Validating Sperm Concentration in Rabbit Cryopreservation Protocol: Implications for Fertility, Litter Size, and Offspring Growth
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Semen Collection, Processing, and Cryopreservation Protocol
2.3. Comparing the In Vivo Reproductive Performance of Nulliparous and Multiparous Rabbit Does Inseminated with Different Sperm Concentrations per Straw
2.4. Statistical Analysis
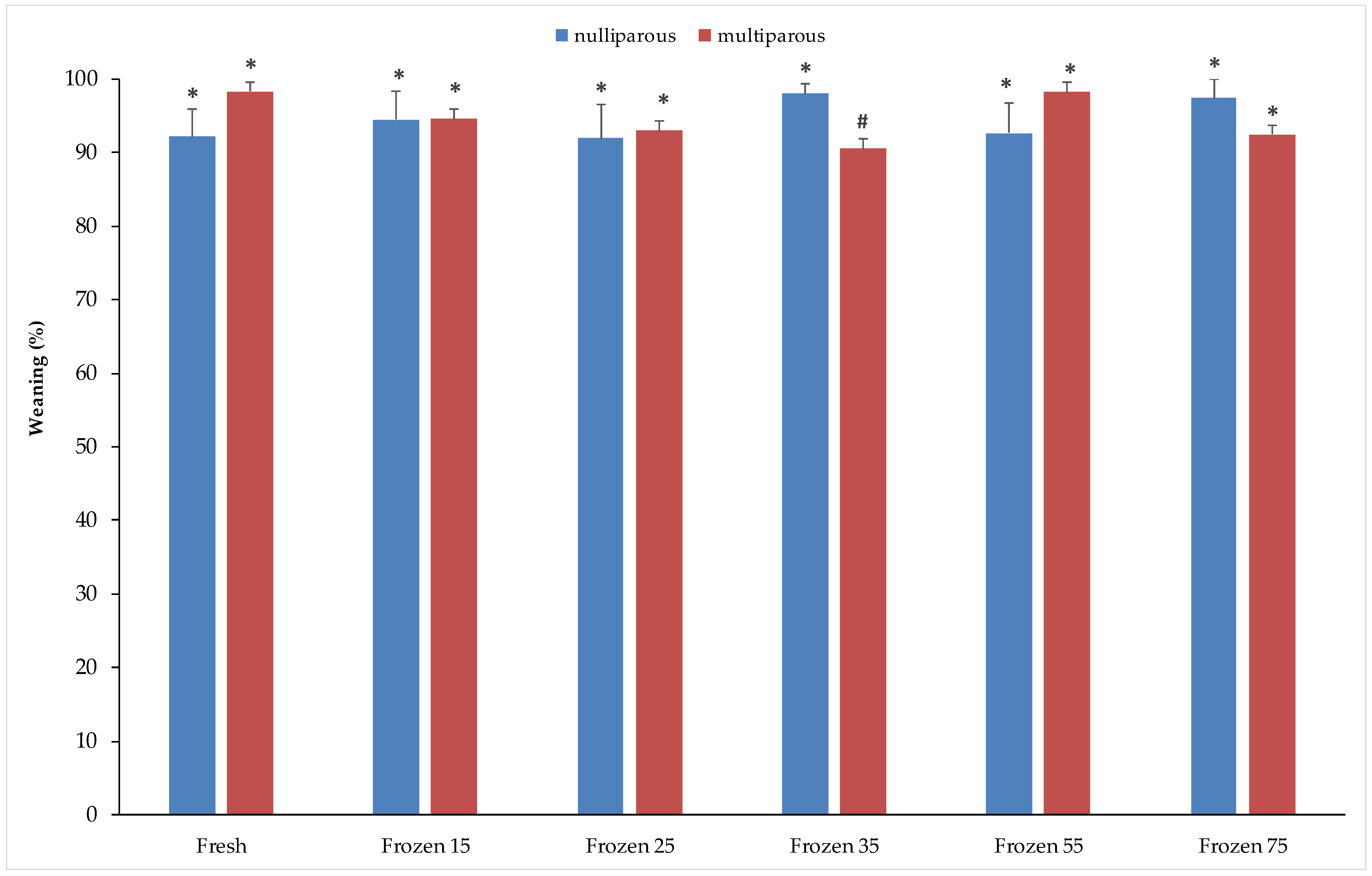
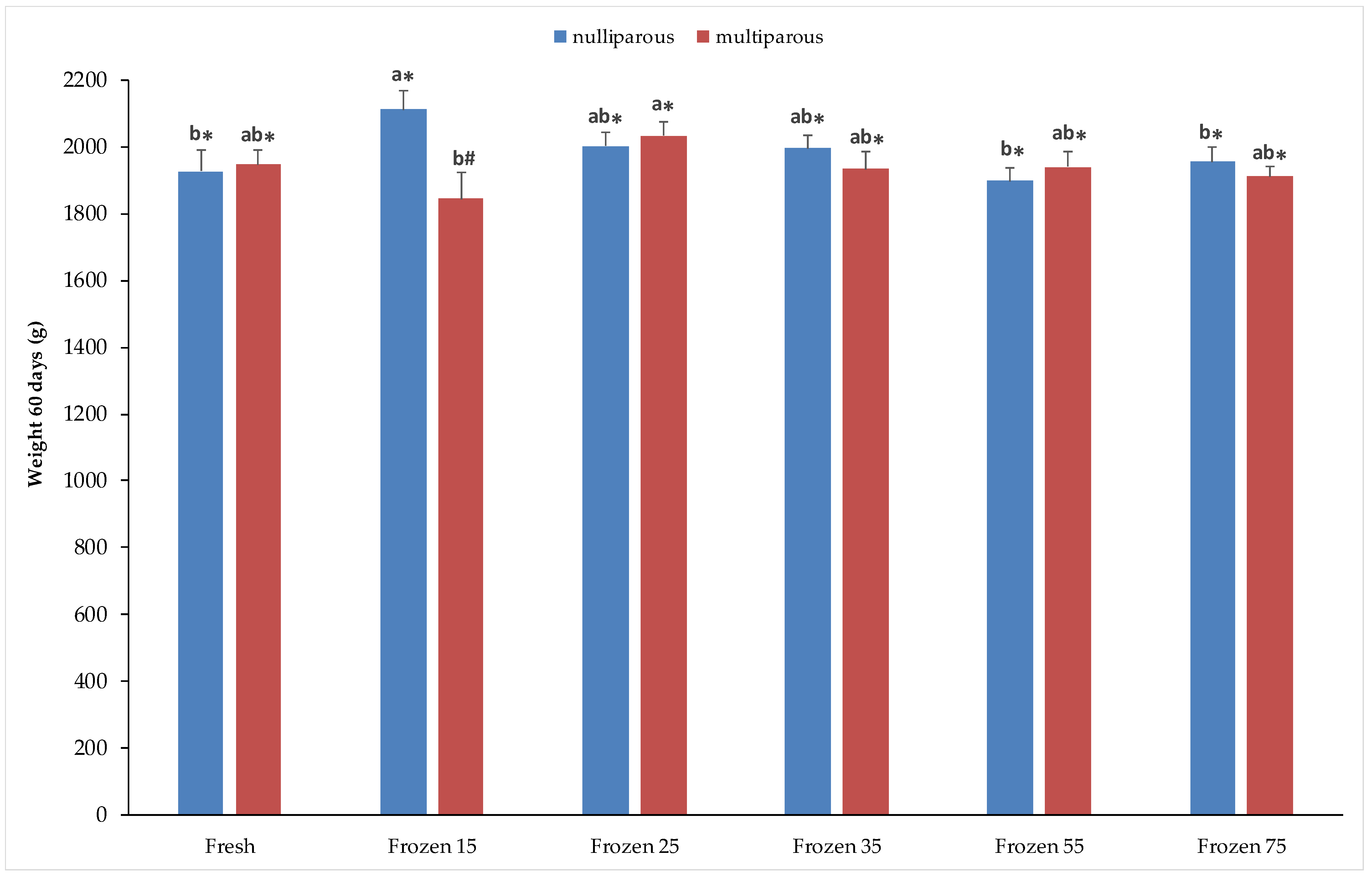
3. Results
3.1. Fertility and Litter Size
3.2. Offspring Growth
4. Discussion
4.1. Fertility and Kindling Rates
4.2. Prolificacy
4.3. Offspring Growth and Development
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mocé, E.; Vicente, J.S. Rabbit sperm cryopreservation: A review. Anim. Reprod. Sci. 2009, 110, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Lavara, R.; David, I.; Mocé, E.; Baselga, M.; Vicente, J.S. Environmental and male variation factors of freezability in rabbit semen. Theriogenology 2013, 79, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Lavara, R.; Mocé, E.; Baselga, M.; Vicente, J.S. Freezability genetics in rabbit semen. Theriogenology 2017, 102, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Kulíková, B.; Oravcová, M.; Baláži, A.; Supuka, P.; Chrenek, P. Factors affecting storage of Slovak native rabbit semen in the gene bank. Zygote 2017, 25, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, K.; Kitajima, S.; Matsuhisa, F.; Niimi, M.; Wang, C.; Fan, J. Strategies for highly efficient rabbit sperm cryopreservation. Animals 2021, 11, 1220. [Google Scholar] [CrossRef] [PubMed]
- Viudes-de-Castro, M.P.; Vicente, J.S. Trends in rabbit insemination extenders for fresh and frozen semen. A review. World Rabbit Sci. 2023, 31, 109–116. [Google Scholar] [CrossRef]
- Iaffaldano, N.; Di Iorio, M.; Rosato, M.P. The cryoprotectant used, its concentration, and the equilibration time are critical for the successful cryopreservation of rabbit sperm: Dimethylacetamide versus dimethylsulfoxide. Theriogenology 2012, 78, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Iaffaldano, N.; Di Iorio, M.; Rosato, M.P.; Manchisi, A. Cryopreservation