Isolation and Biological Characteristics Study of Porcine Reproductive and Respiratory Syndrome Virus GZ2022 Strain
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells, Viruses, Animals, and Ethics Statement
2.2. Isolation and Identification of PRRSV Field Strains
2.3. Viral Plaque Purification and Growth Curve Detection
2.4. Indirect Immunofluorescence Assay (IFA)
2.5. Phylogenetic and Evolutionary Analysis
2.6. Animal Experiments
2.6.1. Virus Inoculation in Piglets and Clinical Monitoring
2.6.2. Viral Load Quantification and Humoral Immune Response Assessment
2.6.3. Necropsy and Gross Pathological Examination
2.7. Statistical Analyses
3. Results
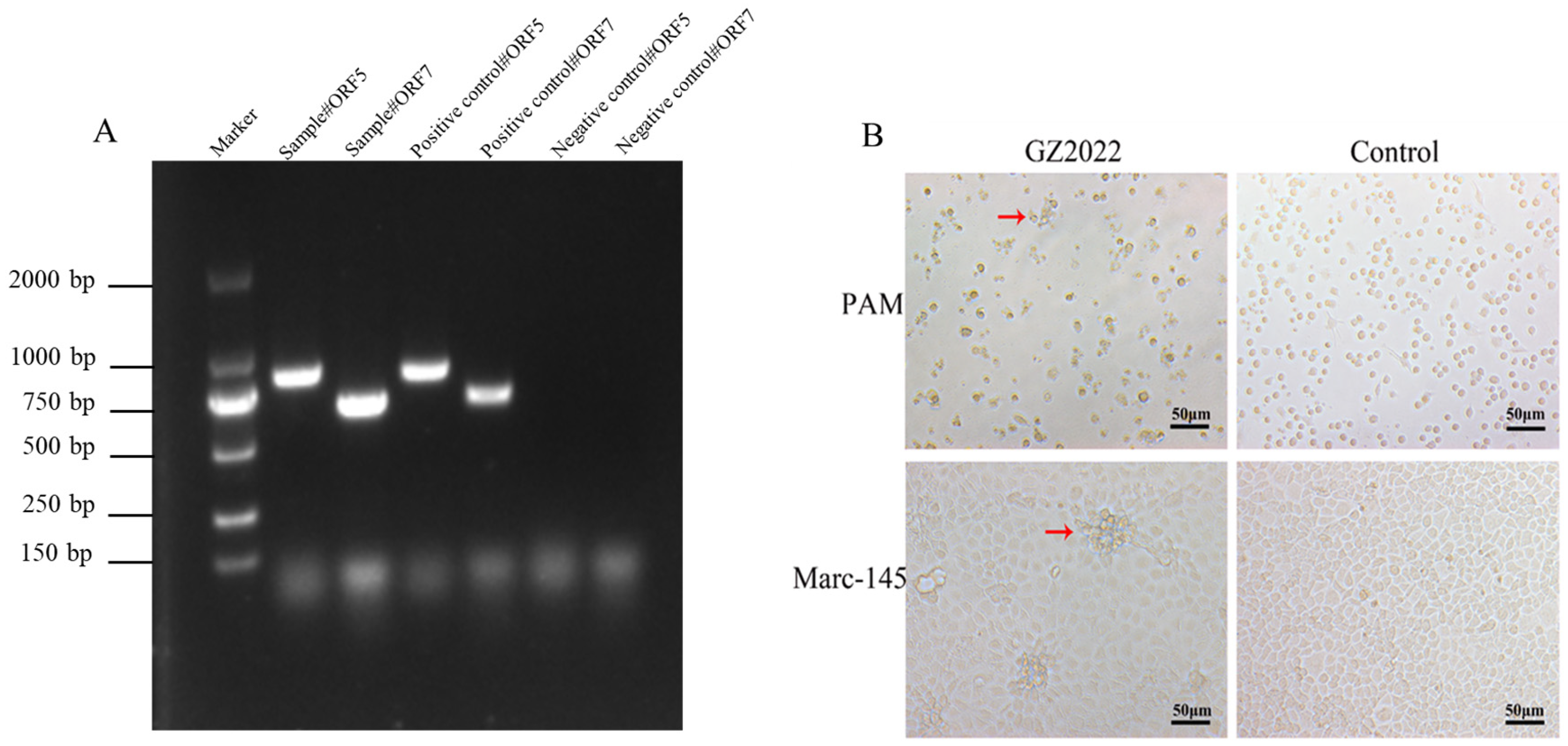
3.1. Virus Isolation and Identification
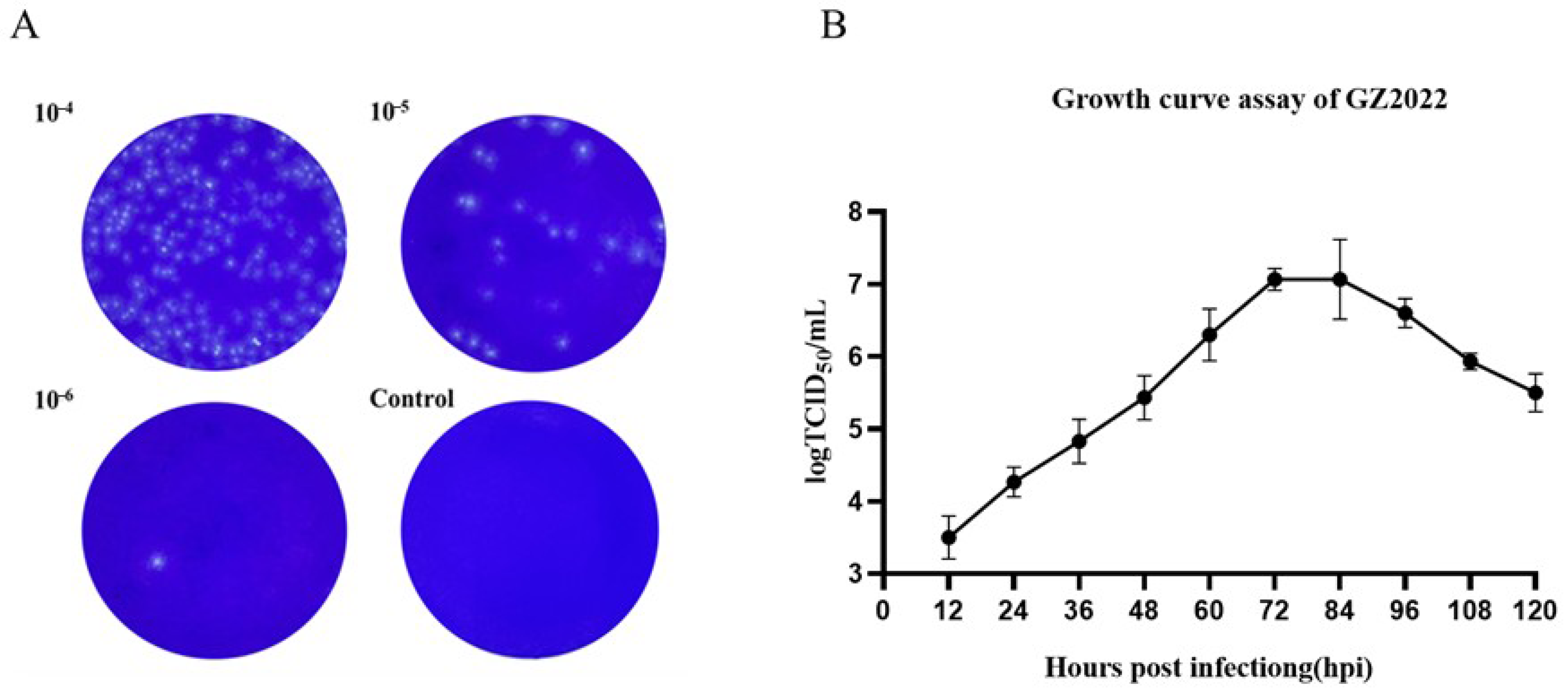
3.2. Plaque Purification and Growth Curve Assay of GZ2022
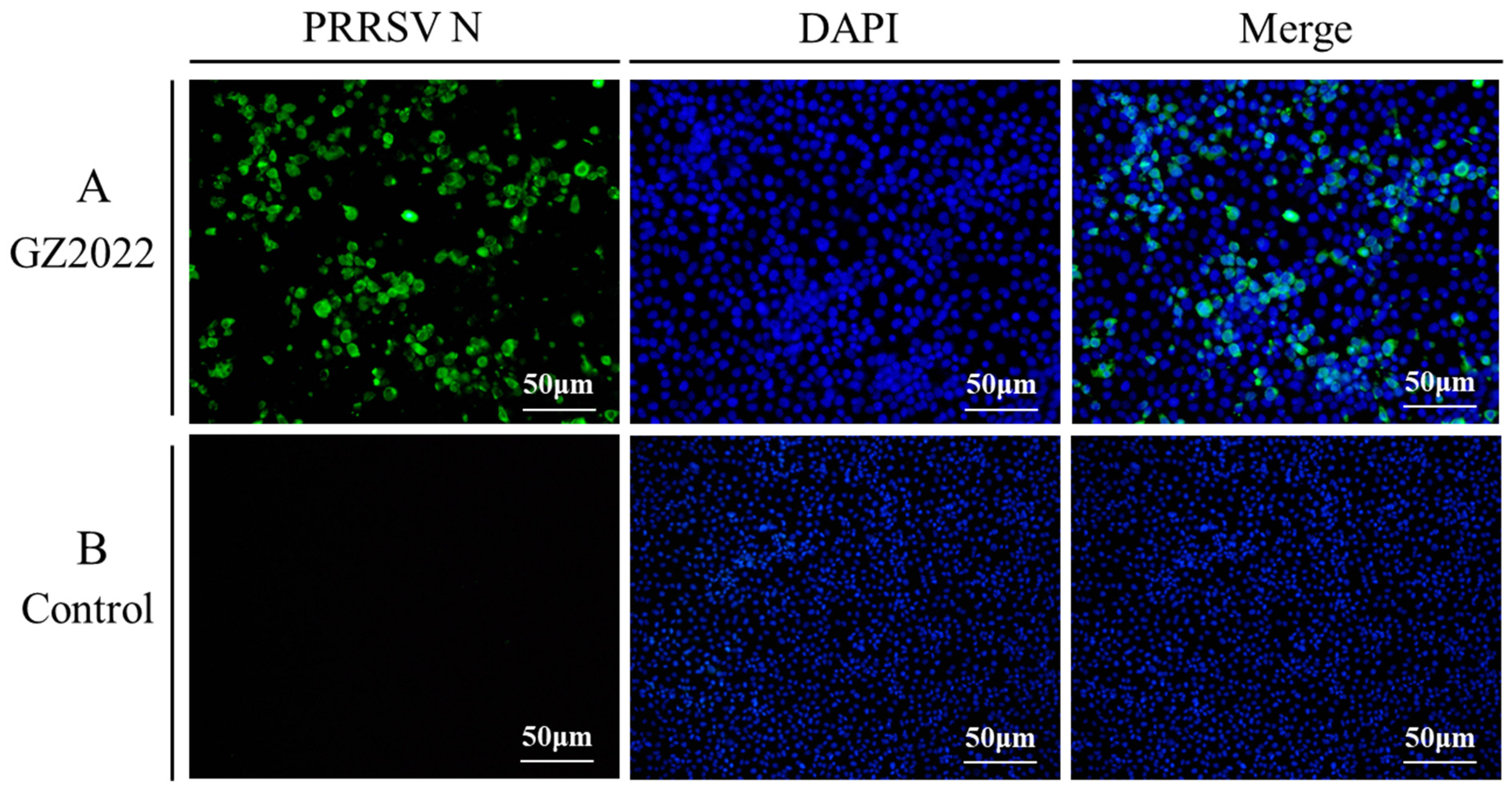
3.3. IFA Results of GZ2022
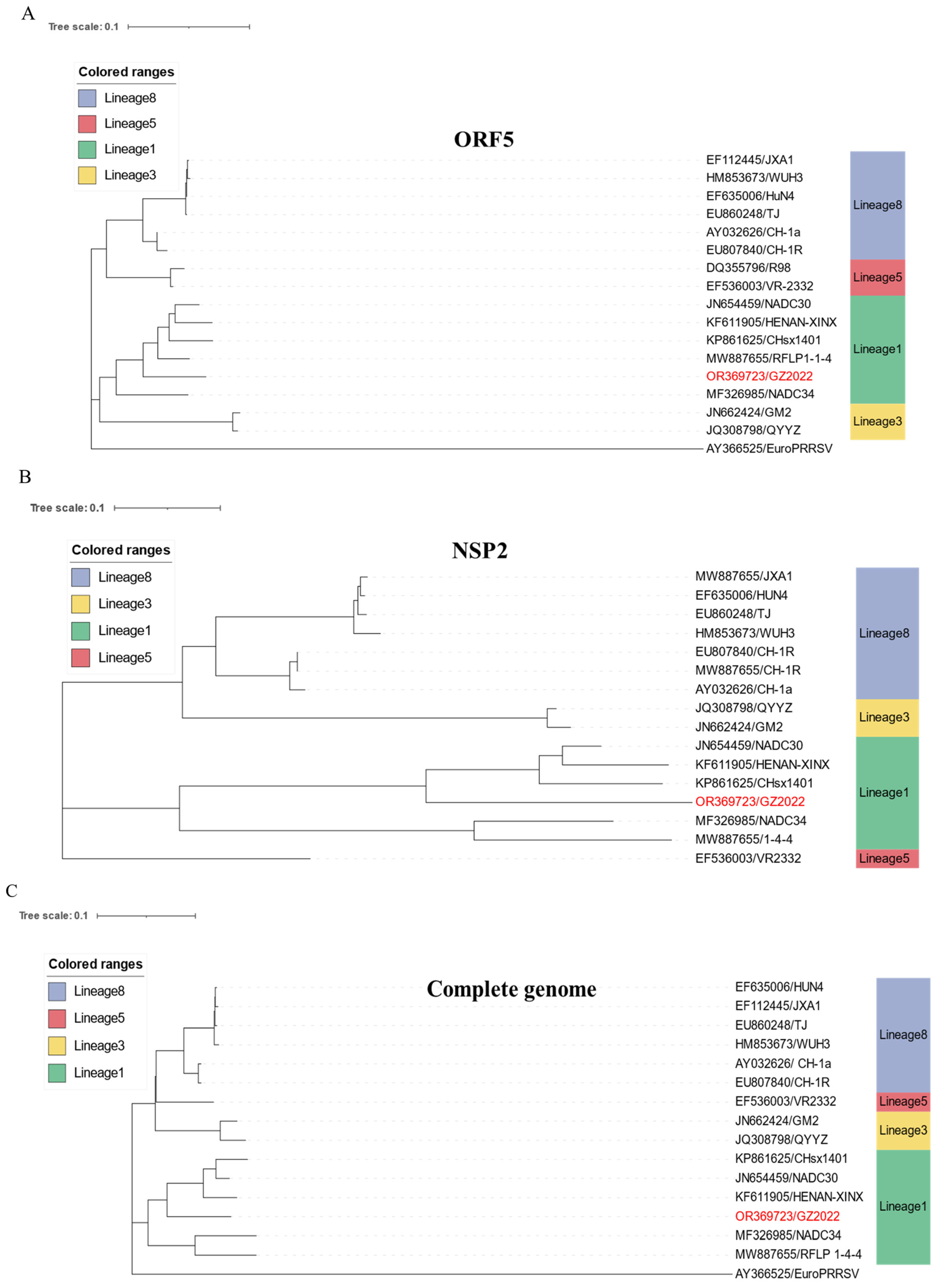
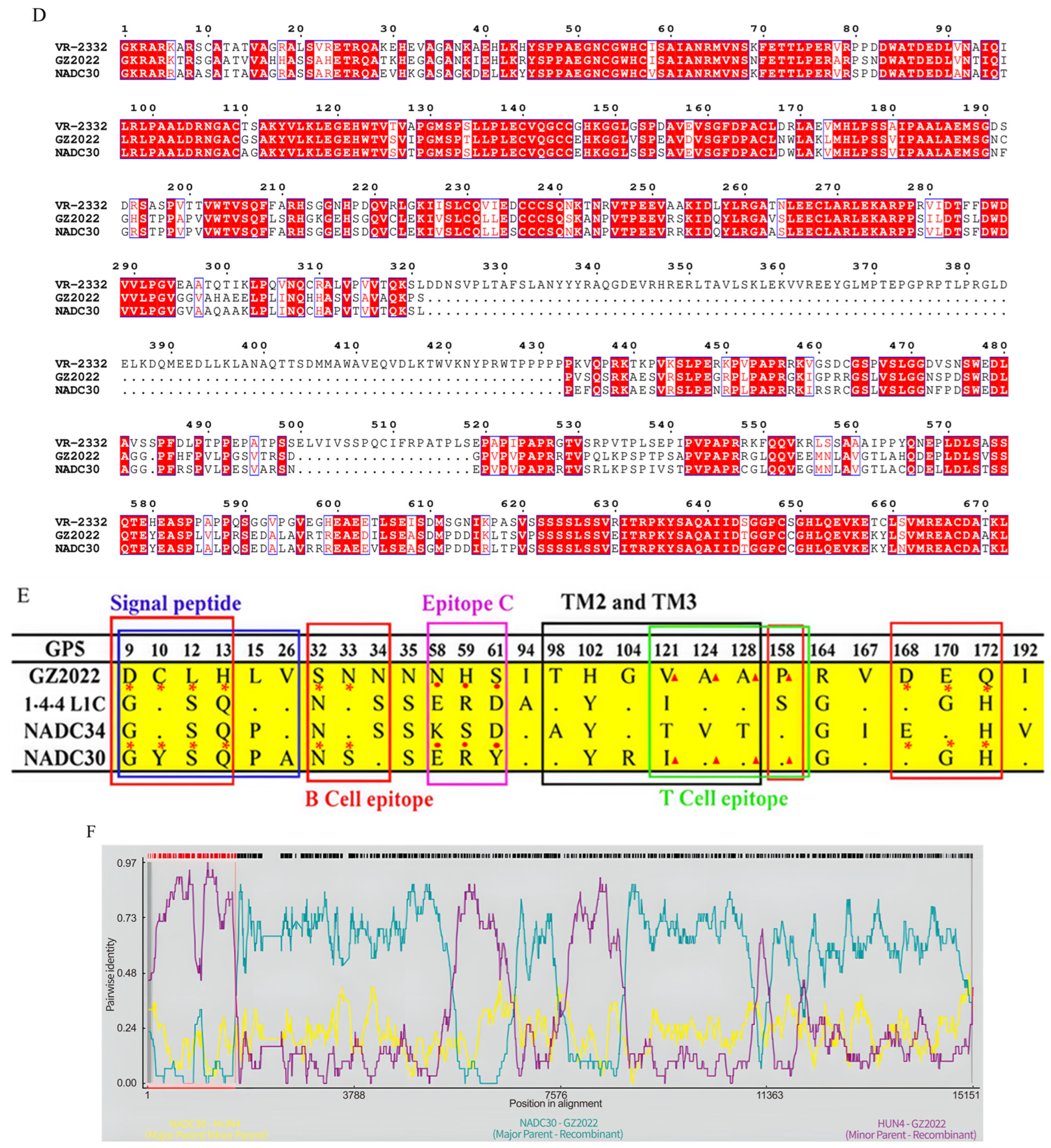
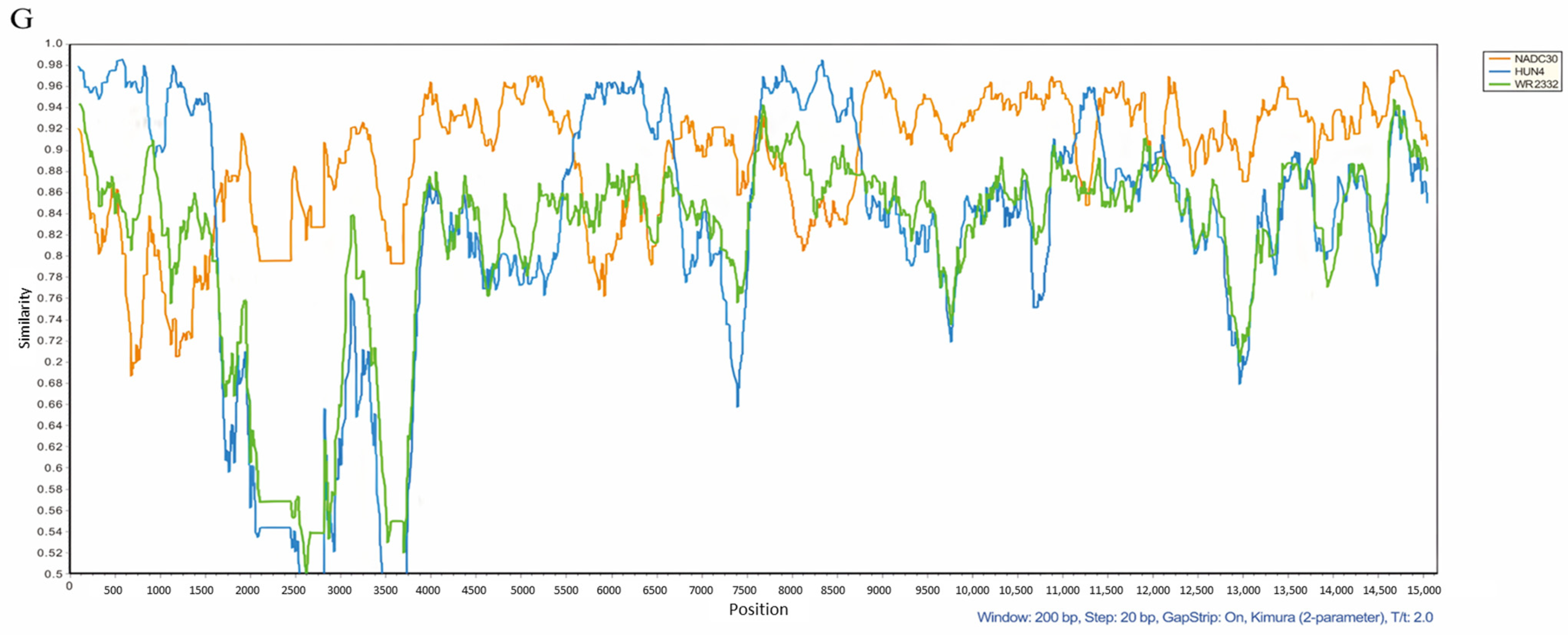
3.4. Genomic Characterization and Recombination Analysis of the GZ2022 Strain
