8 | 0.40 |
| Ti-6Al-4V (Pedicle Screw) | 110,000 | 0.30 |
| Ti-6Al-4V (Fixation Rod) | 110,000 | 0.30 |
| Angles | Nodes | Elements | Angles | Nodes | Elements |
|---|---|---|---|---|---|
| 45° | 166,209 | 87,002 | 56° | 168,027 | 87,710 |
| 47° | 165,931 | 86,450 | 57° | 165,850 | 86,478 |
| 50° | 164,932 | 86,269 | 58° | 164,115 | 85,784 |
| 51° | 164,923 | 86,269 | 59° | 165,859 | 86,478 |
| 52° | 164,923 | 86,269 | 60° | 166,389 | 86,852 |
| 53° | 166,622 | 86,463 | 63° | 161,757 | 83,027 |
| 54° | 161,725 | 83,010 | 65° | 166,622 | 86,436 |
| 55° | 168,027 | 87,710 |
| Angles | Maximum Total Deformation (mm) | Angles | Maximum Total Deformation (mm) |
|---|---|---|---|
| 45° | 0.0066 | 56° | 0.0043 |
| 47° | 0.0055 | 57° | 0.0035 |
| 50° | 0.0046 | 58° | 0.0051 |
| 51° | 0.0034 | 59° | 0.0041 |
| 52° | 0.0041 | 60° | 0.0049 |
| 53° | 0.0045 | 63° | 0.0035 |
| 54° | 0.0033 | 65° | 0.0038 |
| 55° | 0.0064 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Shi, X.; Peng, J.; Zhou, X.; Yang, L.; Zhong, Z.; Liu, H.; Peng, G.; Zheng, C.; Zhang, M. Finite Element Analysis of Stress Distribution in Canine Lumbar Fractures with Different Pedicle Screw Insertion Angles. Vet. Sci. 2025, 12, 682. https://doi.org/10.3390/vetsci12070682
Zhou Z, Shi X, Peng J, Zhou X, Yang L, Zhong Z, Liu H, Peng G, Zheng C, Zhang M. Finite Element Analysis of Stress Distribution in Canine Lumbar Fractures with Different Pedicle Screw Insertion Angles. Veterinary Sciences. 2025; 12(7):682. https://doi.org/10.3390/vetsci12070682
Chicago/Turabian StyleZhou, Ziyao, Xiaogang Shi, Jiahui Peng, Xiaoxiao Zhou, Liuqing Yang, Zhijun Zhong, Haifeng Liu, Guangneng Peng, Chengli Zheng, and Ming Zhang. 2025. "Finite Element Analysis of Stress Distribution in Canine Lumbar Fractures with Different Pedicle Screw Insertion Angles" Veterinary Sciences 12, no. 7: 682. https://doi.org/10.3390/vetsci12070682
APA StyleZhou, Z., Shi, X., Peng, J., Zhou, X., Yang, L., Zhong, Z., Liu, H., Peng, G., Zheng, C., & Zhang, M. (2025). Finite Element Analysis of Stress Distribution in Canine Lumbar Fractures with Different Pedicle Screw Insertion Angles. Veterinary Sciences, 12(7), 682. https://doi.org/10.3390/vetsci12070682