of rabbit semen using non-permeable cryoprotectants: Effectiveness of different concentrations of low-density lipoproteins (LDL) from egg yolk versus egg yolk or sucrose. Anim. Reprod. Sci. 2014, 151, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, M.; Colonna, M.A.; Miranda, M.; Principe, P.; Schiavitto, M.; Cerolini, S.; Manchisi, A.; Iaffaldano, N. Initial cooling time before freezing affects post-thaw quality and reproductive performance of rabbit semen. Anim. Sci. J. 2018, 89, 1240–1244. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, M.; Rusco, G.; Colonna, M.A.; Schiavitto, M.; D’Andrea, M.S.; Cerolini, S.; Iaffaldano, N. Improving the rabbit semen cryopreservation protocol: Comparison between two extenders and inseminating doses. Ann. Anim. Sci. 2020, 20, 887–898. [Google Scholar] [CrossRef]
- Rusco, G.; Słowińska, M.; Di Iorio, M.; Cerolini, S.; Maffione, A.B.; Ciereszko, A.; Iaffaldano, N. Proteomic analysis of rabbit fresh and cryopreserved semen provides an important insight into molecular mechanisms of cryoinjuries to spermatozoa. Theriogenology 2022, 191, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Mocé, E.; Blanch, E.; Talaván, A.; Viudes de Castro, M.P. Reducing the time rabbit sperm are held at 5 °C negatively affects their fertilizing ability after cryopreservation. Theriogenology 2014, 82, 1049–1053. [Google Scholar] [CrossRef] [PubMed]
- Viudes-de-Castro, M.P.; Lavara, R.; Safaa, H.M.; Marco-Jiménez, F.; Mehaisen, G.M.K.; Vicente, J.S. Effect of freezing extender composition and male line on semen traits and reproductive performance in rabbits. Animal 2014, 8, 765–770. [Google Scholar] [CrossRef]
- Viudes-de-Castro, M.P.; Talaván, A.G.; Vicente, J.S. Evaluation of dextran for rabbit sperm cryopreservation: Effect on frozen–thawed rabbit sperm quality variables and reproductive performance. Anim. Reprod. Sci. 2021, 226, 106714. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.E.; Negus, C.; Johinke, D.; Bathgate, R. Adjusting cryodiluent composition for improved post-thaw quality of rabbit spermatozoa. PLoS ONE 2017, 12, e0175965. [Google Scholar] [CrossRef] [PubMed]