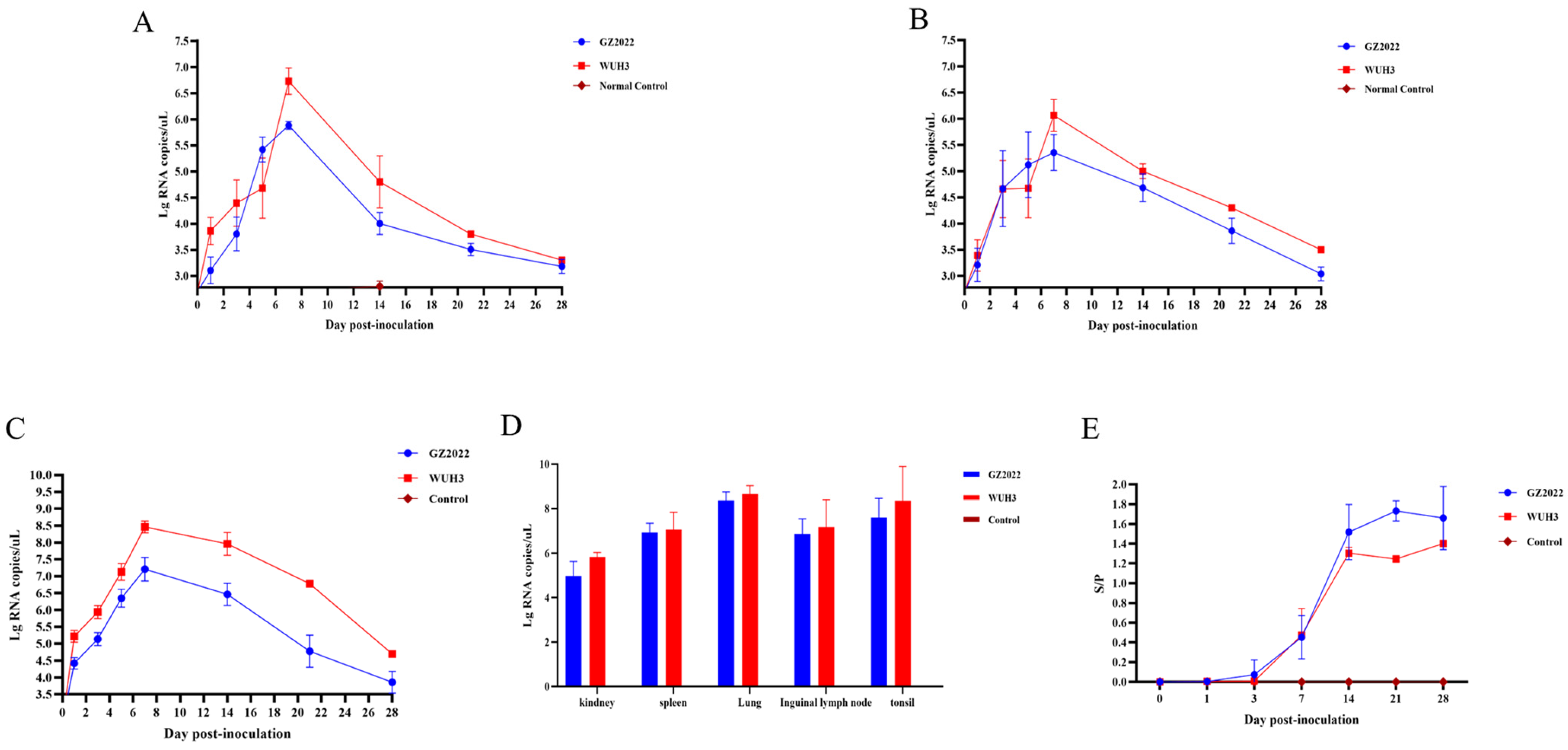
3.5. Divergent Virulence, Clinical Dynamics, and Growth Impacts of GZ2022
3.6. Divergent Viral Shedding, Tissue Tropism, and Antibody Profiles of PRRSV Strains
3.7. Gross Lesions in Piglet Lungs
3.8. Pathological Analysis of Lungs and Hilar Lymph Nodes in Piglets
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, Z.; Chen, X.X.; Li, R.; Qiao, S.; Zhang, G. The prevalent status and genetic diversity of porcine reproductive and respiratory syndrome virus in China: A molecular epidemiological perspective. Virol. J. 2018, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Xu, H.; An, T.; Cai, X.; Tian, Z.; Zhang, H. Recent Progress in Studies of Porcine Reproductive and Respiratory Syndrome Virus 1 in China. Viruses 2023, 15, 1528. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Han, J.; Yang, H. The evolution and diversity of porcine reproductive and respiratory syndrome virus in China. Vet. Microbiol. 2024, 298, 110252. [Google Scholar] [CrossRef]
- Amadori, M.; Razzuoli, E. Immune Control of PRRS: Lessons to be Learned and Possible Ways Forward. Front. Vet. Sci. 2014, 1, 2. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Li, C.; Wang, C.; Liu, Y.; Wang, G.; He, X.; Hu, L.; Liu, Y.; Cui, M.; et al. Secondary Haemophilus parasuis infection enhances highly pathogenic porcine reproductive and respiratory syndrome virus (HP-PRRSV) infection-mediated inflammatory responses. Vet. Microbiol. 2017, 4, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Chae, C. Commercial PRRS Modified-Live Virus Vaccines. Vaccines 2021, 9, 185. [Google Scholar] [CrossRef]
- Huang, B.; Deng, L.; Xu, T.; Jian, Z.; Lai, S.; Ai, Y.; Xu, Z.; Zhu, L.; Jones, C.J. Isolation and pathogenicity comparison of two novel natural recombinant porcine reproductive and respiratory syndrome viruses with different recombination patterns in Southwest China. Microbiol. Spectr. 2024, 12, e0407123. [Google Scholar] [CrossRef]
- Music, N.; Gagnon, C.A. The role of porcine reproductive and respiratory syndrome (PRRS) virus structural and non-structural proteins in virus pathogenesis. Anim. Health Res. Rev. 2010, 11, 135–163. [Google Scholar] [CrossRef]