- Domingo, P.; Olaciregui, M.; González, N.; De Blas, I.; Gil, L. Comparison of different semen extenders and cryoprotectant agents to enhance cryopreservation of rabbit spermatozoa. Czech J. Anim. Sci. 2019, 64, 59–66. [Google Scholar] [CrossRef]
- Mohammed, K.M.; Darwish, G.M.; Rawash, Z.M.; Taha, A.M. Cryopreservation of rabbit semen: Impacts of permeable and non-permeable mixture of cryoprotectant, male group individuality, freezing rate, semen package size and antioxidant bovine serum albumin on rabbit semen freezability. World Rabbit Sci. 2022, 30, 227–238. [Google Scholar] [CrossRef]
- Di Iorio, M.; Lauriola, F.; Rusco, G.; Antenucci, E.; Schiavitto, M.; Iaffaldano, N. Cryopreserving rabbit semen: Impact of varying sperm concentrations on quality and the standardization of protocol. Vet. Sci. 2024, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Contri, A.; Gloria, A.; Robbe, D.; Sfirro, M.P.; Carluccio, A. Effect of sperm concentration on characteristics of frozen-thawed semen in donkeys. Anim. Reprod. Sci. 2012, 136, 74–80. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, A.G.; Martemucci, G.; Colonna, M.A.; Bellitti, A. Post-thaw survival of ram spermatozoa and fertility after insemination as affected by prefreezing sperm concentration and extender composition. Theriogenology 2001, 55, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.; Tamayo-Canul, J.; Anel, E.; Boixo, J.C.; Mata-Campuzano, M.; Martinez-Pastor, F.; Anel, L.; de Paz, P. Sperm concentration at freezing affects post-thaw quality and fertility of ram semen. Theriogenology 2012, 77, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Leahy, T.; Marti, J.I.; Mendoza, N.; Pérez-Pé, R.; Muiño-Blanco, T.; Cebrián-Pérez, J.A.; Evans, G.; Maxwell, W.M.C. High pre-freezing dilution improves post-thaw function of ram spermatozoa. Anim. Reprod. Sci. 2010, 119, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Heitland, A.V.; Jasko, D.J.; Squires, E.L.; Graham, J.K.; Pickett, B.W.; Hamilton, C. Factors affecting motion characteristics of frozen-thawed stallion spermatozoa. Equine Vet. J. 1996, 28, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Crockett, E.C.; Graham, J.K.; Bruemmer, J.E.; Squires, E.L. Effect