- Snijder, E.J.; Meulenberg, J.J. The molecular biology of arteriviruses. J. Gen. Virol. 1998, 79, 961–979. [Google Scholar] [CrossRef]
- Murtaugh, M.P.; Elam, M.R.; Kakach, L.T. Comparison of the structural protein coding sequences of the VR-2332 and Lelystad virus strains of the PRRS virus. Arch. Virol. 1995, 140, 1451–1460. [Google Scholar] [CrossRef]
- Nelsen, C.J.; Murtaugh, M.P.; Faaberg, K.S. Porcine reproductive and respiratory syndrome virus comparison: Divergent evolution on two continents. J. Virol. 1999, 73, 270–280. [Google Scholar] [CrossRef]
- Yoo, D.; Song, C.; Sun, Y.; Du, Y.; Kim, O.; Liu, H.C. Modulation of host cell responses and evasion strategies for porcine reproductive and respiratory syndrome virus. Virus Res. 2010, 154, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Lunney, J.K.; Fang, Y.; Ladinig, A.; Chen, N.; Li, Y.; Rowland, B.; Renukaradhya, G.J. Porcine Reproductive and Respiratory Syndrome Virus (PRRSV): Pathogenesis and Interaction with the Immune System. Annu. Rev. Anim. Biosci. 2016, 4, 129–154. [Google Scholar] [CrossRef]