of cooling of equine spermatozoa before freezing on post-thaw motility: Preliminary results. Theriogenology 2001, 55, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Farrell, P.B.; Foote, R.H.; Simkin, M.E.; Clegg, E.D.; Wall, R.J. Relationship of semen quality, number of sperm inseminate, and fertility in rabbits. J. Androl. 1993, 14, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Castellini, C.; Pizzi, F.; Theau-Clément, M.; Lattaioli, P. Effect of different number of frozen spermatozoa inseminated on the reproductive performance of rabbit does. Theriogenology 2006, 66, 2182–2187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Smith, W. Maternal control of early embryogenesis in mammals. Reprod. Fertil. Dev. 2015, 27, 880–896. [Google Scholar] [CrossRef] [PubMed]
- Denicol, A.C.; Siqueira, L.G.B. Maternal contributions to pregnancy success: From gamete quality to uterine environment. Anim. Reprod. 2023, 20, e20230085. [Google Scholar] [CrossRef] [PubMed]
- Rebollar, P.G.; Pérez-Cabal, M.A.; Pereda, N.; Lorenzo, P.L.; Arias-Álvarez, M.; García-Rebollar, P. Effects of parity order and reproductive management on the efficiency of rabbit productive systems. Livest. Sci. 2009, 121, 227–233. [Google Scholar] [CrossRef]
- Theau-Clément, M. Preparation of the rabbit doe to insemination: A review. World Rabbit Sci. 2007, 15, 61–80. [Google Scholar] [CrossRef]
- Dimitrova, I.; Angelov, G.; Teneva, A.; Uzev, P. Artificial insemination of rabbits. Biotechnol. Anim. Husb. 2009, 25, 1249–1253. [Google Scholar][Green Version]
- Theau-Clément, M. Advances in biostimulation methods applied to rabbit reproduction. World Rabbit Sci. 2000, 8, 61–79. [Google Scholar][Green Version]
- Fortun-Lamothe, L. Energy balance and reproductive performance in rabbit does. Anim. Rep. Sci. 2006, 93, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chavatte-Palmer, P.; Velazquez, M.A.; Jammes, H.; Duranthon, V. Review: Epigenetics, developmental programming and nutrition in herbivores. Animal 2018, 12, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.F. The causes of reduced fertility with cryopreserved semen. Anim. Reprod. Sci. 2000, 60–61, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Prasad, J.K.; Srivastava, N.; Ghosh, S.K. Strategies to minimize various stress-related freeze–thaw damages during conventional cryopreservation of mammalian spermatozoa. Biopreserv. Biobank. 