- Bian, T.; Sun, Y.; Hao, M.; Zhou, L.; Ge, X.; Guo, X.; Han, J.; Yang, H. A recombinant type 2 porcine reproductive and respiratory syndrome virus between NADC30-like and a MLV-like: Genetic characterization and pathogenicity for piglets. Infect. Genet. Evol. 2017, 54, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, C.; Xu, Y.; Yang, Y.; Li, J.; Dai, A.; Huang, C.; Luo, M.; Wei, C.; Jones, C.J. Molecular Characteristics and Pathogenicity of a Novel Recombinant Porcine Reproductive and Respiratory Syndrome Virus Strain from NADC30-, NADC34-, and JXA1-Like Strains That Emerged in China. Microbiol. Spectr. 2022, 10, e0266722. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, Z.; Ding, Y.; Ge, X.; Guo, X.; Yang, H. NADC30-like Strain of Porcine Reproductive and Respiratory Syndrome Virus, China. Emerg. Infect. Dis. 2015, 21, 2256–2257. [Google Scholar] [CrossRef]
- Tian, K. NADC30-Like Porcine Reproductive and Respiratory Syndrome in China. Open Virol. J. 2017, 11, 59–65. [Google Scholar] [CrossRef]
- Li, X.; Wu, J.; Tan, F.; Li, Y.; Ji, G.; Zhuang, J.; Zhai, X.; Tian, K. Genome characterization of two NADC30-like porcine reproductive and respiratory syndrome viruses in China. Springerplus 2016, 5, 1677. [Google Scholar] [CrossRef]
- Ruan, S.; Yu, X.; Wu, H.; Lei, M.; Ku, X.; Ghonaim, A.H.; Li, W.; Jiang, Y.; He, Q. Assessing the antiviral activity of antimicrobial peptides Caerin1.1 against PRRSV in Vitro and in Vivo. Vet. Microbiol. 2024, 297, 110210. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, B.; Xu, L.; Jin, H.; Ge, X.; Guo, X.; Han, J.; Yang, H. Efficacy evaluation of three modified-live virus vaccines against a strain of porcine reproductive and respiratory syndrome virus NADC30-like. Vet. Microbiol. 2017, 207, 108–116. [Google Scholar] [CrossRef]
- Kumar, N.; Barua, S.; Riyesh, T.; Chaubey, K.K.; Rawat, K.D.; Khandelwal, N.; Mishra, A.K.; Sharma, N.; Chandel, S.S.; Sharma, S.; et al. Complexities in Isolation and Purification of Multiple Viruses from Mixed Viral Infections: Viral Interference, Persistence and Exclusion. PLoS ONE 2016, 11, e0156110. [Google Scholar] [CrossRef] [PubMed]
- Pizzi M: Sampling variation of the fifty percent end-point, determined by the Reed-Muench (Behrens) method. Hum. Biol. 1950, 22, 151–190.