2019, 17, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Assan, N. Factors influencing post-weaning growth and mortality in rabbits. Sci. J. Anim. Sci 2018, 7, 486–492. [Google Scholar] [CrossRef]
- Ezzeroug, R.; Belabbas, R.; Naouel, F.; Mina, H.; Djamel, T.; Argente, M.J. Factors affecting weight of kits between birth and weaning in rabbits. Vet. Zoot. 2024, 82, 1–12. [Google Scholar]
- Nusbaumer, D.; Marques da Cunha, L.; Wedekind, C. Sperm cryopreservation reduces offspring growth. Proc. R. Soc. B 2019, 286, 20191644. [Google Scholar] [CrossRef] [PubMed]
- Estudillo, E.; Jiménez, A.; Bustamante-Nieves, P.E.; Palacios-Reyes, C.; Velasco, I.; López-Ornelas, A. Cryopreservation of Gametes and Embryos and Their Molecular Changes. Int. J. Mol. Sci. 2021, 22, 10864. [Google Scholar] [CrossRef] [PubMed]

| Group | Parity | Semen Type | Sperm Concentration (×106/straw) | Notes |
|---|---|---|---|---|
| 1 | Nulliparous | Fresh | ~35 | Control (TCG® diluted) |
| 2 | Multiparous | Fresh | ~35 | Control (TCG® diluted) |
| 3 | Nulliparous | Frozen | 15 | Thawed semen |
| 4 | Nulliparous | Frozen | 25 | Thawed semen |
| 5 | Nulliparous | Frozen | 35 | Thawed semen |
| 6 | Nulliparous | Frozen | 55 | Thawed semen |
| 7 | Nulliparous | Frozen | 75 | Thawed semen |
| 8 | Multiparous | Frozen | 15 | Thawed semen |
| 9 | Multiparous | Frozen | 25 | Thawed semen |
| 10 | Multiparous | Frozen | 35 | Thawed semen |
| 11 | Multiparous | Frozen | 55 | Thawed semen |
| 12 | Multiparous | Frozen | 75 | Thawed semen |
| Reproductive Performance | Treatment | Doe Status | |
|---|---|---|---|
| Nulliparous | Multiparous | ||
| Fertility (%) (n°/total) | Fresh semen | 71.9 a* (23/32) | 81.3 a* (26/32) |
| Frozen semen | |||
| 15 25 35 55 75 | 84.4 a* (27/32) 81.3 a* (26/32) 71.9 a* (23/32) 62.5 a* (20/32) 68.8 a* (22/32) | 68.8 b* (22/32) 78.1 a* (25/32) 78.1 a* (25/32) 81.3 a* (26/32) 50.0 b* (16/32) | |