- Ma, N.; Zhang, M.; Zhou, J.; Jiang, C.; Ghonaim, A.H.; Sun, Y.; Zhou, P.; Guo, G.; Evers, A.; Zhu, H.; et al. Genome-wide CRISPR/Cas9 library screen identifies C16orf62 as a host dependency factor for porcine deltacoronavirus infection. Emerg. Microbes Infect. 2024, 13, 2400559. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, L.; Zhang, J.; Ge, X.; Zhou, R.; Zheng, H.; Geng, G.; Guo, X.; Yang, H.; Murtaugh, M.P. Nsp9 and Nsp10 contribute to the fatal virulence of highly pathogenic porcine reproductive and respiratory syndrome virus emerging in China. PLoS Pathog. 2014, 10, e1004216. [Google Scholar] [CrossRef]
- Sun, Y.; Li, C.; Liu, Z.; Zeng, W.; Ahmad, M.J.; Zhang, M.; Liu, L.; Zhang, S.; Li, W.; He, Q. Chinese herbal extracts with antiviral activity: Evaluation, mechanisms, and potential for preventing PRV, PEDV and PRRSV infections. Anim. Dis. 2023, 3, 35. [Google Scholar] [CrossRef]
- Lu, Y.; Gillam, F.; Cao, Q.M.; Rizzo, A.; Meng, X.J.; Zhang, C. Hepatitis B core antigen-based vaccine demonstrates cross-neutralization against heterologous North American Porcine Reproductive and Respiratory Syndrome Virus (PRRSV-2) strains. J. Virol. Methods 2020, 285, 113945. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zheng, X.; Sun, X.; Wan, B.; Dong, J.; Zhou, Z.; Nan, Y.; Wu, C. Characterization of in vitro viral neutralization targets of highly pathogenic porcine reproductive and respiratory syndrome virus (HP-PRRSV) in alveolar macrophage and evaluation of protection potential against HP-PRRSV challenged based on combination of HP-PRRSV-structure proteins in vitro. Vet. Microbiol. 2024, 292, 110035. [Google Scholar]
- Huang, Y.; Zhai, W.; Wang, Z.; He, Y.; Tao, C.; Chu, Y.; Pang, Z.; Zhu, H.; Jia, H. Analysis of the Immunogenicity of African Swine Fever F317L Protein and Screening of T Cell Epitopes. Animals 2024, 14, 1331. [Google Scholar] [CrossRef]
- Ouyang, Y.; Du, Y.; Zhang, H.; Guo, J.; Sun, Z.; Luo, X.; Mei, X.; Xiao, S.; Fang, L.; Zhou, Y. Genetic Characterization and Pathogenicity of a Recombinant Porcine Reproductive and Respiratory Syndrome Virus Strain in China. Viruses 2024, 16, 993. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, L.; Huang, J.; Yang, Z.; Xu, L.; Gu, S.; Huang, Y.; Zhang, R.; Sun, X.; Zhou, Y.; et al. Genetic characterization of a novel recombined porcine reproductive and respiratory syndrome virus 2 among Nadc30-like, Jxa1-like and TJ-like strains. Vet. Med. Sci. 2021, 7, 697–704. [Google Scholar] [CrossRef]
- Yim-Im, W.; Anderson, T.K.; Paploski, I.A.D.; VanderWaal, K.; Gauger, P.; Krueger, K.; Shi, M.; Main, R.; Zhang, J.; Chao, D.-Y. Refining PRRSV-2 genetic classification based on global ORF5 sequences and investigation of their geographic distributions and temporal changes. Microbiol. Spectr. 2023, 11, e0291623. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Li, D.; Hu, Y.; Gao, P.; Zhang, Y.; Zhou, L.; Ge, X.; Guo, X.; Han, J.; Yang, H.; et al. The Genetic Variation of Porcine Reproductive and Respiratory Syndrome Virus Replicase Protein nsp2 Modulates Viral Virulence and Persistence. J. Virol. 2023, 97, e0168922. [Google Scholar] [CrossRef]
- An, T.Q.; Li, J.N.; Su, C.M.; Yoo, D. Molecular and Cellular Mechanisms for PRRSV Pathogenesis and Host Response to Infection. Virus Res. 2020, 286, 197980. [Google Scholar] [CrossRef]
- Qiu, M.; Li, S.; Li, S.; Sun, Z.; Lin, H.; Yang, S.; Cui, M.; Qiu, Y.; Qi, W.; Yu, X.; et al. The GP2a 91/97/98 amino acid substitutions play critical roles in determining PRRSV tropism and infectivity but do not affect immune responses. J. Virol. 2025, 99, e0004825. [Google Scholar] [CrossRef]
- Zhou, L.; Kang, R.; Yu, J.; Xie, B.; Chen, C.; Li, X.; Xie, J.; Ye, Y.; Xiao, L.; Zhang, J.; et al. Genetic Characterization and Pathogenicity of a Novel Recombined Porcine Reproductive and Respiratory Syndrome Virus 2 among Nadc30-Like, Jxa1-Like, and Mlv-Like Strains. Viruses 2018, 10, 551. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Du, Y.; Yu, Z.; Zhang, Q.; Liu, Y.; Tang, J.; Shi, J.; Feng, W.-H. Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus Nsp4 Cleaves VISA to Impair Antiviral Responses Mediated by RIG-I-like Receptors. Sci. Rep. 2016, 6, 28497. [Google Scholar] [CrossRef]
- Chang, C.C.; Yoon, K.J.; Zimmerman, J.J.; Harmon, K.M.; Dixon, P.M.; Dvorak, C.M.; Murtaugh, M.P. Evolution of porcine reproductive and respiratory syndrome virus during sequential passages in pigs. J. Virol. 2002, 76, 4750–4763. [Google Scholar] [CrossRef] [PubMed]
- Barfoed, A.M.; Blixenkrone-Møller, M.; Jensen, M.H.; Bøtner, A.; Kamstrup, S. DNA vaccination of pigs with open reading frame 1–7 of PRRS virus. Vaccine 2004, 22, 3628–3641. [Google Scholar] [CrossRef]