| Kindling rate (%) (n°/total) | Fresh semen | 65.6 a* (21/32) | 71.9 a* (23/32) |
| Frozen semen | |||
| 15 25 35 55 75 | 81.3 a* (26/32) 65.6 ab* (21/32) 56.3 b* (18/32) 53.1 b* (17/32) 56.3 b* (18/32) | 59.4 ab# (19/32) 56.3 ab* (18/32) 68.8 ab* (22/32) 68.8 ab* (22/32) 43.8 b* (14/32) | |
| Total born (mean ± SEM) | Fresh semen | 7.0 ± 0.4 * | 9.6 ± 0.5 # |
| Frozen semen | |||
| 15 25 35 55 75 | 6.3 ± 0.5 * 6.7 ± 0.4 * 7.3 ± 0.3 * 6.6 ± 0.6 * 6.7 ± 0.8 * | 9.2 ± 0.6 # 9.2 ± 0.7 # 8.4 ± 0.5 * 8.2 ± 0.4 # 7.8 ± 0.7 * | |
| Live born (mean ± SEM) | Fresh semen | 6.0 ± 0.5 * | 8.9 ± 0.6 # |
| Frozen semen | |||
| 15 25 35 55 75 | 5.2 ± 0.7 * 6.5 ± 0.4 * 6.7 ± 0.5 * 6.2 ± 0.8 * 5.7 ± 0.9 * | 8.8 ± 0.6 # 8.9 ± 0.7 # 8.0 ± 0.5 * 7.9 ± 0.4 # 7.7 ± 0.7 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Iorio, M.; Rusco, G.; Lauriola, F.; Antenucci, E.; Roncarati, A.; Cerolini, S.; Schiavitto, M.; Iaffaldano, N. Validating Sperm Concentration in Rabbit Cryopreservation Protocol: Implications for Fertility, Litter Size, and Offspring Growth. Vet. Sci. 2025, 12, 678. https://doi.org/10.3390/vetsci12070678
Di Iorio M, Rusco G, Lauriola F, Antenucci E, Roncarati A, Cerolini S, Schiavitto M, Iaffaldano N. Validating Sperm Concentration in Rabbit Cryopreservation Protocol: Implications for Fertility, Litter Size, and Offspring Growth. Veterinary Sciences. 2025; 12(7):678. https://doi.org/10.3390/vetsci12070678
Chicago/Turabian StyleDi Iorio, Michele, Giusy Rusco, Fabrizio Lauriola, Emanuele Antenucci, Alessandra Roncarati, Silvia Cerolini, Michele Schiavitto, and Nicolaia Iaffaldano. 2025. "Validating Sperm Concentration in Rabbit Cryopreservation Protocol: Implications for Fertility, Litter Size, and Offspring Growth" Veterinary Sciences 12, no. 7: 678. https://doi.org/10.3390/vetsci12070678
APA StyleDi Iorio, M., Rusco, G., Lauriola, F., Antenucci, E., Roncarati, A., Cerolini, S., Schiavitto, M., & Iaffaldano, N. (2025). Validating Sperm Concentration in Rabbit Cryopreservation Protocol: Implications for Fertility, Litter Size, and Offspring Growth. Veterinary Sciences, 12(7), 678. https://doi.org/10.3390/vetsci12070678