- Hu, W.; Tang, D.; Zeng, Z.; Wang, B.; Zhou, M.; Mao, Y.; Zhou, P.; He, S. Research progress on the molecular mechanism of immune escape of porcine reproductive and respiratory syndrome virus. Virology 2025, 602, 110298. [Google Scholar] [CrossRef]
- Chen, N.; Trible, B.R.; Kerrigan, M.A.; Tian, K.; Rowland, R.R.R. ORF5 of porcine reproductive and respiratory syndrome virus (PRRSV) is a target of diversifying selection as infection progresses from acute infection to virus rebound. Infect. Genet. Evol. 2016, 40, 167–175. [Google Scholar] [CrossRef]
- Faaberg, K.S.; Hocker, J.D.; Erdman, M.M.; Harris, D.H.; Nelson, E.A.; Torremorell, M.; Plagemann, P.G. Neutralizing antibody responses of pigs infected with natural GP5 N-glycan mutants of porcine reproductive and respiratory syndrome virus. Viral Immunol. 2006, 19, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Wu, W.; Jiang, N.; Ge, X.; Zhang, Y.; Han, J.; Guo, X.; Zhou, L.; Yang, H. Highly Pathogenic PRRSV-Infected Alveolar Macrophages Impair the Function of Pulmonary Microvascular Endothelial Cells. Viruses 2022, 14, 452. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Gao, J.C.; Xiong, J.Y.; Guo, J.C.; Yang, Y.B.; Jiang, C.G.; Tang, Y.-D.; Tian, Z.-J.; Cai, X.-H.; Tong, G.-Z.; et al. Two Residues in NSP9 Contribute to the Enhanced Replication and Pathogenicity of Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus. J. Virol. 2018, 92, e02209-17. [Google Scholar] [CrossRef]
- Labarque, G.G.; Nauwynck, H.J.; Van Reeth, K.; Pensaert, M.B. Effect of cellular changes and onset of humoral immunity on the replication of porcine reproductive and respiratory syndrome virus in the lungs of pigs. J. Gen. Virol. 2000, 81, 1327–1334. [Google Scholar] [CrossRef]
- Shi, M.; Lam, T.T.; Hon, C.C.; Murtaugh, M.P.; Davies, P.R.; Hui, R.K.; Li, J.; Wong, L.T.; Yip, C.W.; Jiang, J.W.; et al. Phylogeny-based evolutionary, demographical, and geographical dissection of North American type 2 porcine reproductive and respiratory syndrome viruses. J. Virol. 2010, 84, 8700–8711. [Google Scholar] [CrossRef]
- Liu, D.; Zhou, R.; Zhang, J.; Zhou, L.; Jiang, Q.; Guo, X.; Ge, X.; Yang, H. Recombination analyses between two strains of porcine reproductive and respiratory syndrome virus in vivo. Virus Res. 2011, 155, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Carvajal, J.M.; Rodríguez-Gómez, I.M.; Ruedas-Torres, I.; Zaldívar-López, S.; Larenas-Muñoz, F.; Bautista-Moreno, R.; Garrido, J.J.; Pallarés, F.J.; Carrasco, L.; Gómez-Laguna, J.; et al. Time Series Transcriptomic Analysis of Bronchoalveolar Lavage Cells from Piglets Infected with Virulent or Low-Virulent Porcine Reproductive and Respiratory Syndrome Virus 1. J. Virol. 2022, 96, e0114021. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, J.; Vu, H.L.X. Porcine Reproductive and Respiratory Syndrome Virus Reverse Genetics and the Major Applications. Viruses 2020, 12, 1245. [Google Scholar] [CrossRef]
- Guo, J.; Li, C.; Lu, H.; Wang, B.; Zhang, L.; Ding, J.; Jiao, X.; Li, Q.; Zhu, S.; Wang, A.; et al. Reverse genetics construction and pathogenicity of a novel recombinant NADC30-like PRRSV isolated in China. Front. Vet Sci. 2024, 11, 1434539. [Google Scholar] [CrossRef]


| Day | NADC30-Like Strain (GZ2022) | Highly Pathogenic Strain (WUH3) | PRRSV Classical Strain | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1: 2 | 1: 4 | 1: 8 | 1: 2 | 1: 4 | 1: 8 | 1: 2 | 1: 4 | 1: 8 | |
| 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 21 | 40% (2/5) | 20% (1/5) | 0 | 20% (1/5) | 0 | 0 | 20% (1/5) | 0 | 0 |
| 28 | 60% (3/5) | 40% (2/5) | 20% (1/5) | 20% (1/5) | 20% (1/5) | 0 | 20% (1/5) | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Yu, B.; Li, Q.; Ma, H.; Yu, Z.; Ma, P.; Ruan, S.; Yu, X.; He, Q.; Li, W. Isolation and Biological Characteristics Study of Porcine Reproductive and Respiratory Syndrome Virus GZ2022 Strain. Vet. Sci. 2025, 12, 651. https://doi.org/10.3390/vetsci12070651
Yang X, Yu B, Li Q, Ma H, Yu Z, Ma P, Ruan S, Yu X, He Q, Li W. Isolation and Biological Characteristics Study of Porcine Reproductive and Respiratory Syndrome Virus GZ2022 Strain. Veterinary Sciences. 2025; 12(7):651. https://doi.org/10.3390/vetsci12070651
Chicago/Turabian StyleYang, Xinmei, Bin Yu, Qing Li, Hailong Ma, Zhengjun Yu, Pei Ma, Shengnan Ruan, Xuexiang Yu, Qigai He, and Wentao Li. 2025. "Isolation and Biological Characteristics Study of Porcine Reproductive and Respiratory Syndrome Virus GZ2022 Strain" Veterinary Sciences 12, no. 7: 651. https://doi.org/10.3390/vetsci12070651
APA StyleYang, X., Yu, B., Li, Q., Ma, H., Yu, Z., Ma, P., Ruan, S., Yu, X., He, Q., & Li, W. (2025). Isolation and Biological Characteristics Study of Porcine Reproductive and Respiratory Syndrome Virus GZ2022 Strain. Veterinary Sciences, 12(7), 651. https://doi.org/10.3390/vetsci12070651








